The purpose of this study is to help in the choice of an appropriate reconstructive technique by reference to the dimensions of the defect, the required functional and esthetic outcomes, and retention of adequate surgical safety margins to prevent primary tumor recurrence.
Material and methodsA total of 158 patients were treated. We indicate how the most appropriate reconstructive method may be chosen, with reference to the size and position of the cancer and depth of tissue infiltration.
ResultOf all patients, 89 (56.3%) had T1 (lesions up to 2cm long, less than 1/3). The remaining patients had T2 lesions >2cm, from 1/3 to 2/3 of lip involvement (50 patients), T3 lesions>4cm, more than 2/3 of lip involvement (18), and a T4 lesion>5.5cm with commissure involvement (1).
ConclusionWe share the widespread view that a surgeon who performs a reconstruction using the minimal tissue components required to close the lesion will achieve the best results. Reconstruction does not influence prognosis and overall should be oriented to the defect. Careful, clean, and safe resection of lip carcinoma, with creation of healthy margins, can be followed by functional and esthetic lip reconstruction.
El objetivo de este estudio es orientar al cirujano en la correcta elección de una técnica reconstructiva según la dimensión del defecto, de los resultados estéticos-funcionales necesarios y tendente a conservar los margenes quirúrgicos de seguridad indispensables para prevenir la recidiva del tumor primitivo.
Materiales y métodosAnalizaremos los casos de cáncer de labio tratados en 158 pacientes, indicaremos el método apropiado para la reconstrucción con referencia a la dimensión y localización del cáncer, y a la profundidad del tejido infiltrado.
ResultadosDe todos los pacientes que hemos analizado, 89 (56,3%) pertenecían al grupo clasificado como T1 (lesiones hasta 2cm de largo, menos de 1/3 del labio implicado), 50 pacientes pertenecían al grupo T2 (lesiones>2cm, desde 1/3 hasta 2/3 del labio involucrado), 18 pacientes pertenecían al grupo T3 (lesiones>4cm, más de 2/3 de labio implicado) y un paciente pertenecía al grupo T4 (lesiones>5,5cm, con la comisura incluida).
ConclusiónCoincidimos con la idea de que el cirujano reconstruye utilizando la cantidad mínima de tejido para corregir la deformidad y obtener los mejores resultados, pero la reconstrucción no puede influir en el pronóstico, solo debe orientar el tipo de defecto.
Una extirpación segura de el tumor del labio, meticulosa, con el mantenimiento de los márgenes sanos, debe ser complementada con una cirugía reconstructiva del labio en su totalidad, es decir, en toda su estética y su funcionalidad.
Lip squamous cell carcinoma constitutes 15–30% of all oral cavity cancers, and the lower lip is involved in 80% of cases.1–4 Lower lip cancer (excluding non-melanoma skin cancer) constitutes 5% of all head-and-neck cancers.5 Most patients are Caucasian males over 50 years of age, live in rural areas, and have jobs associated with high-level exposure to UV radiation.6,7
Lip cancer is a slowly developing tumor, and the frequency of lymph node metastasis is 3–29%.8 Metastasis is initially noted in level I nodes (the sub-mental and submandibular nodes), later extending to nodes at lower levels.9 The 5-year survival rate is 85–99% in patients with stage T1N0 cancer, but decreases markedly (to 25–50%) in patients who are in the cN+ stage at presentation.10,11 The pronounced effect of N+ stage on prognosis reflects immune status.
Macroscopic growth of squamous cell carcinoma may involve the entire vermilion border, the skin component of the lip, and the commissure and oral mucosa. Indeed, advanced cancer can involve the jawbone, featuring vessel and neural invasion. The standard therapy for lower lip squamous cell carcinoma is surgery12,13; many authors have developed different techniques for reconstruction of surgical defects, and the extent of resection required to ensure safe margins has attracted a great deal of attention.
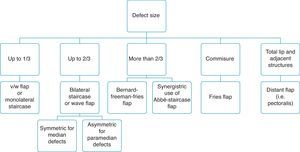
It is accepted that a good reconstructive approach must achieve various esthetic goals, and this may be challenging when a defect affects over 2/3 of the lip length.14,15 Many lower lip reconstructive techniques have been described, and no ideal method of reconstruction has yet been devised.
In the last 150 years, over 200 different reconstructive techniques have been described, ranging from typical V/W-shaped excision to treat lesions up to 1cm long to the creation of different types of flaps (used alone or in combination) to close bigger defects, even the entire lower lip.16 The aim of the present study was to standardize a reconstructive surgical approach oriented to the lower lip defect emphasizing, principles timing and esthetic results.
Patients and methodsDue to the retrospective nature of this study, it was granted an exemption in writing by the University of Modena and Reggio Emilia IRB.
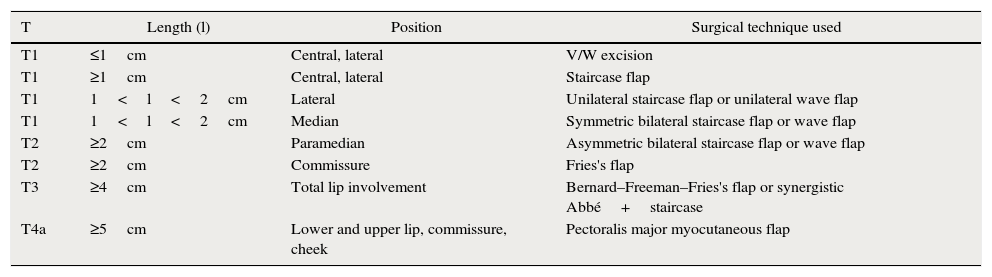
From January 1995 to March 2012, 158 patients (133 males and 25 females) were treated for lower lip squamous cell carcinoma. The mean patient age at the time of tumor excision and reconstruction was 75.63±13.02 years. All subjects exhibited one or more known risk factors for lip cancer (i.e., chronic exposure to the sun and/or cigarette smoking). Lower lip lesions were classified primarily by length and biopsied using suitably sized surgical punches. Of all patients, 89 were of tumor grade T1, 50 were T2, 18 were T3, and 1 was of grade T4. Clinical and ultrasonographic tests were run to investigate neck lymph node status and tumor stage. Each lesion was classified in terms of dimensions, position, and operability. The tumor characteristics were considered during choice of an adequate treatment algorithm (Tables 1 and 2).
Flaps created for patients varying in T stage and in the size and position of the defect.
| T | Length (l) | Position | Surgical technique used |
|---|---|---|---|
| T1 | ≤1cm | Central, lateral | V/W excision |
| T1 | ≥1cm | Central, lateral | Staircase flap |
| T1 | 1<l<2cm | Lateral | Unilateral staircase flap or unilateral wave flap |
| T1 | 1<l<2cm | Median | Symmetric bilateral staircase flap or wave flap |
| T2 | ≥2cm | Paramedian | Asymmetric bilateral staircase flap or wave flap |
| T2 | ≥2cm | Commissure | Fries's flap |
| T3 | ≥4cm | Total lip involvement | Bernard–Freeman–Fries's flap or synergistic Abbé+staircase |
| T4a | ≥5cm | Lower and upper lip, commissure, cheek | Pectoralis major myocutaneous flap |
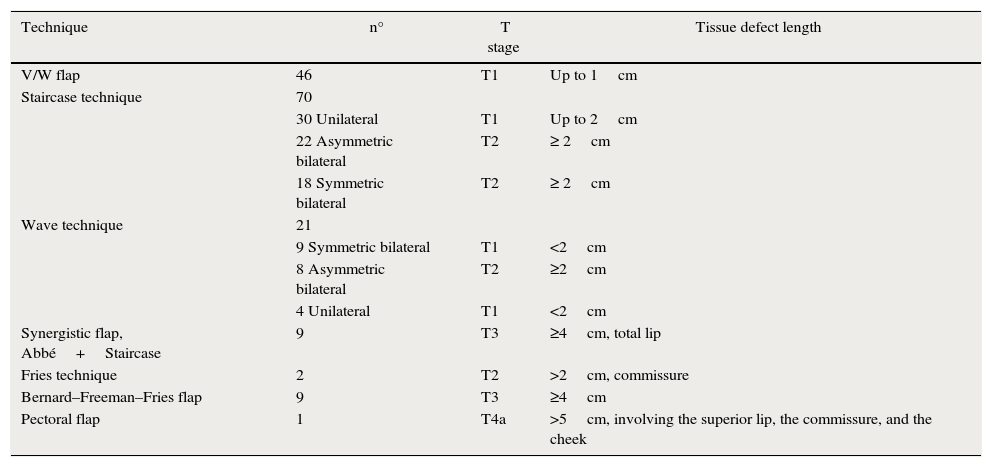
Numbers of flaps constructed by defect type.
| Technique | n° | T stage | Tissue defect length |
|---|---|---|---|
| V/W flap | 46 | T1 | Up to 1cm |
| Staircase technique | 70 | ||
| 30 Unilateral | T1 | Up to 2cm | |
| 22 Asymmetric bilateral | T2 | ≥ 2cm | |
| 18 Symmetric bilateral | T2 | ≥ 2cm | |
| Wave technique | 21 | ||
| 9 Symmetric bilateral | T1 | <2cm | |
| 8 Asymmetric bilateral | T2 | ≥2cm | |
| 4 Unilateral | T1 | <2cm | |
| Synergistic flap, Abbé+Staircase | 9 | T3 | ≥4cm, total lip |
| Fries technique | 2 | T2 | >2cm, commissure |
| Bernard–Freeman–Fries flap | 9 | T3 | ≥4cm |
| Pectoral flap | 1 | T4a | >5cm, involving the superior lip, the commissure, and the cheek |
All patients were evaluated prior to surgery for their ability to tolerate anesthesia (ASA codes were generated). Information on general medical status, medical history, presence of chronic disease, drugs taken, and age was gathered. These data were combined with information on the clinical and pathological characteristics of each lesion to choose optimal techniques for defect reconstruction.
It was imperative that each lesion be radically excised, leaving an adequate safety margin on all sides (always 6–10mm if margins were not examined by a pathologist working in the operating theater, and 3mm otherwise). It has been reported that 3mm of healthy tissue is sufficient when a cryostat test of the margins is performed intra-operatively. However, 6–10mm is necessary when this is not performed. For tumors up to 1cm (46 cases), when no cryostat test was performed, we kept a margin of 6mm.17
Some smaller lesions were treated under local assisted anesthesia (with ECG monitoring and maintenance of O2 saturation). Mepivacaine (10ml, 2.0% [w/v] solution) with a 1:100.000 dilution of epinephrine was used to this end. Other patients were treated under general anesthesia with nasothrachel intubation.
Mucosa and muscle incisions were closed with 4/0 Vicryl (polygalactin 910; Ethicon, Inc. [Johnson and Johnson], Somerville, NJ) and skin incisions with 4/0 or 5/0 monofilament Prolene (polypropylene; Ethicon, Inc.). Skin sutures were removed on day 7, and intraoral sutures on day 14. All patients received antibiotics (1g amoxicillin and 1g clavulanic acid every 12h for 4 days). On request, patients were also given paracetamol 1g three times daily or ibuprofen 600mg three times daily. Cancer surgery was followed by lip reconstruction at the same time.
The follow-up period was 15–125 months, with checkups every 3 months in the first year, every 6 months in the second and third years, and annually thereafter.
Surgical techniquesThe V/W incision full thickness is performed in all lesion, median or lateral, smaller than 1cm. Defect edges are advanced and primary closure was always researched.
Sutures are placed both on mucosa and skin side. Skin sutures are removed after 7 days and intraoral after 14 days.
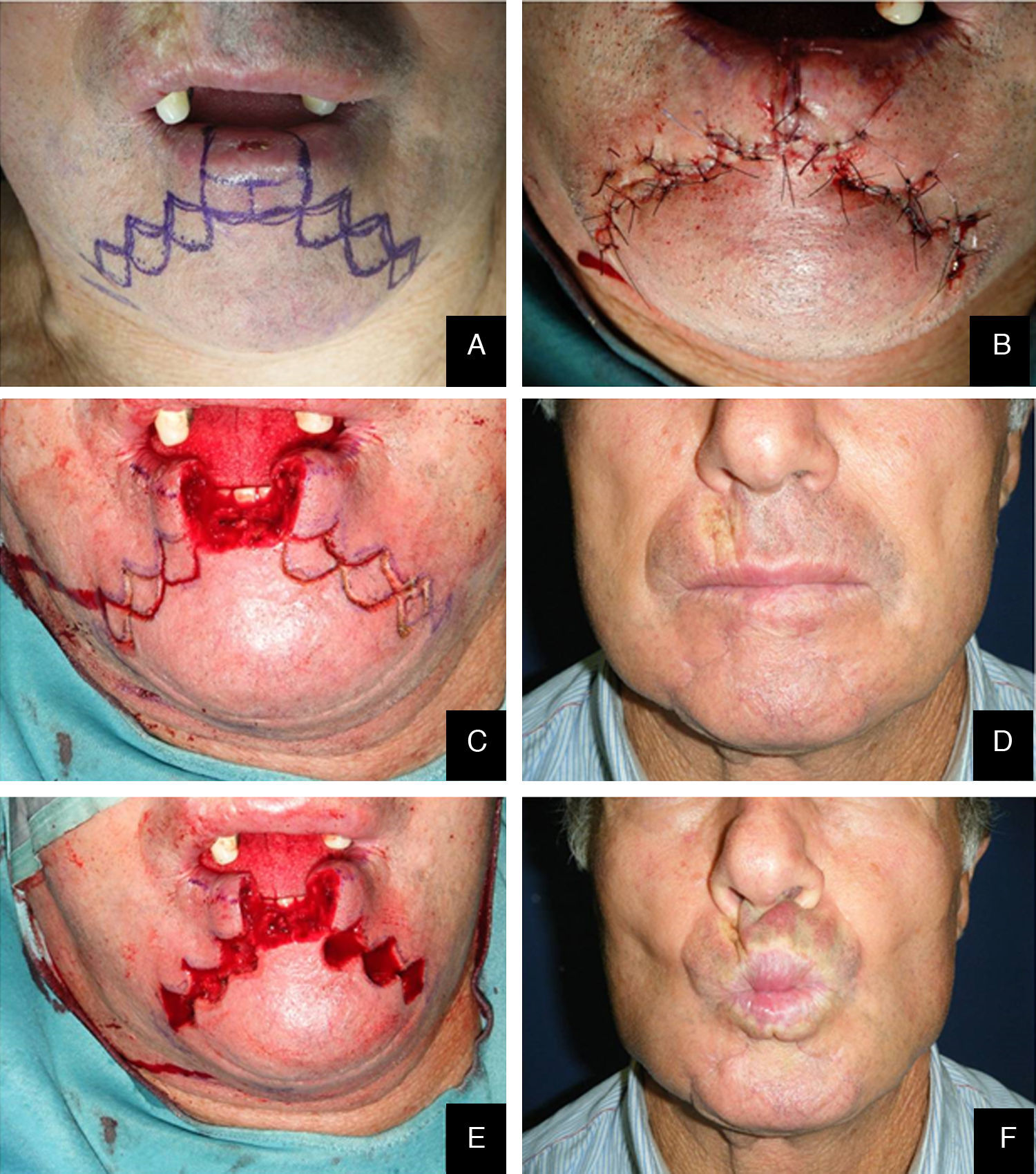
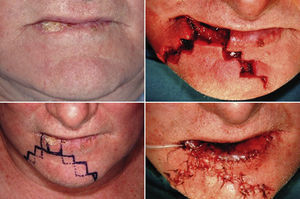
We performed the Staircase technique devised by Johanson et al. and modified by Dado and Angelats, Stiernberg, Kuttenberger and Hardt and Salgarelli et al.2,18–21 with preservation of the orbicularis oris, depressor anguli oris, and depressor labii inferioris. The tumor was removed using a full-thickness excision.
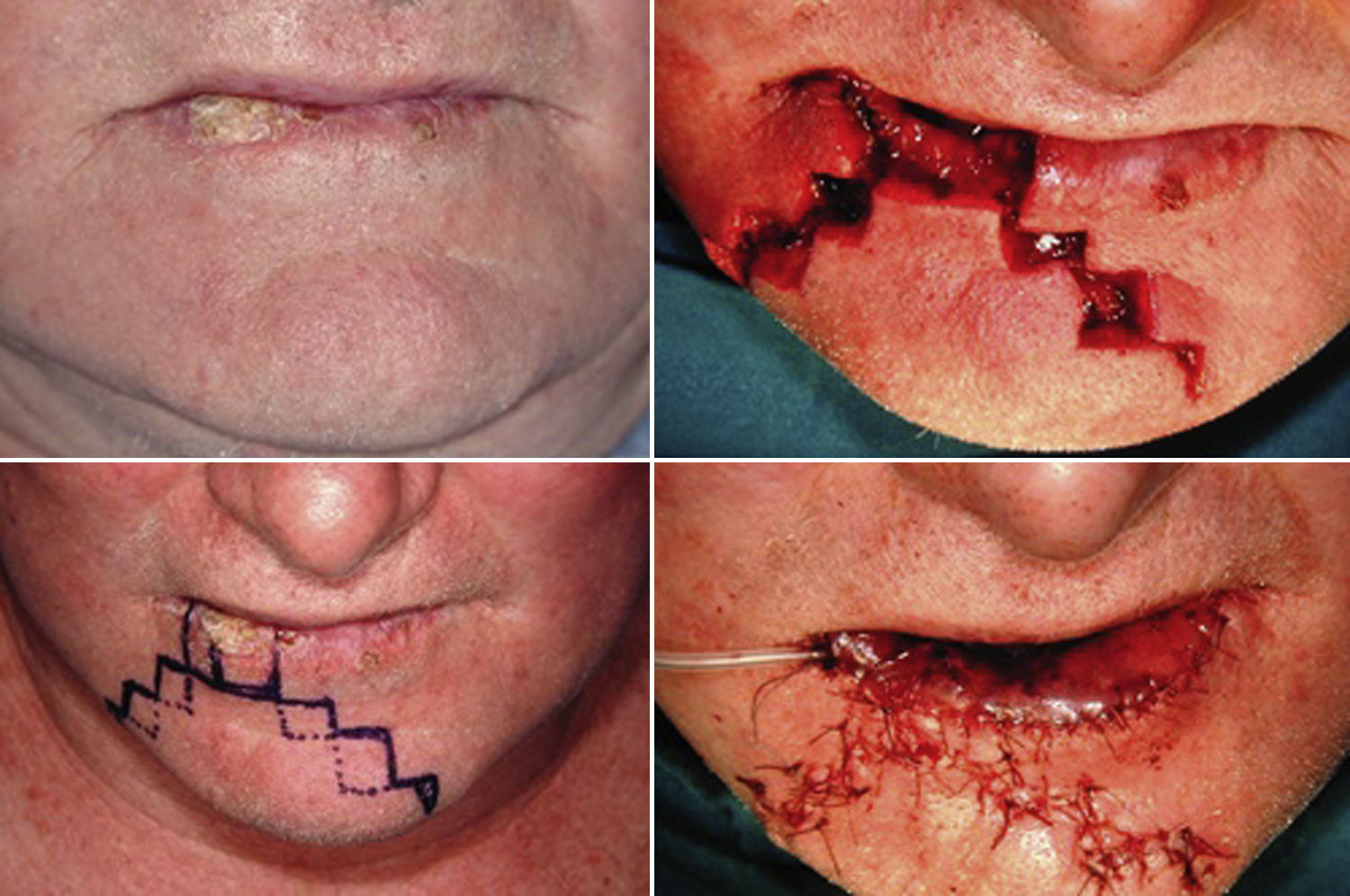
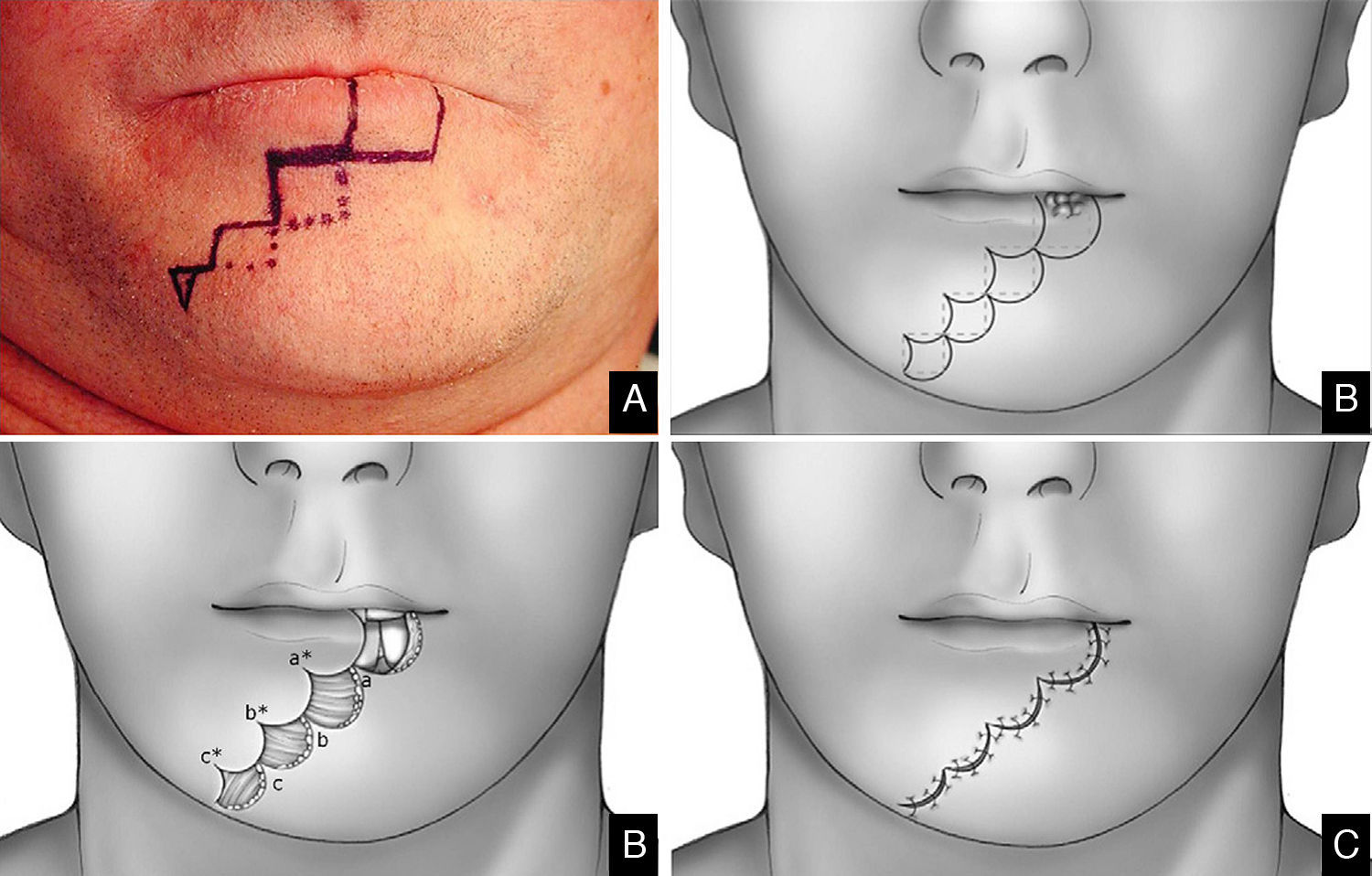
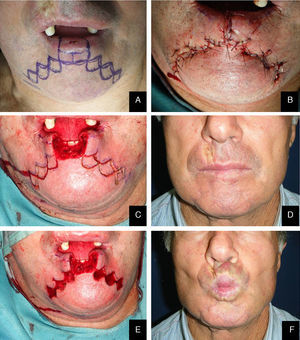
Lateral defects less than 2cm in size are generally treated with a unilateral flap, and median defects are closed with bilateral symmetric flaps. If the defect is paramedian and greater than 2cm in width, two asymmetric flaps are used. In the first stage of reconstruction, an incision is made starting at the lower margin of the defect and is extended laterally parallel to the vermilion border. The length of this incision should be a few millimeters less than the length of the defect if only one flap is prepared; it should be divided between the two sides if bilateral flaps are used. The next stage is the same for both the monolateral and bilateral staircase technique. An incision is made from the lateral side of the horizontal incision and extended vertically downward toward to the chin for 8 to 10mm. From this point, an additional horizontal incision is made and extended laterally for 3mm less than the previous step. Two or 3 steps are usually required to close the defect. The incision ends with a Burow triangle with an inferiorly located apex and a base approximately corresponding to two thirds of the last horizontal incision; triangles are excised at a plane superficial to the muscle layer, which is left intact. Thereafter, the rectangles below the steps must be removed (Figs. 1–3).
In the last stage, the flaps are advanced and approximated using a layered closure.
Sutures are placed starting from the mucosa toward the skin in areas of full-thickness tissue loss, and routine (subcutaneous tissue and skin) suturing is done in the Burow triangles and excised rectangles, taking care to place corner sutures at the corners of the steps.
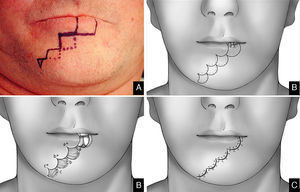
The wave technique is basically derived from the staircase one described above. The goal of this modification is to use broken lines and round lines is to create less visible scars.22
We begin by delimiting the lesion in a rectangular shape, and the inferior line is extended laterally, parallel to the vermilion border. The length of this mark should be a few millimeters less than the length of the full-thickness defect if only one flap is prepared; it should be divided between the two sides if bilateral flaps are used. In the case of symmetric bilateral flaps, the length of the defect is equally divided between both sides. In the case of asymmetric bilateral flaps, the length of the defect is divided between the two sides, keeping the longest flap homolateral to the lesion. The next stage is the same for both monolateral and bilateral flaps. A line is made from the lateral side of the horizontal line and extended vertically downward toward to the chin for 8/10mm. From this point, an additional horizontal line is made and extended laterally 3mm less than the previous step. The last stage of the flap planning is modification of the rectangular design in a wave shape. The nodal point of the new design is that each straight line becomes curved. The superior and lateral sides form a single arc designed outside of the rectangle, while the medial sides appear as two opposite arcs inside at the rectangle. It is important to note that all of the opposite arcs must have the same radius of curvature. Two or three waves are usually required to close the defect.
In the last stage, the flaps are advanced medially toward the defect and approximated using a layered closure that reveals the wave shape (Figs. 5 and 6).
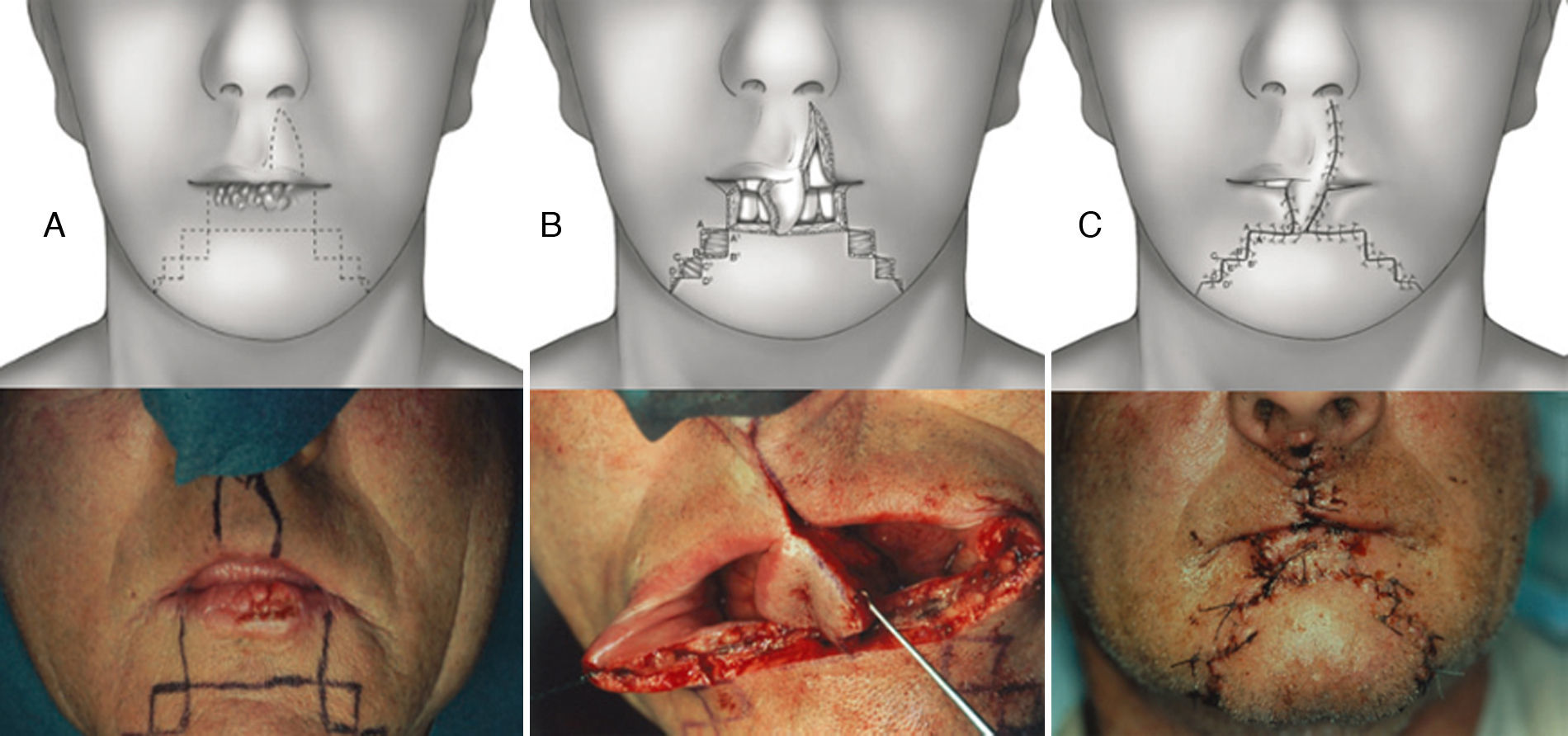
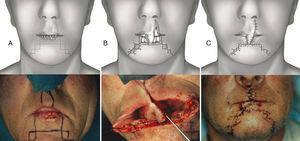
Synergistic use of Abbé and staircase flap. (A) Preoperative planning for the Sabattini-Abbe¿ flap on the medial part of the upper lip and bilateral symmetrical staircase flaps in the inferior lip: scheme and clinical case. (B) The Sabattini-Abbe¿ flap is totally incised on the side away from the pedicle and rotated into the lip defect; the staircase flaps are incised: scheme, note preservation of muscle layer, and clinical case. (C) The staircase flaps are advanced and approximated to the Sabattini-Abbe¿ flap, and sutures are placed: scheme and clinical case.
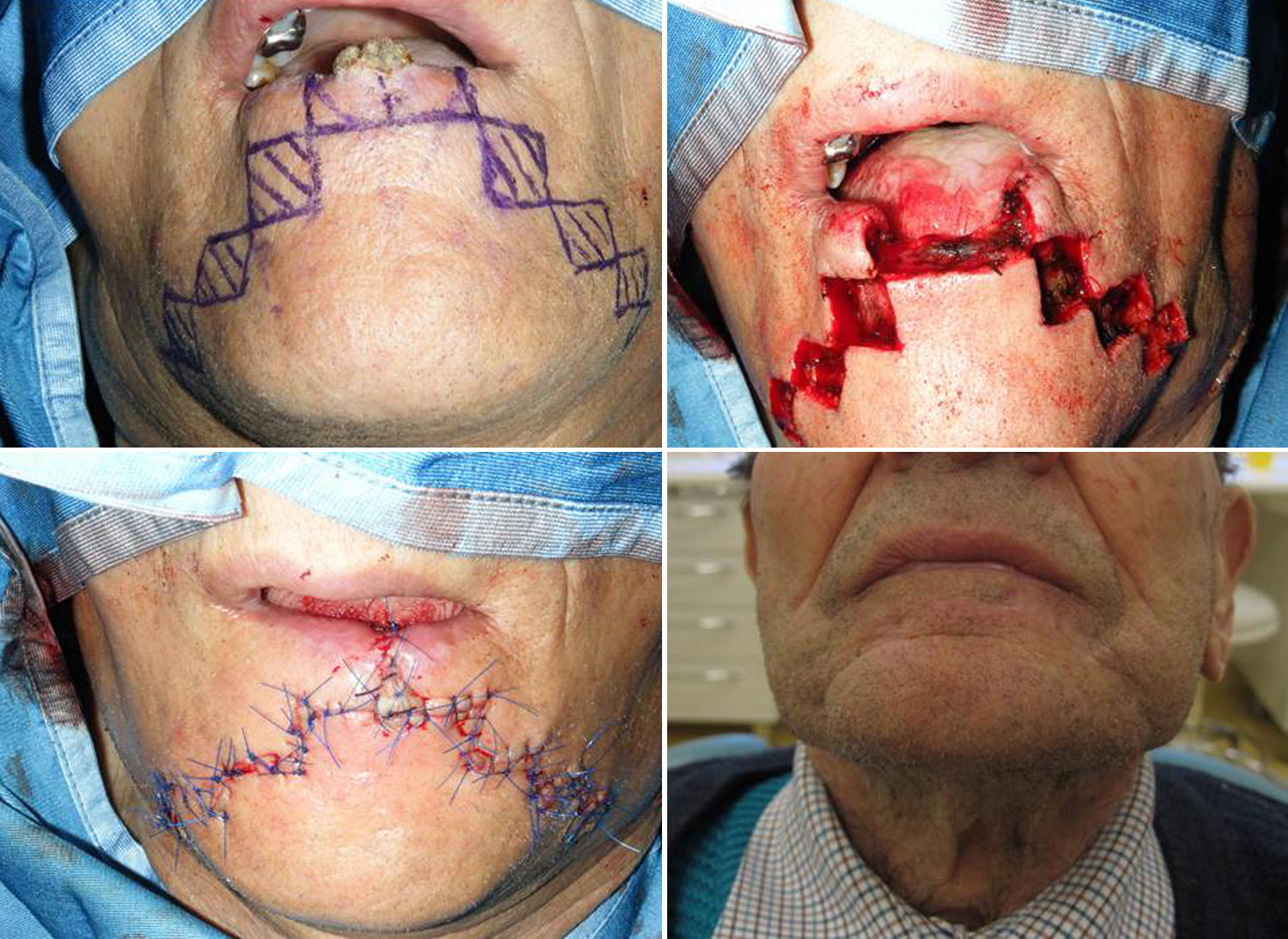
The synergistic flap technique23,24 is used to close defects wider than 2/3 of the lip. After full-thickness box resection in the first step, a Sabattini-Abbe’ flap is used to replace the central third of the defect. The median upper lip flap is totally incised on the side away from the pedicle, and the labial artery position is noted. On the other side, the lip is incised until only a small cuff of subcutaneous tissue and muscle surround the vascular pedicle. This allows for easy rotation of the flap into the central lower lip defect. Closure of the upper lip is performed in layers. Care must be taken when suturing around the pedicle, because blood flow may be compromised. Persistent cyanosis calls for mandatory suture removal in this area.
In the second step, a bilateral symmetric staircase flap is used to complete the lower lip reconstruction (described above) (Figs. 6 and 7).
In the last stage, the flaps are advanced and approximated to the Sabattini-Abbe¿ flap, using a layered closure; a small detachment is made, if necessary. During the suturing, the most important moment is the refurbishment of the vermillion. The vermillion border is the normally sharp demarcation between the lip (red colored) and the adjacent normal skin.
It represents the change in the epidermis from highly keratinized external skin to less-keratinized internal skin. The first point is usually located right on the vermillion and then proceeds to suture the skin and the mucosa; this closure has the best outcome because it reestablishes continuity of the vermillion border and adequate size of the opening.
The pedicle of the Sabattini-Abbe¿ flap is divided after 14–28 days; any necessary trimming, particularly refurbishment of vermilion on the pedicle flap side, is performed at this time (Fig. 7).
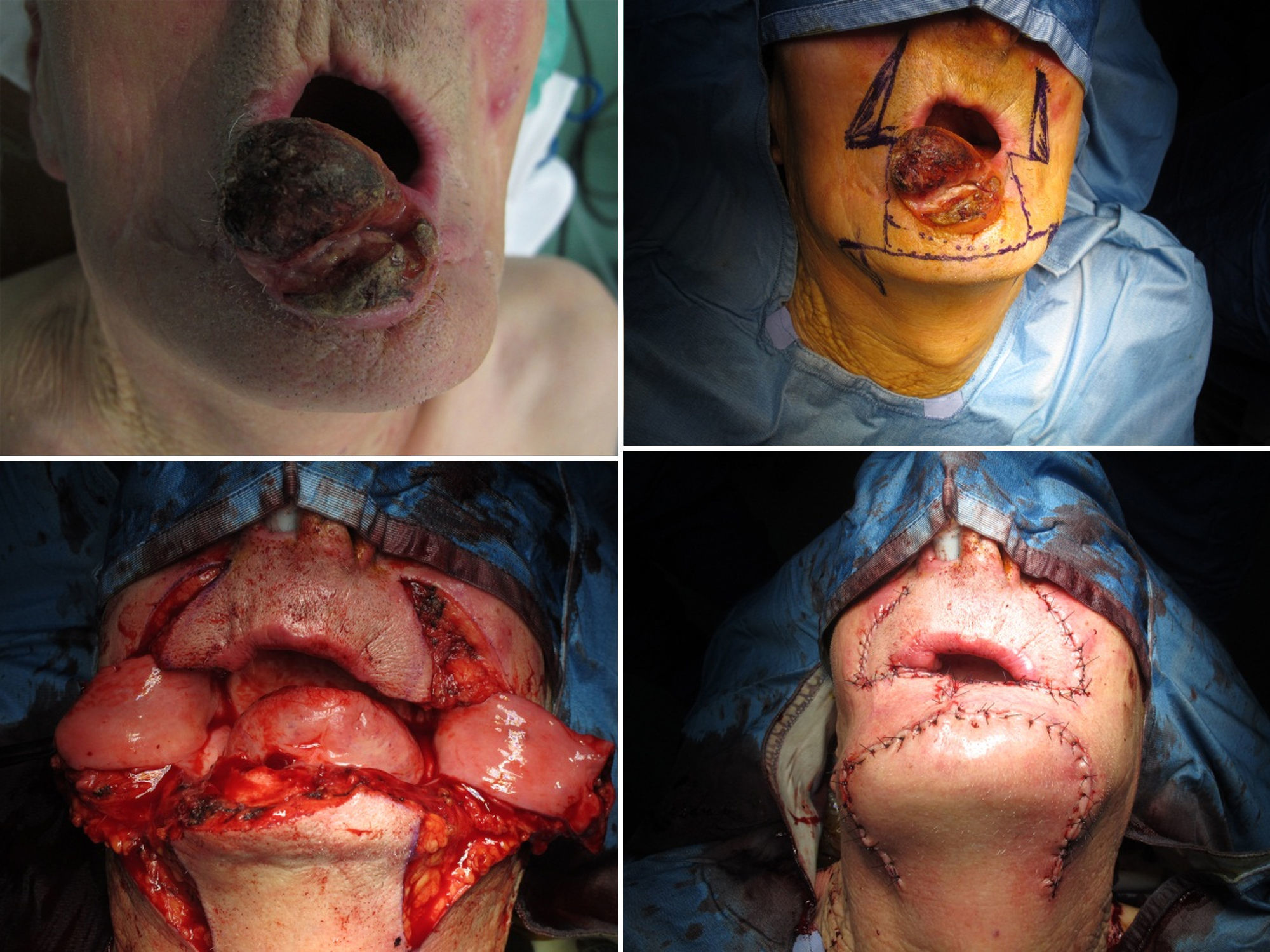
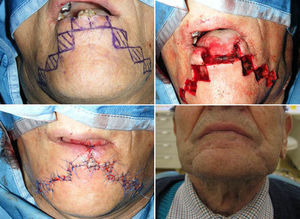
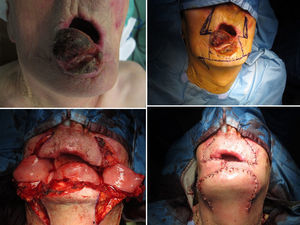
The Bernard–Freeman–Fries25–27 technique is performed under general anesthesia with nasotracheal intubation. Defect is removed with the design of a quadrilateral shape, compatibly with extension. Secondarily, labial commissures are separated on each side, with incision directed through the cheek and Burow triangle design, laterally the nose wing and closer to the nasal-labial line. Two additional triangles with apex converging to the midline are designed in the chin. It is important to preserve the mucosal layer because it acts as guidance for vermilion reconstruction. Bilateral flaps mobilization permits to close the defect with the suture on the medial line. Tissue are sutured by planes, full or mid thickness, depending on the flap area (Figs. 8 and 9). The last stage consists in the plastic reconstruction of the lost vermilion. Sutures are removed in 30 days.
ResultsOf all patients, 89 (56.3%) had T1 (lesions less than 2cm long, less than 1/3). The remaining patients had T2 lesions≥2cm, up to 2/3 of lip involvement (50 patients), T3 lesions≥4cm, more than 2/3 of lip involvement (18), and a T4 lesion≥5.5cm with commissure involvement (1).
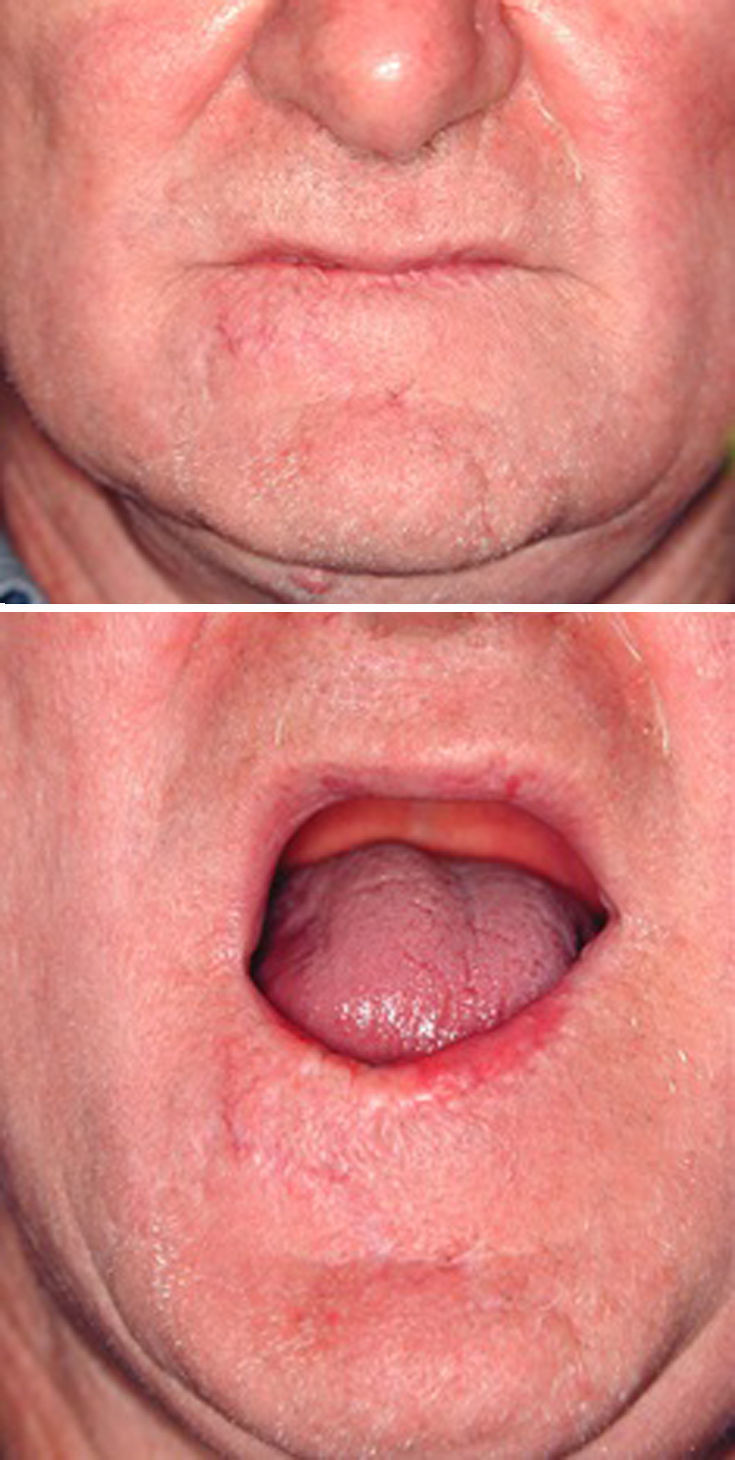
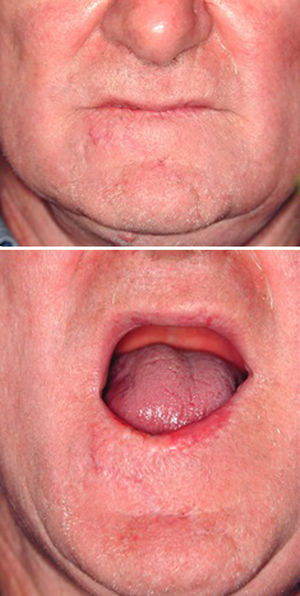
Neck lymph node data were positive (cN+) in 16 patients who were treated with modified radical neck dissection (MRND). Three T2N1 and six T3N1 patients underwent MRND. Three T3N2a and four T3N2c underwent bilateral MRND. Only one local tumor relapse was observed during follow-up, in a 39-year-old renal transplant patient under high-level immunosuppressive drug therapy. All patients achieved acceptable esthetic results, and functional deficits resolved after healing, except the defect corrected with pectoralis flap reconstruction where only closure was researched, with minimal attention being paid to esthetics or functionality, due to lesion wide extension (T4) and patient age.
DiscussionSurgical removal is the treatment of choice for squamous cell carcinoma of the lower lip, although radiotherapy or brachytherapy can be used to treat small lesions29; tissue loss is treated using a variety of techniques, depending on the extent and location (median or lateral) of the defect.2 The functional goals of reconstructive lip surgery include creation of a watertight seal to prevent drooling, preservation of the ability to insert dentures and access dentition, preservation of lip sensation, preservation of a symmetrical appearance of the lip at rest, and preservation of dynamic voluntary and involuntary functions (particularly smiling).
Functional and esthetic lip reconstruction has a very long history but remains surgically challenging. Today, these aspects of the work must be a primary concern of every reconstructive surgeon. An ideal scar must be flat, narrow, and level with the surrounding skin, and it must match the lip color well and lie within or parallel to relaxed skin tension lines. Moreover, it is often necessary to repair quite large defects if intraoperative pathological examination of defect margins yields positive results. The prognosis improves if the margins are healthy and the resection radical. Recurrence is observed when, histologically, the stroma is widely infiltrated by connective tissue, and desmoplasia is evident.
A V/W flap was constructed for 2911% of all patients, all with defect lengths ≤1cm.16 Two scars developing upon healing were treated with Z-plastic in a second surgical procedure.
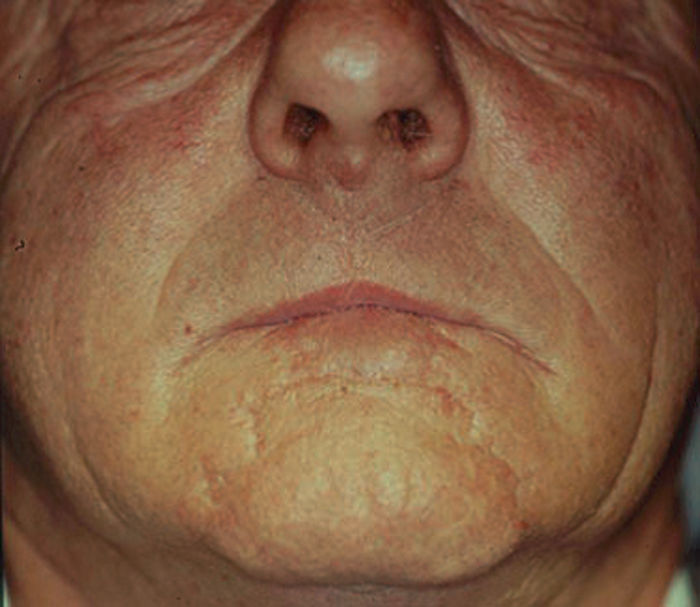
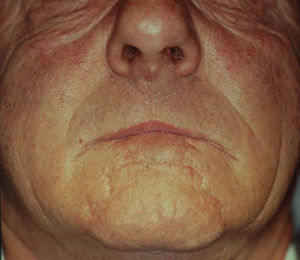
We decided to correct 91 defects involving up to 60% of the lower lip using staircase or wave flaps. Such flaps are respectful of anatomy, with the exception of the commissure, in patients with cancer of grade T2 or less. The flaps are very flexible and easy to construct, and they afford several distinct advantages. The flaps can be constructed under local anesthesia. Scarring is minimal and can be corrected later, 6 months after the first intervention. In elderly patients, the scar blends well with the wrinkles of age (Fig. 3). The most common post-operative complication is temporary local paresthesia, which self-resolves after an average interval of 6 months. Staircase flaps were constructed for 44.3% of patients.2,18–21 This approach was very adaptable, and such flaps were used to correct median, paramedian, or lateral defects.
The wave technique22 uses the “steps” of the staircase flap, with retention of dimensions and extensions, but transforms straight lines into curves to achieve better esthetic results. The original Burow's triangle is now rounded, allowing the flap to readily slide to cover lost tissue (Fig. 4).
Techniques that break up the line of the scar or render scar margins irregular afford better camouflage. Thus, we replaced the straight lines of the staircase technique by the curves of the wave technique, improving esthetic results. Curves render scars less visible. In our view, this approach is particularly valuable because it is not necessary to transect the orbicularis oris, the depressor labii inferioris, or the depressor anguli oris, thus preventing adverse functional or cosmetic sequelae. Further, this technique is a single-stage procedure, unlike other techniques (such as the Abbé approach) in which transposition flaps must be later converted to free flaps (Fig. 5). The wave flap was constructed for 13.3% of patients, with indications similar to those who were treated by construction of staircase flaps.
We also performed widely used Bernard–Freeman–Fries flap,25–27 but if possible, such surgery should be reserved for lip reconstruction in T3 patients because of high rates of associated functional and esthetic defects. The approach is particularly useful for reconstruction of severe lip defects and is relatively easy to perform. In contrast to the staircase technique, scarring is marked after a Bernard-Freeman-Fries repair, and smiling may be difficult (Figs. 8 and 9). When a defect involved 60–100% of the lower lip, a Bernard–Freeman–Fries flap was the favored solution and was constructed for 5.7% of patients. However, if the lip commissure is involved, a Fries flap should be formed.28
If over 2/3 of the lower lip is lost (over 60%), a combination of a Sabattini–Abbé flap and a bilateral staircase flap should be considered2,15,23 (Fig. 6). Cross-lip reconstruction involves transfer of full-thickness lip tissue, and restoration of the orbicularis oris sphincter is thus required (Fig. 6A). The first signs of motor reinnervation begin to appear a few months after flap transfer. Motor unit potentials become increasingly normal within 1 year after surgery. Superior lip tissue is used to fill the tissue defect of the lower lip and formation of a staircase flap to approach the margins of the defect; this procedure was used to treat 5.7% of patients. An inevitable consequence of use of this procedure is the presence of pedicles on the Sabattini–Abbé flap, which were divided after 14–28 days, depending on healing time and the quality (in terms of blood supply and venous congestion) of any flap, particularly the pedicle flap. During healing of the latter flap, patients were given a liquid diet ingested through a straw. The latter flap uses full-thickness tissue from the upper lip and half-thickness tissue from the mental region to close the defect (Fig. 6B and C). The results are very good (Fig. 7); motor nerve activity returns to normal within 1 year.
We have chosen to apply this technique considering age of patient (9 cases), reserving Bernard-Freeman-Fries in older ones (9 cases).
In one patient with squamous cell carcinoma affecting many adjacent structures (T4), a pectoralis flap was constructed to close the defect, with minimal attention being paid to esthetics or functionality.
In our present study, we report no disease recurrences. This might be associated with the fact that a high proportion of our patients had T1 lesions, over half of which were less than 1cm in length. Excision of 6–10mm of adjacent healthy tissue provided an adequate margin of safety.29,30 Safety was further assured by treating widespread cheilitis (pre-cancerosis) by vermigliectomy. We adhered to these principles and treated our patients to a consistently high standard.
We also report that one case, a transplanted patient under immunosuppressive therapy, was involved by disease recurrence.31
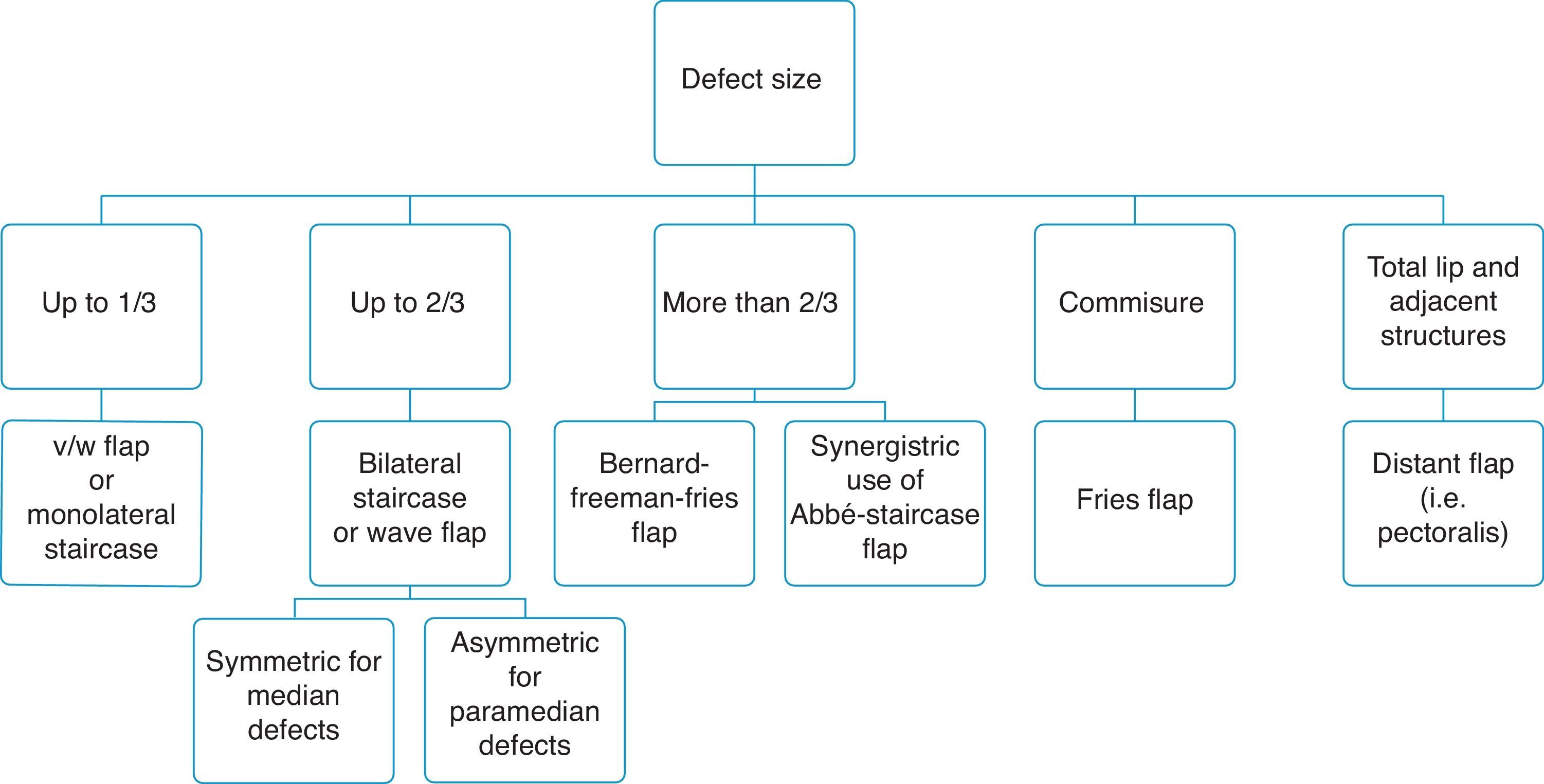
Moreover, we have prepared a simple flow chart to guide appropriate flap choice (Fig. 10).
ConclusionsWe share the widespread view that a surgeon who performs a reconstruction using the minimal tissue components required to close the lesion will achieve the best results. Reconstruction does not influence prognosis and overall should be oriented to the defect. Careful, clean, and safe resection of lip carcinoma, with creation of healthy margins, can be followed by functional and esthetic lip reconstruction.
Conflict of interestNo conflict of interests exists.
FundingNo sources or funding for this research are to be declared. The nature of the study is retrospective and observational; no experimentation on human subject was done.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.