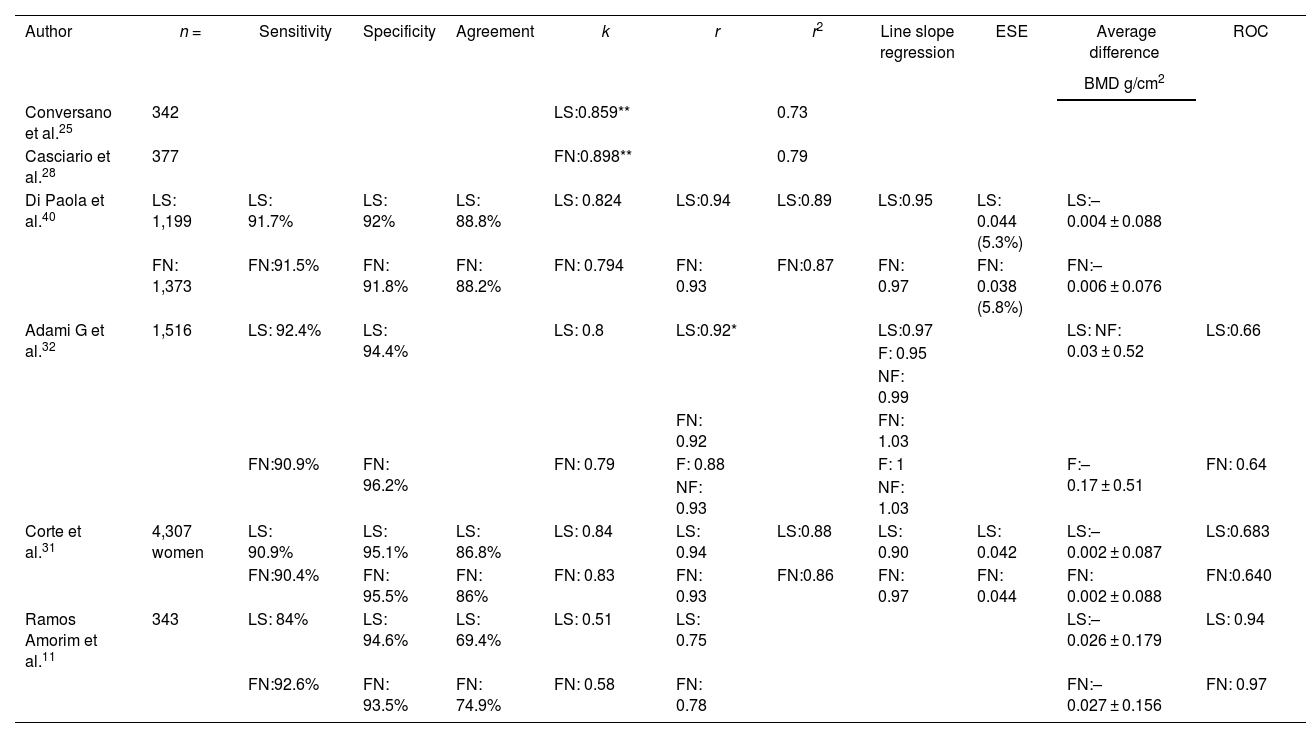
Osteoporosis is a skeletal disorder characterized by decreased bone mineral density (BMD) and a disruption of bone microarchitecture, leading to an increased fracture risk. Traditionally, BMD has been measured using X-ray densitometry (DXA). However, Radiofrequency Echographic Multi Spectometry (REMS) has emerged as a promising technique for the assessment of osteoporosis. Various studies have evaluated the feasibility and precision of REMS, showing significant correlations with DXA in different anatomical sites and populations, including post-menopausal women, renal transplant patients, and those with rheumatoid arthritis, among others. REMS has also demonstrated the ability to detect bone artifacts and provide reliable measurements in their presence. While DXA remains the gold standard for diagnosing osteoporosis, REMS has proven to be an effective and promising tool in assessing BMD and fracture risk. Its capability to rule out artifacts and offer precise measurements in diverse populations highlights its potential as a complement or alternative in the evaluation of osteoporosis.
La osteoporosis es una enfermedad esquelética caracterizada por una disminución de la densidad mineral ósea (DMO) y la alteración de la microarquitectura ósea, lo que resulta en un incremento del riesgo de fractura. Tradicionalmente, se ha medido haciendo una densitometría por rayos X (DXA). Sin embargo, la multiespectrometría ecográfica por radiofrecuencia (REMS) ha emergido como una técnica prometedora para la evaluación de esta enfermedad. Diferentes estudios han evaluado la factibilidad y la precisión del REMS, y han mostrado correlaciones significativas con DXA en diferentes sitios anatómicos y distintas poblaciones, incluyendo mujeres postmenopáusicas, hombres, pacientes con artritis reumatoide y trasplantados renales, entre otros. El REMS también ha demostrado su capacidad para detectar artefactos óseos y proporcionar mediciones confiables en su presencia. Aunque la DXA sigue siendo el estándar de oro para el diagnóstico de la osteoporosis, el REMS se ha revelado como una herramienta eficaz y prometedora en la valoración de la DMO y el riesgo de fractura. Su capacidad para descartar artefactos y ofrecer mediciones precisas en diversas poblaciones resalta su potencial como complemento o alternativa a la evaluación de la osteoporosis.