The BAFF complex (B cell activator factor) composed by the BAFF cytokine, APRIL and their receptors – BAFF-R (BR3), TACI, BCMA – influences B-lymphocyte maturation, peripheral survival and immunoglobulins class isotype switching, with multiple potential clinical implications. In this review we discuss BAFF biologic functions and it relevance in several clinical disorders – autoimmune, neoplastic, infectious and BAFF therapies. BAFF/APRIL serum levels are increased in autoimmune diseases where their concentrations are related with disease antibodies titles, activity, progression and inclusive organic compromise, making its inhibition an important therapeutic target.
El complejo BAFF (factor activador de células B) compuesto por la citocina BAFF, APRIL y sus receptores —BAFF-R (BR3), TACI y BCMA— influyen en la sobrevida periférica, en la maduración de los linfocitos B y en el cambio de clase de las inmunoglobulinas, con múltiples implicaciones clínicas potenciales. Las funciones biológicas de BAFF y su relevancia en varios desórdenes clínicos —autoinmunes, neoplásicos, infecciosos, incluyendo las terapias BAFF dirigidas— son revisadas y discutidas en el presente artículo. Los niveles séricos de BAFF/APRIL se encuentran incrementados en las enfermedades autoinmunes en las que sus concentraciones se relacionan con los títulos de anticuerpos, actividad, progresión de la enfermedad e incluso compromiso orgánico, haciendo de su inhibición un blanco terapéutico atractivo.
Cytokines are a group of proteins of low molecular weight (usually less than 30kDa) produced and secreted during innate and acquired immune responses, which act as a signaling system that allow complex interactions between lymphoid, inflammatory and hematopoietic cells.1 By their binding to specific membrane receptors present in the target cells, they initiate a cascade of intracellular signaling which alters the gene expression pattern, thus regulating important biological functions, such as the growth, activation, survival, differentiation, and even the death of the cells.1,2
The cytokine B-cell activating factor (BAFF), or B-lymphocytes activating factor, has been recognized in the last years as a homeostatic cytokine vital for the maturation and survival of the peripheral B lymphocytes, and the development and activation of lymphoid organs. This cytokine exerts important regulatory functions by inducing pleiotropic responses through its interaction with 3 receptors: tumor necrosis factor (TNF), receptor homologue transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI), B-cell maturation antigen (BCMA) and BAFF-receptor (BAFF-R) whose expression is restricted to B (BL) and T (TL) lymphocytes.3,4
The importance of BAFF has been previously demonstrated in murine models, in which the deficiency of this protein results in a decrease in the number of peripheral B cells and a low capacity for the humoral response, whereas an overexpression of BAFF has been linked with the development of autoimmune phenomena and hematologic neoplasms.4,5 High levels of BAFF have been associated with the presence of autoantibodies, such as anti-double-stranded DNA (anti-dsDNA) antibodies in systemic lupus erythematosus (SLE), anti-SSA antibodies in the primary Sjögren's syndrome (pSS) and the rheumatoid factor (RF) in rheumatoid arthritis (RA).3
Through this narrative review, it will be shown the existing information about the function of BAFF, the regulation of its expression, its cell receptors, its implication in immunologic diseases and the use of a biological therapy directed against BAFF in the treatment of systemic autoimmune diseases.
MethodologyIt was conducted a review of the literature, based on relevant articles about the BAFF cytokine. Databases such as PUBMED and EMBASE were used for the bibliographic search. The search did not have limits of date and key words and MESH terms such as BAFF, Blys, APRIL, BCMA, TACI, systemic autoimmune diseases were introduced.
The following terms were used for the search of current therapies: belimumab, atacicept, tabalumab, blisibimod, rituximab, in autoimmune diseases.
The following selection criteria were established for the literature found: systematic reviews of the literature and clinical trials that were of interest for the authors. Articles in English, Spanish and French were included.
After reviewing the articles, the key ideas were extracted in order to be able to expose in a simple and complete manner the role of the BAFF cytokine in the autoimmune diseases and to simplify the treatment alternatives.
DefinitionThe BAFF, also known as BlyS, TALL-1, zTNF4 and THANK, TNFSF13B, was identified independently by several research groups in 1999, hence its multiple names.6,7 It is a member of the TNF ligands superfamily, produced and secreted, mainly, by myeloid cells (macrophages, monocytes, neutrophils, dendritic cells), activated T cells, non-lymphoid cells (astrocytes, epithelial cells of salivary glands, fibroblast-like synoviocytes, bronchial cells, nasal cells) and stromal cells (local niches that modulate the survival and function of the B cells and the plasma cells in health and disease).8,9 Its synthesis occurs in response to multiple stimuli, among them: interferon (IFN)-α or IFN-γ, interleukin-10 (IL-10), lipopolysaccharides10,11 and the activation of Toll-like receptors (TLR) TLR-4, TLR-9 and the CD40 ligand (CD40L),12 produced during inflammation or chronic infections. Normally, the BAFF cytokine is not expressed in B cells, however, it has been detected in infectious and lymphoproliferative processes such as the infection with Epstein–Barr virus and chronic lymphoid leukemia.13–16
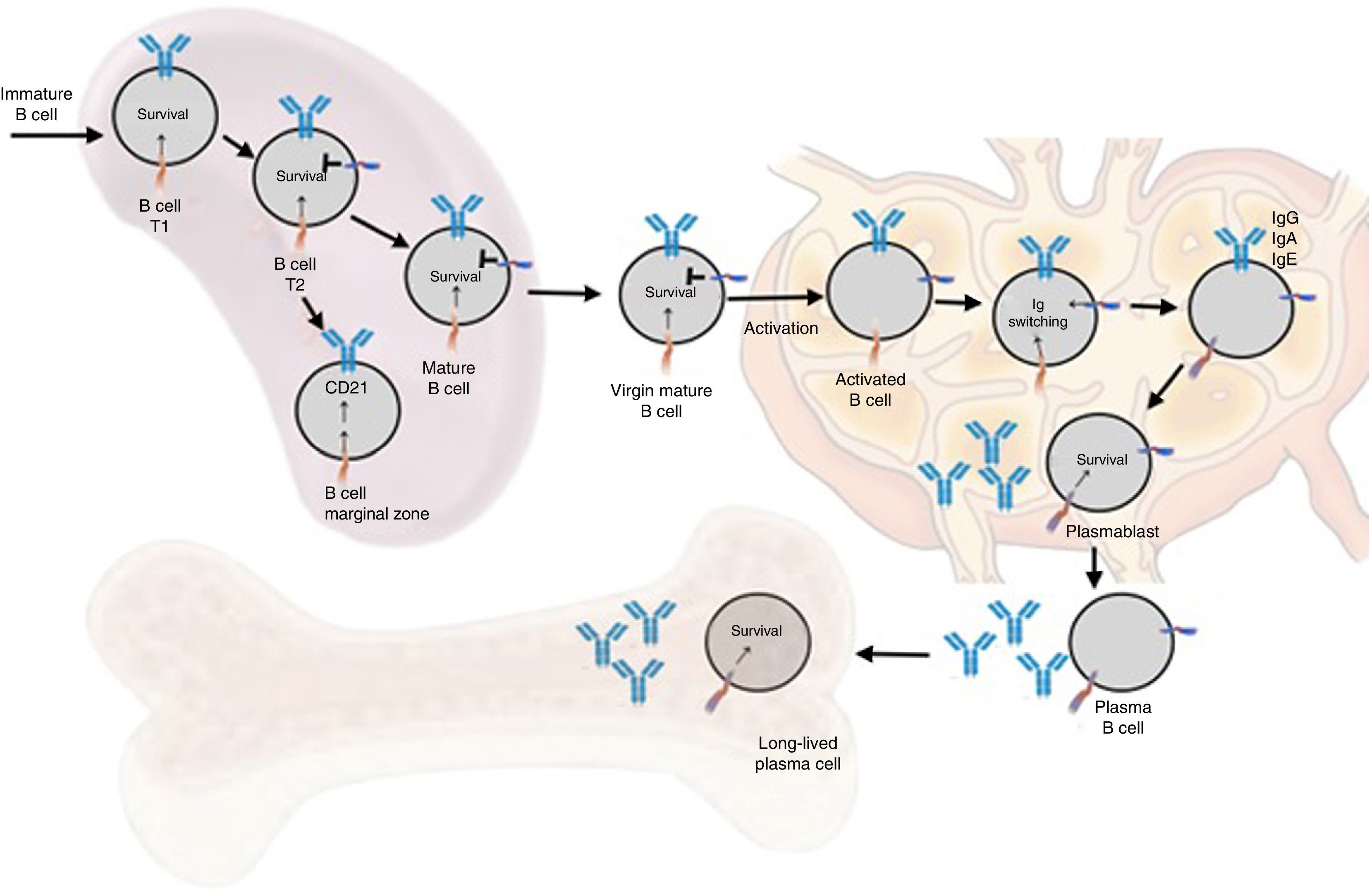
Animal models of BAFFIn 1999, starting from murine models, the powerful regulatory role of BAFF in multiple functions of murine B cells (BAFF and BAFF-R deficient), in which maturation was altered beyond the type 1 transitional stage (T1) began to be elucidated.17 These initial models were subsequently transferred to human in vitro models, and it was found that, in contrast with the murine B cells, the effects of BAFF on the survival of the resting and mature human transitional B cells, at least in vitro, were less pronounced than the effects of BAFF on the murine B cells17,18 (Table 1). In the latter, BAFF also supports the survival of plasma cells, but not of those of humans or monkeys, which evidences species differences regarding the response to BAFF. With the exception of these few species-specific differences, it can be concluded that this cytokine is essential for the survival of human and murine B cells at different stages of development and differentiation.14–16
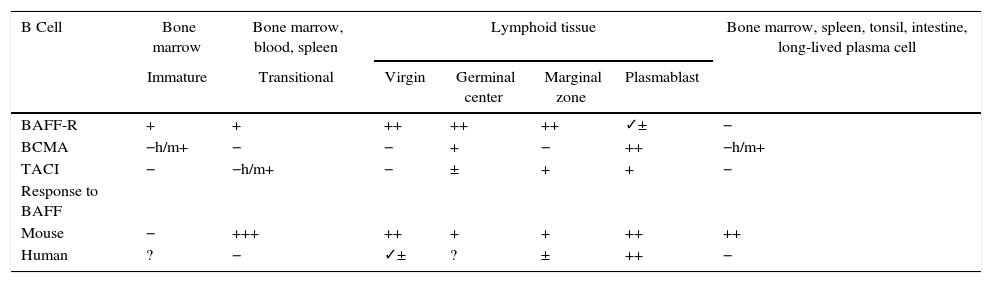
Expression of BAFF and BAFF receptors during the development and differentiation of human and murine B cells.
| B Cell | Bone marrow | Bone marrow, blood, spleen | Lymphoid tissue | Bone marrow, spleen, tonsil, intestine, long-lived plasma cell | |||
|---|---|---|---|---|---|---|---|
| Immature | Transitional | Virgin | Germinal center | Marginal zone | Plasmablast | ||
| BAFF-R | + | + | ++ | ++ | ++ | ✓± | − |
| BCMA | −h/m+ | − | − | + | − | ++ | −h/m+ |
| TACI | − | −h/m+ | − | ± | + | + | − |
| Response to BAFF | |||||||
| Mouse | − | +++ | ++ | + | + | ++ | ++ |
| Human | ? | − | ✓± | ? | ± | ++ | − |
The transgenic models or deficient of BAFF, a proliferation-inducing ligand (APRIL) and of the different receptors (BAFF-R, TACI and BCMA)15 allowed to substantiate a series of murine phenotypes (Table 2) and in summary they have shown that both BAFF and its main receptor (BAFF-R) control the development and survival of transitional type 2 B cells (T2) and of the marginal zone. APRIL regulates aspects of the immunoglobulin (Ig) class switching independently of the CD-40 and promotes the survival of plasma cells. TACI helps the humoral immune response of the innate B cells to repetitive antigens and, somehow, regulates the expansion of B-cell reserve. And the BCMA contributes to the maintenance of the plasma and memory cells.15
Phenotype of the genetically modified mice for BAFF, APRIL and their receptors.
| Murine model | Phenotype |
|---|---|
| BAFF−/− mouse | -Altered B cell maturation beyond -Decreased Ig levels -Decreased T-cell dependent/independent immune response -Modest increase of the graft survival, improved with non-effective low doses of cyclosporine |
| APRIL−/− mouse | -Alteration in switching of IgA isotype -Alteration of survival of tetanus toxoid specific plasma cells in bone marrow-normal survival of nitrophenol-specific long-lived plasma cells in bone marrow -Increase in the percentage of CD44hiCD62Llow effector memory T cells |
| BAFF transgenic mouse | -Development of B cells hyperplasia starting from stage -Development of autoimmunity independent of T cells but dependent of MyD88, involving the production of autoantibodies, glomerulonephritis, inflammation and destruction of salivary glands -Decreased production of saliva; B, B1 cells present in salivary gland Expansion of regulatory and effector T cell compartments |
| APRIL transgenic mice | -Development of B, B1 cells neoplasms -Increased survival of CD4+ and CD8+ T cells and increased production of IL-2 by the CD8+ T cells -Increased survival of superantigen-reactive T cells linked to increased expression of BCL-2 -Increased T cell proliferation -Decreased percentage of T cells in peripheral lymph nodes |
AFFR−/− mice | -Equal to BAFF−/− -Decreased longevity in germinal centers -Alteration of Ig class switching |
| BAFFR mutant A/WySnJ mouse | -Equal to BAFF−/− but less severe -Autoimmunity develops as the mouse ages -Alteration in antibody-dependent T-cells responses |
| TACI−/− mouse | -Development of B-cell hyperplasia in stage B1 -Increased B cell proliferation rate -Defective antibody response independent of type II T cells -Development of B-cell lymphomas -Alteration in class switching to IgA -Increased number of CD4+ T cells in Peyer's patches -Failure to induce the proliferation of cytotoxic T cells after being transferred to B-cell deficient mice |
| BCMA−/− | Altered survival of long-lived plasma cells in bone marrow |
APRIL, a proliferation-inducing ligand; BAFF, B-cell activating factor; BAFF-R, BAFF receptor; BCMA, B-cell maturation antigen; BCL-2, B-cell lymphoma 2; IL-2, interleukin-2; MYD88, myeloid differentiation primary response protein 88; T1, transitional type 1; TACI, transmembrane activator and calcium-modulator and cytophilin ligand interactor.
The BAFF Tg mice exhibit a model of lupus-like disease induced by BAFF, as they develop phenotypic characteristics reminiscent of manifestations similar to SLE and to other autoimmune conditions: increase of compartments of B cells, effector T cells and lymphoid organs, high titers of multiple antibodies including anti-dsDNA, RF and hypergammaglobulinemia, circulating immune complexes and glomerulonephritis with deposits of Ig. As they age, they develop lymphoid cell infiltration of salivary glands with decreased salivary flow, very similar to what happens in the pSS.18
Other murine models of SLE in which elevated levels of BAFF have been found are the New-Zealand Black×New-Zealand White (NZB×NZW) F1 and the MRL-lpr/lpr mice. These mice susceptible to SLE have shown decreased disease activity in response to cytokine BAFF antagonists.19
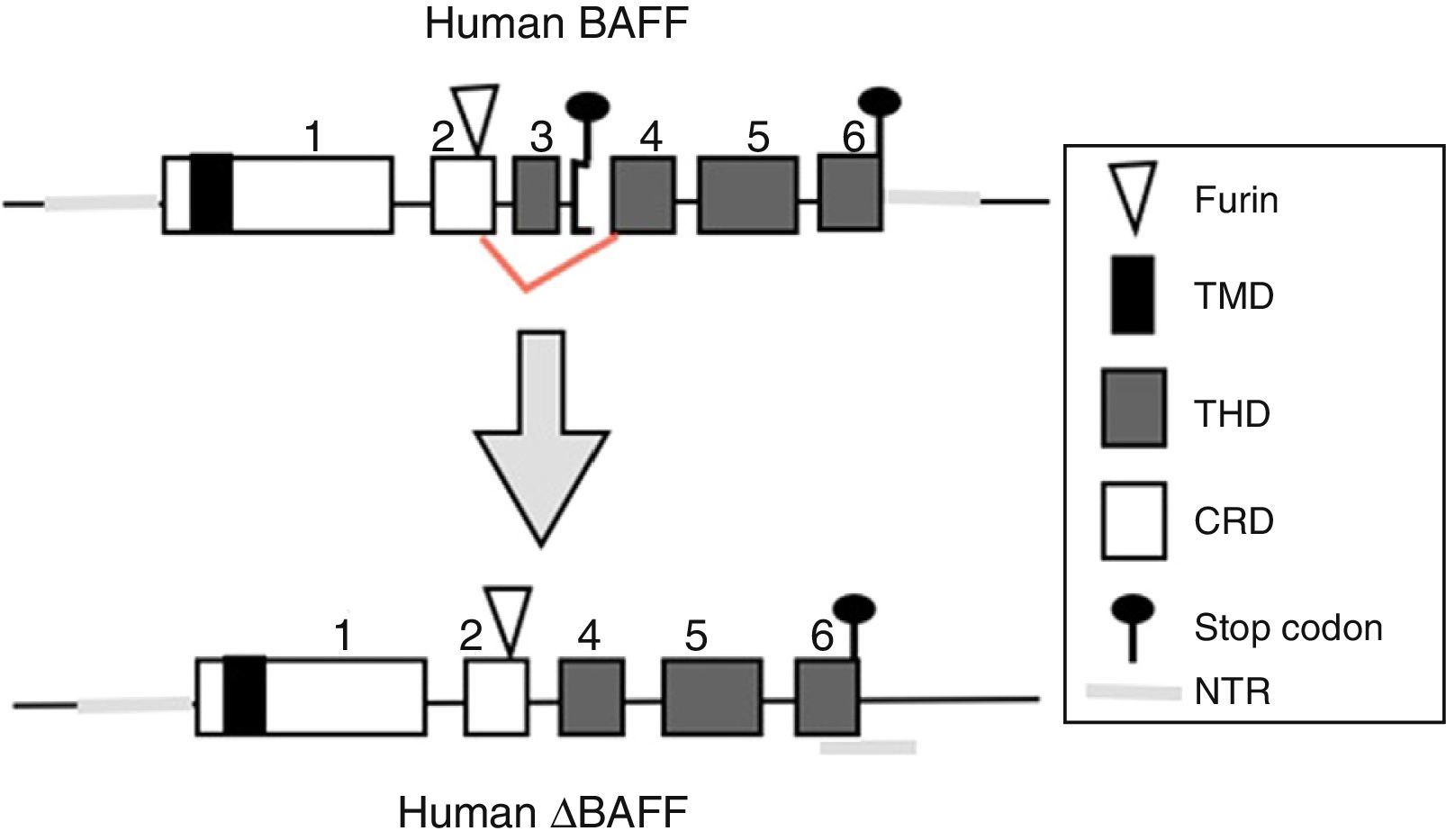
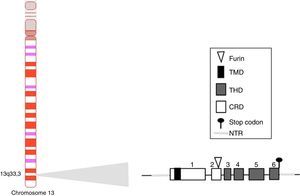
BAFF: from the gene to the BAFF protein in humansThe BAFF gene in humans (Fig. 1) is located on the chromosome 13q33.3 and consists of 6 exons and 5 introns, corresponding to 39kb. Exon 1 encodes for the transmembrane domain and the flanking regions, exon 2 for the furin processing site, and exons 3–6 encode for the TNF homology domain (THD), which binds to the receptors.14
The BAFF promoter of 1020 base pairs can be activated by multiple transcription factors, including family members of NFAT and of the nuclear factor kappa light chains enhancer of activated B cells (NF-kB) (p50, p52, c-Rel and p65).20 Two additional transcription factors belonging to the TNF receptor family have been described: CD40 and BR3.8,9 These latter interact with c-Rel of the NF-kB pathway to activate the transcription of BAFF. This BAFF transcript encoded by the gene includes 1204 pairs of bases, with a reading code of 858 base pairs.9,21
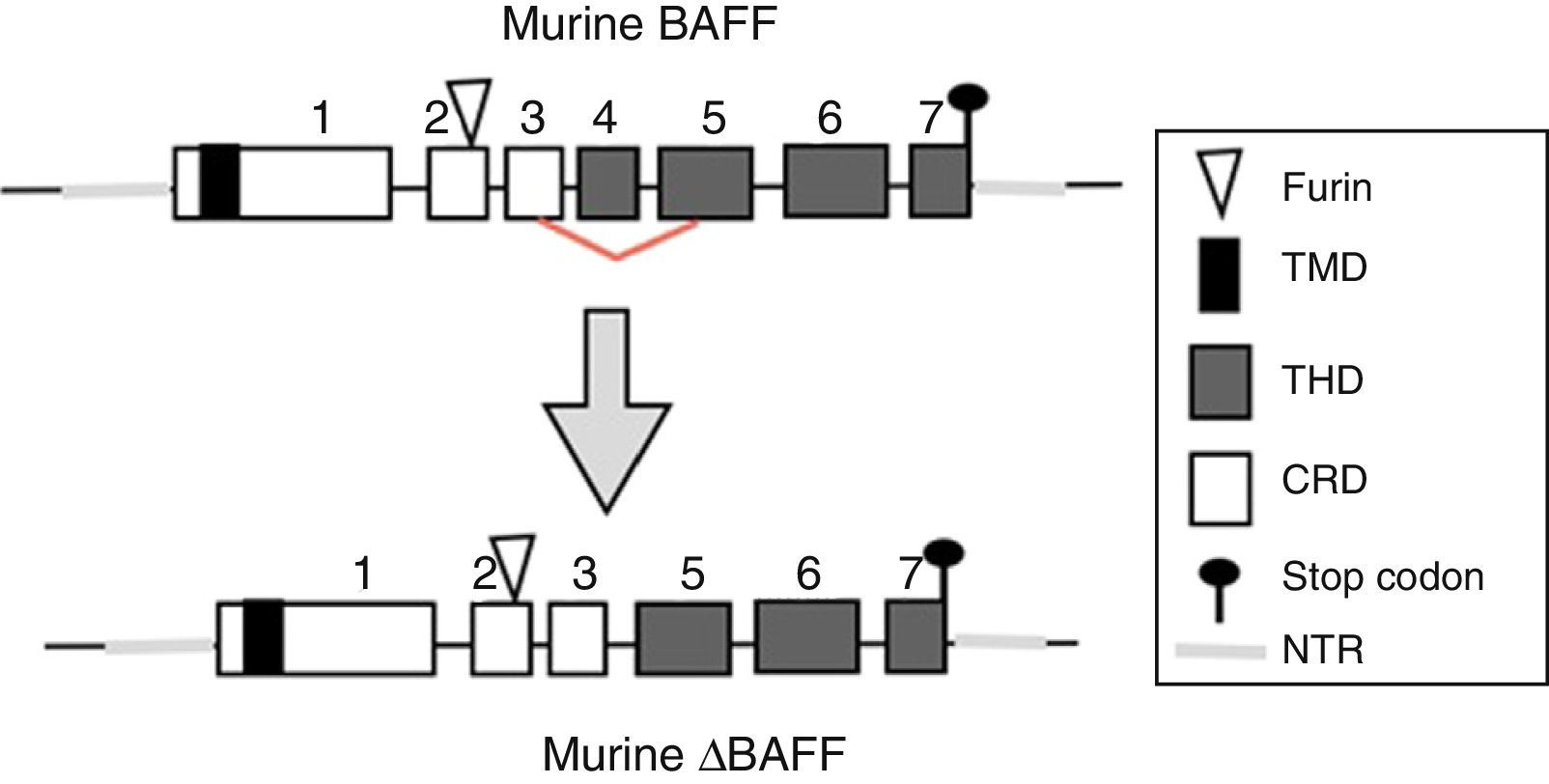
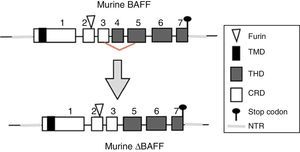
The BAFF gene in miceIn mice, the BAFF gene (Fig. 2) is located on the chromosome 8 A1.1 and consists of 7 exons and 6 introns corresponding to 31kb. Unlike the human, it encodes an additional exon with a stretch of 30 amino acids located between the furin site and the TNF homology domain (THD).14 Its activation depends on several transcription factors. The first transcription factor described was p65 (of the NF-kB pathway) which can act in collaboration with the p300 coactivator protein.22 The expression of BAFF can be induced by Smad3 and Smad4 and inhibited by Smad7. The Smad proteins are homologous both of the protein of the Drosophila fly, the mothers against decapentaplegic protein, where “decapen-taplegic” refers to a protein of the fly homologous to the human bone morphogenetic protein and the protein of the nematode species Caenorhabditis elegans SMA (from the “sma” gene of the word “small”, small body in view of its ability to alter the body size). The Smad3 protein binds in the promoter to 3 potential sites called smad binding elements, which is indispensable for the activation of BAFF in murines.23
The BAFF cytokine as a proteinBAFF is a type II transmembrane protein of 285 amino acids, with a cytoplasmic domain of 46 amino acids, characterized by being rich in cysteine in its ligand binding region, a transmembrane hydrophobic region and a domain of 218 amino acids that contain 2 potential sites of N-glycosylation. The sequence of the extracellular domain of BAFF shows high homology to APRIL (33% identical aa, 48% homologous). The BAFF can be secreted as a soluble trimeric ligand by proteolytic processing in the furin consensus site. At neutral or basic pH, 20 trimers of soluble recombinant human BAFF are associated in 60-mers with a virus-like structure. This association is dependent on a single loop in the TNF family. The physiological significance of the 60-mer is unknown, but it can bind to receptors and is moderately more active than the trimers in in vitro assays. At an acidic pH or when is fused to the N-terminal extension as a myc tag (technique recombinant with the gene c-myc), the 60-mer is dissociated into trimers.6,14
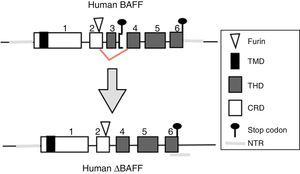
BAFF isoformsWhile the full-length mRNA encodes the biologically active form of the protein, there are different isoforms, described in both species, which are formed by splicing or alternative splicing (Fig. 3). Among the isoforms of BAFF, is found the delta-BAFF (ΔBAFF) identified in 2003,24 which does not include the exon 3 in humans nor the 4 in mice, where it retains the reading frame, despite the loss of 57 base pairs. In mice this alternative splicing results in the encoding of an amino residue at position 155, which leads to a glycosylation on the new site, resulting in a higher weight for the BAFF isoform. The ΔBAFF expressed in mice has a failure in A-A1 loop that impairs the ability to bind to BAFF, TACI and BAFF-R receptors. It also has the ability to bind to BAFF by disulphide bonds and to form intracellular heteromultimers, which bind poorly to the BAFF receptors, limiting the homotrimerization of BAFF and playing a suppressive role due to their competitive coassociation, by modulating the release of BAFF.24 Thus, the expression of the ΔBAFF isoform has an opposite effect on the survival and the number of BL in the marginal zone.25 In humans the transcript has been expressed in lymphatic tissue, astrocytes and myeloid cells.24,25
There is also another isoform in humans called Δ4BAFF (Δ5BAFF in murines), which, unlike the ΔBAFF, it has a cleavage of exon 4 that occurs by the formation of a new stop codon within exon 5 in the junction, causing that exons 5 and 6 are not translated, with conservation of the N124 glycosylation site and the furin cleavage site, which makes that it probably does not form trimers or bind to the BAFF receptors.26 However, this variant could act as a transcription factor for the same gene in association with the p50 protein in the NF-κB pathway. This has been observed in lymphoproliferative and autoimmune diseases with predominance of B cells.26 The presence of Δ4BAFF is essential for the release of soluble BAFF by the IFN-γ stimulated monocytes and the survival of B lymphocytes in chronic lymphocytic leukemia.26 In consensus, both ΔBAFF and Δ4BAFF play a role in the regulation of the immune response, both in physiological states and in some disorders, such as the autoimmune diseases.27
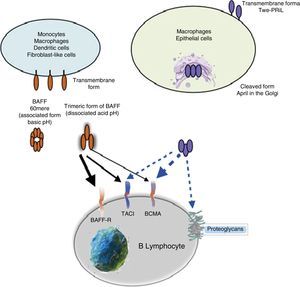
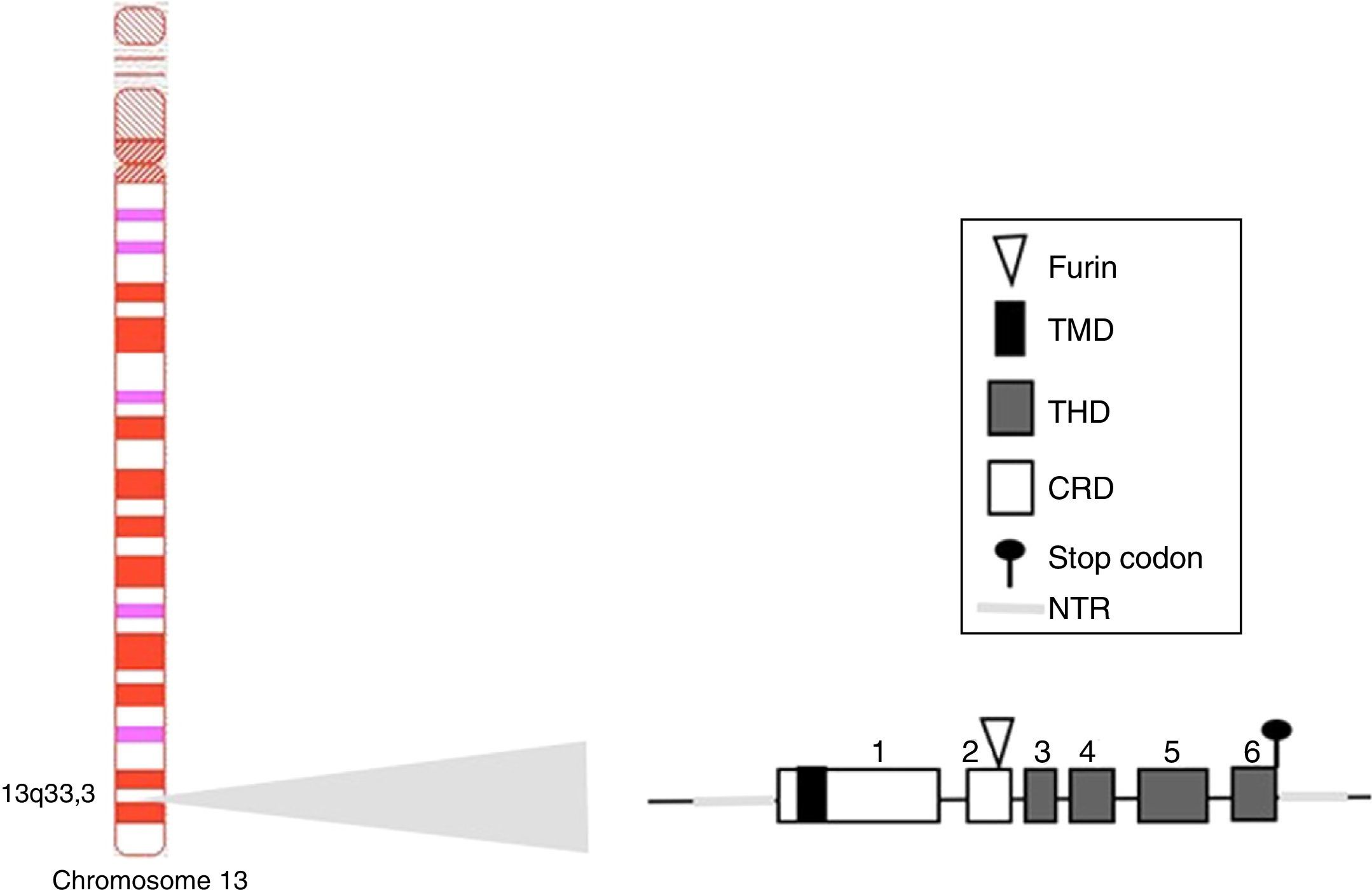
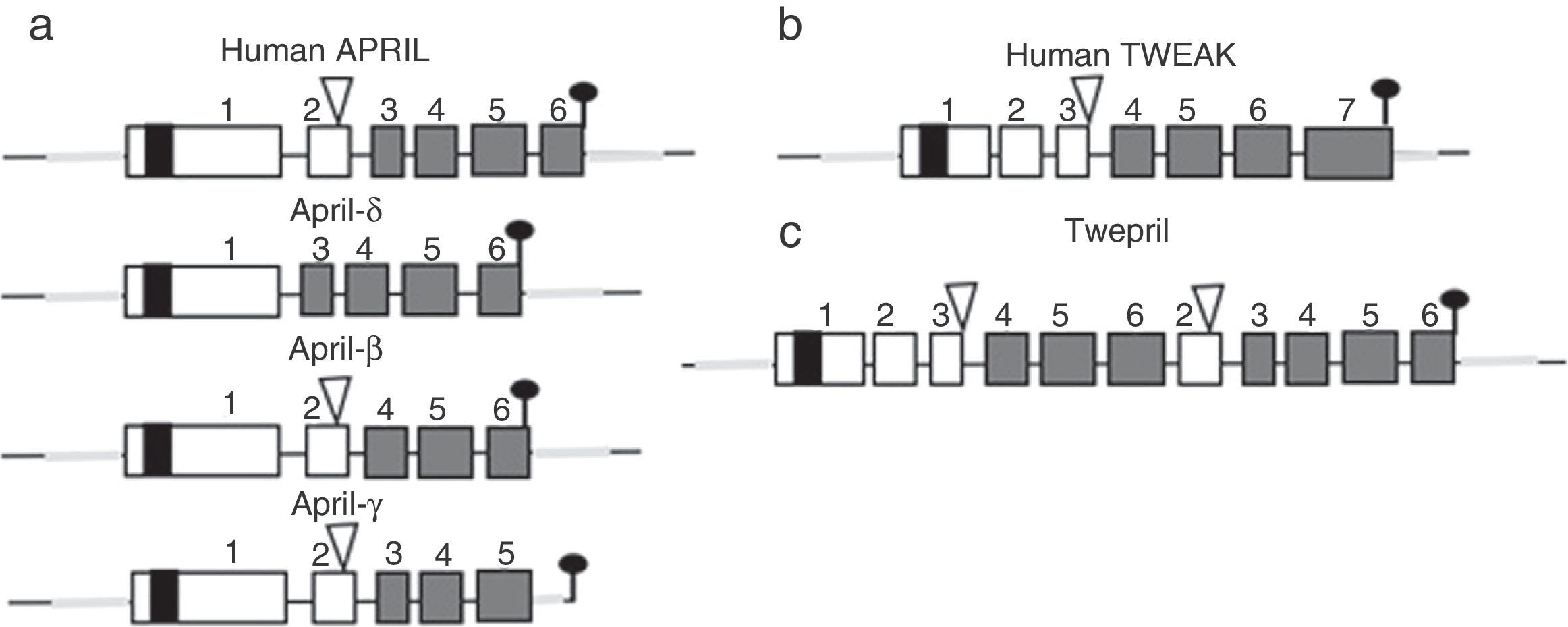
Other family members: APRIL and TWEAKWithin this family of cytokines, there is another member of the TNF family, which shares many of the signaling pathways and is called APRIL, located on chromosome 17 p13 (Fig. 4a), which generates 7 transcript variants. Unlike BAFF, APRIL is cleaved in the Golgi apparatus by the enzyme furin convertase before being released, and it is only found in its secretory form.27 Like BAFF, it expresses in the same cells and shares some of its receptors: TACI and BCMA.28,29 In this way, BAFF-R is practically exclusive to bind BAFF, although even a poor union of APRIL has been described by some authors.14
Variants of human APRIL (a proliferation-inducing ligand) and TWEAK (tumor necrosis factor [TNF]-like weak inducer of apoptosis). Exons are represented in boxes; introns in thick gray lines. THD, TNF homology domain; CRD, cysteine-rich domain; TMD, transmembrane domain; NTR, 5′ and 3′ non-translated regions.
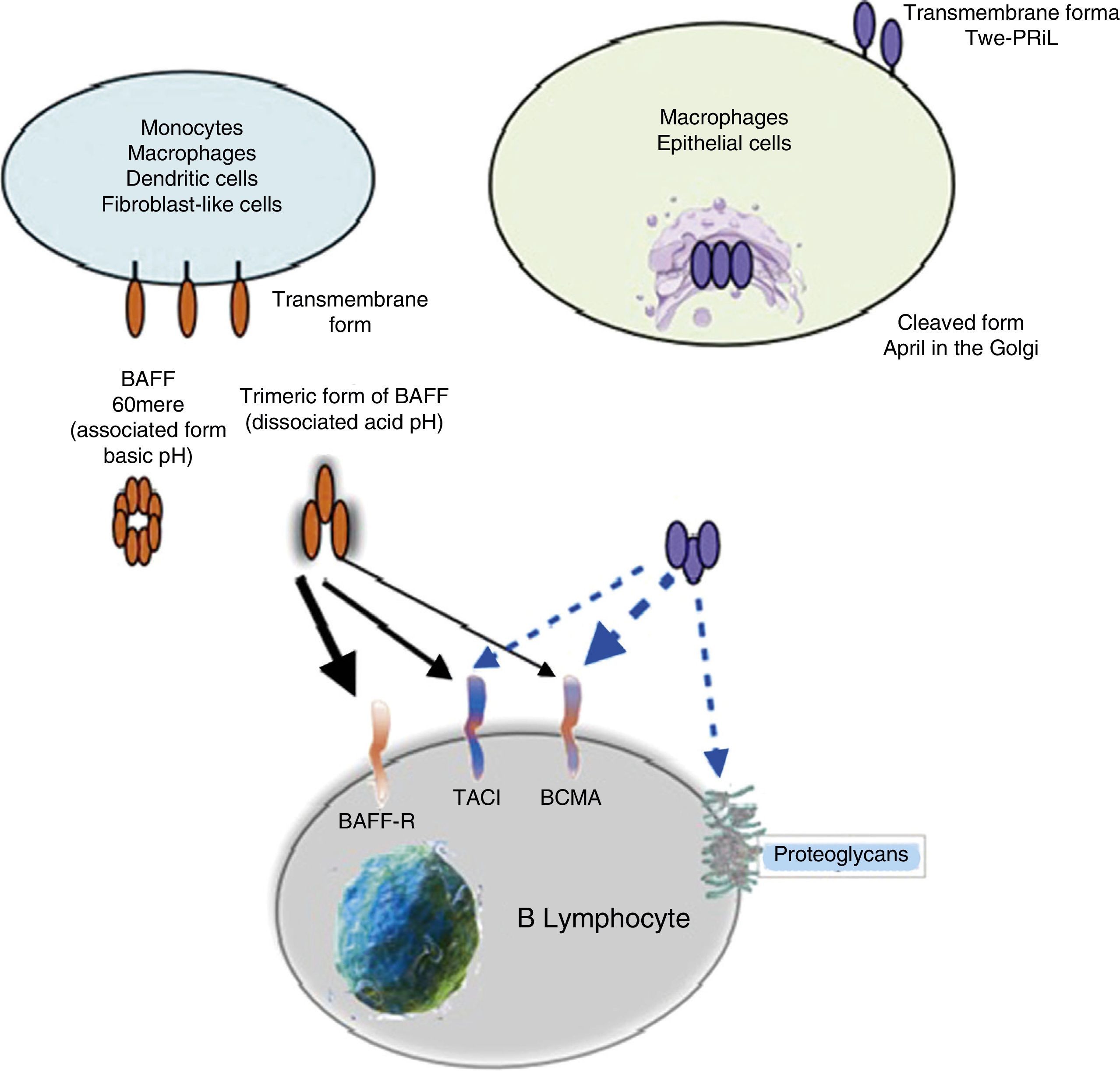
APRIL has also the ability to bind to sulfated glycosaminoglycans, such as those present in syndecan-1 (CD138) or to other proteoglycans expressed both in lymphoid and non-lymphoid cells. The relevance of this binding is uncertain, but it may serve to accumulate or multimerize APRIL in the extracellular matrix or in the surface of the syndecan positive cells, which facilitates access to the TACI or BCMA receptor (Fig. 5).30
Being found in soluble form and structurally very similar to BAFF, it can form heterotrimers with it in the following manner: 3 APRIL or 2 APRIL+1 BAFF, which can be active forms. These are formed in the cell membrane or in Golgi apparatus, and are more frequently detected in autoimmune diseases.31
In addition, some APRIL polymorphisms have been identified, 2 of which are associated with autoimmune diseases such as SLE, located in the region of the extracellular domain.32 APRIL, like BAFF, is also important in cancer and other autoimmune diseases such as systemic sclerosis and multiple sclerosis.33 In active SLE the serum concentrations of APRIL increase in parallel with the anti-dsDNA antibodies.34 In RA, the dendritic cells appear to be the main responsible for the increase in the serum levels of APRIL35 and in multiple sclerosis it has been found and increase in APRIL mRNA, especially in monocytes, TL and peripheral blood.36
APRIL also has isoforms that are formed by uncommon splicing events such as APRIL-δ (Fig. 4a) in which the exon 1 of the human APRIL combines with an alternative acceptor site in the site of exon 3, generating a non-cleavable membrane-bound isoform, no linked to glycosaminoglycans.14 APRIL-β is formed by omission of exon 3. This isoform is homologous to ΔBAFF which by analogy negatively regulates the APRIL activity.14 The APRIL-γ is formed by splicing of a cryptic intron in exon 6 in which there is a truncation of 4 amino acids in the C-terminal region, being replaced by a single residue (this isoform has not been studied).14
In both species (humans and mice) the APRIL gene is located in contiguity with the 3′ end of the TNF-like weak inducer of apoptosis (TWEAK), which is located upstream of the APRIL gene on the chromosome 17 p13 (Fig. 4b).7
Two functional protein transcripts of the TWEAK protein have been described: the TWEAK-ligand (TWEAK-L) and the TWEAK. The TWEAK-L binds to its TWEAK receptor (TWEAK-R or FN14) and generates functions of cell proliferation, migration, survival and death.37 It is a type ii transmembrane protein synthesized in the endoplasmic reticulum with an extracellular C-terminal site with a residue of N-glycosylation and a intracellular N-terminal associated to the phosphorylation of protein kinase C.38 It has been found expressed intracellularly in macrophages, dendritic cells, natural killer, TL and even in endothelial cells, astrocytes and platelets.39
On the other hand, the TWEAK is a type i transmembrane protein of 102 amino acids and its activity is mediated by the interaction with the cysteine-rich domain of the receptor FN1437 by inducing trimerization of the receptor, this implies a preference for the TNF receptor associated factor (TRAF) pathway, where TRAF2 and TRAF5 activate the NF-κB.40 In autoimmunity, TWEAK has been associated with RA, and it has been found in high concentrations in the synovial tissue: the BL are one of the greatest sources of TWEAK.41 In SLE, the TWEAK protein promotes the production of inflammatory mediators such as interferon γ – inducible protein-10, regulated on activation, normal T expressed and secreted and monocyte chemotactic protein-1 in the renal cells, increases the cellular infiltrate and tissue inflammation and plays a role in the initiation or exacerbation of autoimmunity.42 In multiple sclerosis there is an increase of the expression not only of TWEAK, but also of its receptor FN14, where the microglia secretes it and causes a loss of myelin an neuronal damage.14
There is also an isoform formed by intergenic splicing between exon 6 of TWEAK and exon 2 of APRIL: the TWE-PRIL (Fig. 4c), which contains the complete THD (TNF homology domain) of APRIL, which confers it the same specificity of receptor as APRIL, although its function has not been yet elucidated.14,43
ReceptorsThe BAFF is a ligand of unusual receptors, since they are type iii transmembrane proteins that lack of a signal sequence. There are 3 receptors described: BAFF-R, TACI and BCMA (Table 3), whose expression is quite restricted and they express in different times during the development of B cells (Fig. 5).14 BCMA, TACI and BAFF-R are expressed by BL, while TACI and BAFF-R are also expressed by activated TL. On the other hand, BAFF-R is the only one that has only BAFF as a ligand while BCMA and TACI bind to APRIL.14 These 3 receptors lack of death-associated domains, common in some molecules of the TNF ligand superfamily, and instead they interact with members of the family of TRAF proteins, which indicates their participation in survival and differentiation pathways rather than in cell death.14,15
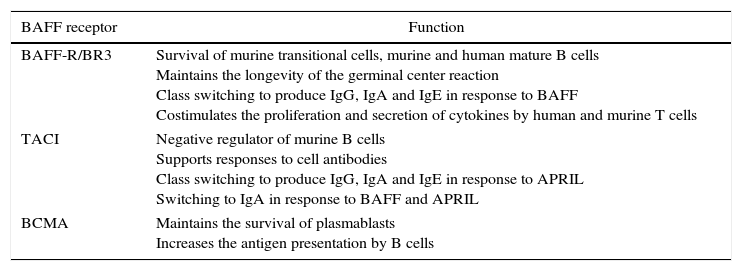
Specific functions of BAFF-R, TACI and BCMA in human and murine B cells.
| BAFF receptor | Function |
|---|---|
| BAFF-R/BR3 | Survival of murine transitional cells, murine and human mature B cells Maintains the longevity of the germinal center reaction Class switching to produce IgG, IgA and IgE in response to BAFF Costimulates the proliferation and secretion of cytokines by human and murine T cells |
| TACI | Negative regulator of murine B cells Supports responses to cell antibodies Class switching to produce IgG, IgA and IgE in response to APRIL Switching to IgA in response to BAFF and APRIL |
| BCMA | Maintains the survival of plasmablasts Increases the antigen presentation by B cells |
APRIL, a proliferation-inducing ligand; BAFF, B-cell activating factor; BAFF-R, BAFF receptor; BCMA, B-cell maturation antigen; BR3, BAFF receptor 3; Ig, immunoglobulin; TACI, transmembrane activator and calcium-modulator and cytophilin ligand interactor.
Many of the expressed BAFF molecules are separated from the cell surface and circulate as soluble active homotrimers that will bind to BAFF-R. A small proportion of free circulating BAFF is associated in multimers of 20 trimers that bind to and activate TACI, or it can be activated bound to the membrane.15 BCMA is a receptor with high affinity for APRIL, while in humans it binds to BAFF with low affinity.15
Diverse studies show that only the BAFF-R plays a clear role in the cell maturation. This was demonstrated in a A/WySnJ mouse strain, which presented a natural mutant gene for BAFF-R that resulted in a phenotype similar to BAFF deficient mice, reflected as a significant reduction of the peripheral mature BL and a poor immune response.44,45 Likewise, it has been described that the expression of BAFF-R is determinant in the formation of the germinal centers.28
BCMA and TACI appear to be related to the effector phase of the humoral immune response, mediating the plasma cells differentiation. BCMA is, in addition, essential for the survival of the long-lived plasma cells of the bone marrow.14
BAFF-R, TACI and BCMA are expressed in the spleen, thymus, peripheral blood lymphocytes and B cells; BAFF-R and BCMA are also expressed in the lymph nodes; BAFF-R and TACI are also expressed in activated T cells. On the other hand, TACI is the only one expressed in the small intestine, while BCMA can also be expressed in the liver and the adrenal glands.33
BAFF-R, TACI and BCMA show specific but overlapping expression patterns, and the functional analysis has revealed distinct roles for these 3receptors in the mediation of the BAFF and APRIL signals.14 Regarding the BL, the expression of BAFF, TACI and BCMA is not evident until this cell reaches a transitional maturation status.14 TACI is the predominant receptor in the short-lived plasma cells while BCMA predominates in the long-lived plasma cells.14 Each receptor activates its own signaling pathway; BAFF-R is the only receptor that activates the alternative pathway of NF-kB.15
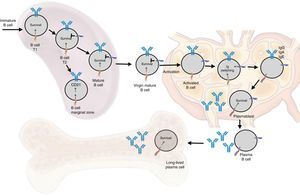
Functions in normal immune responseOntogenesis of the B lymphocyte: the role of BAFF and its receptorsThe generation of the reserve of mature BL involves a sequential development of hematopoietic stem cells into pro-B cells, which are transformed into pre-B cells and then into immature cells y (Fig. 6). The latter are exported to the periphery where they undergo a subsequent selection that involves a series of events: first they enter the spleen at an immature T1 stage, then they go into a T2 stage, to pass, later, to a stage of mature or marginal zone B-cell; during the differentiation they require a functional B-cell receptor. In addition to the signal of the functional B-cell receptor, a signal of survival given by BAFF during their differentiation is required (the deletion of BAFF results in a loss of more than 90% of mature cells). At this stage, the BAFF-R receptor is crucial to mediate the survival of the B cells of the marginal zone and the positive regulation of CD21, while TACI acts as a negative regulator.15 When the mature B cells encounter a T-dependent antigen, they differentiate into high affinity effector cells called memory B cells and Ig-secreting cells or long-lived plasma cells. This process occurs in specialized structures called germinal centers, located in secondary lymphoid tissues. Is at this stage of late differentiation when the expression of BCMA seems to be relevant for the survival of these long-lived plasma cells.14,16
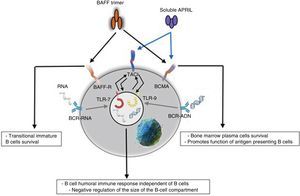
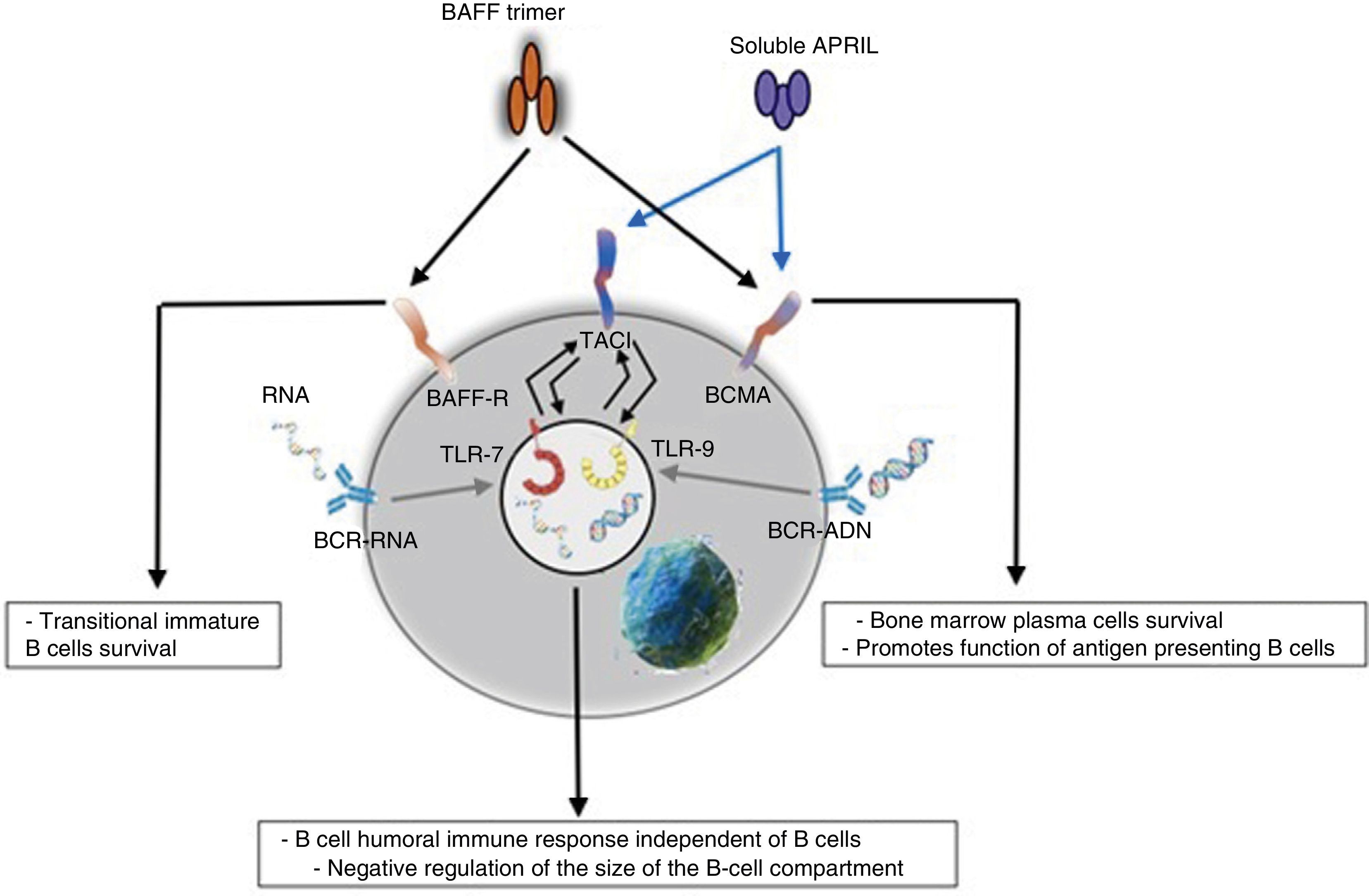
Innate responseThe BAFF/APRIL ligand and receptor system interacts with the innate immunity through its signaling receptors. After their internalization by the Fc-gamma IIa receptor (FcγRIIa) in plasmocytoid dendritic cells, the immune complexes constituted by autoantibodies and autoantigens which contain RNA or DNA are able to join and activate intracellular receptors TLR-7 and TLR-9 (Fig. 7), increase the activation of the latter and lead to the secretion of IFN-α, promoting inflammation. Since the expression of BAFF/APRIL is induced by IFN, especially the IFN-α, the latter induces its secretion by cells of the innate immune system such as neutrophils, monocytes, macrophages, dendritic cells and follicular dendritic cells.19
It has also been described a close relationship between TLR-7 and TLR-9 and the TACI, since both can up-regulate its expression through the stimulation of both receptors.19
These events are which lead some authors to suppose that BAFF could be the link between the activation of the innate immunity and the modification of the adaptive immune response under physiological condition, as well as in autoimmune diseases mediated by BL.19
Activation of T cellsWhile the majority of studies have focused on the stimulatory function of BAFF on B cells,14,15 a costimulatory function on the TL that influence the activation, survival and differentiation of the effector T cells has also been documented.30
However, the BAFF also increases the number of regulatory T cells, indicating that any activator effect of BAFF on the T cells can be annulled by the expansion of regulatory T cells.19 This shows that there are mechanisms of BAFF independent of T cells, corroborated by transgenic models of SLE in which the BAFF Tg mice without T cells develop a SLE indistinguishable from those which have enough T cells, where probably the TLR-induced expression of TACI would play a role.19
The role of BAFF in autoimmunitySystemic lupus erithematosusThe role of the BAFF system in SLE is given by the importance of this cytokine in the maturation and survival of BL.18 The mice that overexpress BAFF have a large number of B cells and autoantibodies that eventually develop autoimmune diseases similar to lupus.18 This has been proved by studies in BAFF deficient mice, which develop immaturity of B cells and immunodeficiency. In addition, single nucleotide polymorphisms have been found in the promoter, coding and regulatory regions of the TNFSF13B region (the human gene of BAFF),46,47 but only in one study it has been seen a relationship of susceptibility to SLE in the positions 871C>T and 2701T>A.27
In SLE both the BAFF and APRIL serum levels are elevated in individuals with the disease, as compared with control individuals without it, so they could be used as markers of active disease.48 They even have a positive correlation (particularly the BAFF) with the clinimetric assessment of disease activity scores such as SLEDAI. But it should be taken into account that the BAFF levels vary in accordance with a number of factors, among which we can include: the race, the population polymorphism, the binding to glycoproteins (proteoglycans) in the case of APRIL, or the renal clearance where the renal excretion of BAFF can increase in cases of glomerulonephritis, resulting in a decrease of the serum levels.48–50 There is also a regulation of the BAFF serum levels with respect to the concentration of mRNA: it is found in high concentrations, but with decreased levels of BAFF, so it could be due to a compensatory mechanism or to a loss of BAFF in the urine.51 That is why among the multiple factors which may be associated with the induction and activity of the disease, is included the BAFF, which is contemplated as one of the therapeutic targets for the modulation of the disease.48–51
There is currently only one study showing the mRNA expression in patients with lupus nephritis and in subjects pre-transplanted due to other cause different than lupus nephropathy, where it was found that the BAFF and APRIL mRNA levels were significantly higher in patients with lupus nephropathy in the glomeruli and in their receptors in the tubulointerstitial area.52 However, there are no reports in the literature of similar findings or that evaluate the protein expression by immunohistochemistry and its correlation with variables of severity or activity of the lupus nephritis. Table 4 shows the main studies that express the effect of BAFF in SLE.48–51
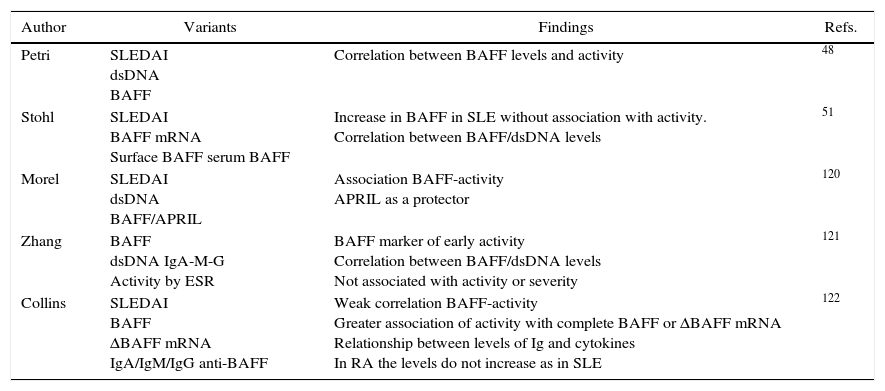
Summary of studies that show alteration of BAFF cytokine in SLE.
| Author | Variants | Findings | Refs. |
|---|---|---|---|
| Petri | SLEDAI dsDNA BAFF | Correlation between BAFF levels and activity | 48 |
| Stohl | SLEDAI BAFF mRNA Surface BAFF serum BAFF | Increase in BAFF in SLE without association with activity. Correlation between BAFF/dsDNA levels | 51 |
| Morel | SLEDAI dsDNA BAFF/APRIL | Association BAFF-activity APRIL as a protector | 120 |
| Zhang | BAFF dsDNA IgA-M-G Activity by ESR | BAFF marker of early activity Correlation between BAFF/dsDNA levels Not associated with activity or severity | 121 |
| Collins | SLEDAI BAFF ΔBAFF mRNA IgA/IgM/IgG anti-BAFF | Weak correlation BAFF-activity Greater association of activity with complete BAFF or ΔBAFF mRNA Relationship between levels of Ig and cytokines In RA the levels do not increase as in SLE | 122 |
ΔBAFF, delta BAFF; dsDNA, double stranded DNA; RA, rheumatoid arthritis; BAFF, B-cell activating factor; SLE, systemic lupus erythematosus; SLEDAI, systemic lupus erythematosus activity index; ESR, erythrocyte sedimentation rate.
The pSS is a chronic autoimmune process that mainly affects the exocrine glands and entails an alteration of their function. It is characterized by a mononuclear infiltrate of periductal location which compromises even the secretory units.53
The mechanisms responsible include an abnormal expression of BAFF. This abnormality is mainly given by the excessive production of IFN-α in the cellular infiltrate, mediated by the apoptosis of epithelial cells, which leads to the induction of the expression of cytokines such as BAFF and to the stimulation of autoreactive BL. These findings indicate that the epithelial cells play a fundamental role in the autoimmune lesions in pSS and, more specifically, they would have the potential to produce locally in an autocrine manner the BAFF, which creates a microenvironment propitious for the interaction between cytokines, epithelial cells, dendritic cells, hyperactive TL and BL, which altogether cause abnormalities of the organ-specific immunoregulation.54,55
The ectopic formation of lymphocyte accumulations similar to germinal centers, which occurs in 17% of patients with pSS, is a complex process given by the interaction of different factors, among which the BAFF has been identified.56,57 Being BAFF one of the most important mediators of the neogenesis of these germinal centers in pSS, there is an amplification of the signaling of BL, that promotes their local proliferation and differentiation into antibody-producing plasma cells.58,59 The level of BAFF is also correlated with the titles of anti-SSA and RF, indicating that the BAFF has a primary or secondary role in the modulation of the production of autoantibodies.59
The BAFF transgenic mice, which develop SLE-like and subsequently form glandular infiltrates and clinical manifestations similar to pSS, as they age, explain how BAFF leads to a excessive signaling of the survival of autoreactive BL, skipping the control point in the spleen.60
Integrating in a pathophysiological model of pSS, the cytokine BAFF is secreted by the epithelial cells after the stimulation by type iIFN, which in turn is influenced by viral infections, which suggests the role of these infections as a trigger of the disease. That is how the epithelial cells not only express and present autoantigens, but they can concomitantly activate the BL by secreting BAFF locally.54,55 This is of great importance in the therapeutic approaches in pSS, in which one of the main targets are the BL, and BAFF antagonism can potentially emerge as a new interesting alternative in this disease.53
Rheumatoid arthritisThe B cell plays a nominal role in the pathophysiology of RA, being the cell responsible for the production of autoantibodies such as the RF, antibodies against type iii collagen and citrullinated antipeptide,61 as well as they are essential for the production of cytokines and the stimulation of the TL.62 The fact that the mice without B cells do not develop collagen-induced arthritis and that the anti-CD20 therapy is effective are additional indicators of its importance in the development of this disease.62
Despite the individual variations, different studies document that high levels of BAFF correlate with the disease activity.63–65 The proportions of patients with elevated serum levels of BAFF range between 19 and 40%.66 This heterogeneity can be explained by the immunosuppressants and the variations in the doses of steroids.66
BAFF and APRIL have positive and negative regulatory effects in RA, a disease in which the BL contribute to the formation of 3 different types of lymphoid microarchitectures in the inflamed synovia: ectopic germinal centers, aggregates of T-B cells that lack of germinal center reactions and disorganized diffuse infiltrates.61
Phenotypically, the synovitis associated with formation of ectopic germinal centers is characterized by higher levels of APRIL produced by the CD83+ dendritic cells, while the BAFF is found in similar levels in all types of tissues and it derives exclusively from the CD68+ macrophages, which means that APRIL rather than BAFF correlates with the variability of the tissular function of B cells.35
As for the expression of the receptors, it is known that they are independent of the type of synovitis: the expression of BAFF-R is more abundant than the one of TACI, but it does not correlate with the lymphoid structure, while in a different way the TACI is found in the T cells present in lymphoid aggregates and is absent in the synovitis of the germinal center, ant the BCMA is expressed in the BL and TL infiltrates.61
From the therapeutic point of view, the treatment of the different types of synovitis with TACI: Fc (atacicept) was able to decrease the formation of germinal centers and inhibited the increased production of IFN (showing the role of APRIL and BAFF in the associated lymphoorganogenesis process). However, this effect was not documented in synovitis that did not show formation of germinal centers and which had T TACI+ cells, where an increase in the tissular production of proinflammatory cytokines and IFN-γ was observed. Thus, BAFF and APRIL might regulate the synovial inflammation in RA through the modulation of the function of B and T cells with pro- and anti-inflammatory functions, the latter mediated by T cells with TACI receptors.35
As for the chronology of the expression of BAFF and its receptors in the initial stages of RA (very early and early RA) it has been established that the expression of TACI, the levels of BAFF and its gene expression occur very early at the synovial level at the onset of the RA (since the first weeks), and progressively decrease with the establishment of the disease. While the BAFF-R is significantly increased in later stages of RA (early and established).67
Systemic sclerosisThe chronic activation of B cells is not only critical for the induction of autoantibodies, but also for the development of cutaneous sclerosis in systemic sclerosis.68 Although the pathogenesis still remains uncertain, it is known that the polyclonal cell activation and the abnormalities of the B cell characterized by the production of autoantibodies, which are correlated with certain specific manifestations of the disease, play an important role.68
Elevated levels of BAFF have been documented in systemic sclerosis (in up to 66% of cases).69 This elevation of BAFF is correlated by the extension of the cutaneous fibrosis measured by the modified Rodnan score, the onset or worsening of the organic involvement and with the diffuse variety, compared with the limited, and consequently decreases with the steroid treatment.69,70 These data show that BAFF is associated with the disease activity.
The APRIL levels are also elevated compared with the controls, and are correlated with higher incidence of pulmonary fibrosis, but not with the BAFF levels.71
Based on these data we can establish the severity and the patients profiles based on high serum levels of BAFF (marker of severe cutaneous sclerosis) and APRIL (marker of pulmonary fibrosis).70,71
Role of BAFF in other diseases and cell activation different from B lymphocytesNeoplasmsAs a regulator, BAFF also has a potential in the cell survival, growth and migration, and it can contribute to the process of neoplastic transformation, which puts BAFF/APRIL and their receptors as an important factor in both solid and hematological tumors.4,5 In these cases, the expression of BAFF can be given by the tumor cells (autocrine) or by the neighbor cells (paracrine) in the tumor microenvironment, protecting these cells from death, either spontaneous or induced by chemotherapeutic agents. These findings indicate that the blockage of BAFF and its receptors in malignant B cells might be a plausible therapeutic strategy in the area of Oncology.4,5
It is expected and well established that the levels of BAFF are increased in lymphoid cancers. However, and interestingly, it has been detected in non-lymphoid cells of epithelial type.
Increased serum levels of BAFF, derived from neutrophils, have been found in oral cancer,72 associated with the invasive migration in hypoxic cell lines in breast cancer and with an increase of the levels of serum TNF, angiogenesis and poor prognosis in multiple myeloma.73,74
As for their prognostic value, both BAFF and BAFF-R could be useful in chronic lymphocytic leukemia combined with CD38, and the zeta-chain-associated protein kinase-70. In follicular lymphoma they correlate with reduced progression-free survival.75
The different isoforms of BAFF/APRIL, previously described, could play a relevant role in the genesis of lymphoproliferative disorders: Δ4BAFF could behave as a transcription factor that might lead to an exaggerated autocrine production of BAFF in chronic lymphocytic leukemia.76 Whereas the different isoforms of APRIL (β, γ, δ, ¿) have been detected in pre-B acute lymphoblastic leukemia.76
Concerning the role of BAFF receptors, they have been also found overexpressed, as the TACI in multiple myeloma and thyroid carcinoma, which has been involved as a prognostic marker for lymphoma.77
As an exception, in prostate cancer, the BAFF secreted by the epithelial cells has a protective role on the survival of the periglandular lymphocytes and it limits the expansion of the tumor.78
Infectious diseasesWithin the viral infections it has been seen an induction of BAFF in infections with hepatitis C, human immunodeficiency virus, respiratory syncytial virus and H1N1.79–81 It has been described that the positive regulation of BAFF, after a viral infection, is TNF dependent, being the monocytes the responsible for the release of BAFF as a response to the treatment with IFN.82 The success of the therapy with IFN to control these infections has been attributed, in part, to the increase in BAFF signaling.83
The different types of viruses infect different cell targets and this is related with the level of secretion of BAFF, which differs from one cell type to another. For example, the macrophages, the dendritic cells and the neutrophils are able to increase the production of BAFF, while the epithelial cells that are part of the mucosa have a lower secretion, as a result of an infection, without losing their function as effector cells of a local response.83 In the infection with Epstein Barr virus there is a upregulation of BAFF. In fact, some lines of BL cells positive for Epstein Barr virus produce levels of BAFF comparable with those of the myeloid cells.84
The importance of this BAFF/APRIL system in the defense against bacterial infections is demonstrated in patients with common variable immunodeficiency, where mutations in BAFF-R, which are frequently manifested by recurrent bacterial infections at the level of the upper and lower respiratory tract are documented.85 However, there are individuals with mutations of the same receptor who are asymptomatic, which indicates that there are other environmental or genetic factors that contribute to this phenotype.85 Regarding the murine models, BAFF-R deficient mice exhibit a deficit of humoral immunity to TL Ag-independent, including encapsulated bacteria such as Streptococcus pneumoniae.86 In newborns, their lower ability to mount a protective response to bacterial polysaccharidases could be due to a lower expression of TACI, BCMA and BAFF-R in the BL, and to a decrease in the ability to change the antibody response.87
Therapies directed against BAFF in autoimmune diseasesThe importance of the BAFF/APRIL system and its receptors in the pathogenesis of autoimmunity has been described in the previous sections. These findings suggest that the anti-BAFF/APRIL therapy is an attractive therapeutic target in these diseases.
BelimumabIt is a human IgG1-λ which targets the soluble BAFF, inhibiting its activity and reducing its concentration.88 This leads to the apoptosis of the autoreactive B cells, preventing the proliferation and self-replication.89,90
Pharmacodynamically, belimumab binds specifically to the BAFF in solution, preventing its union to TACI, BCMA and BAFF-R. This drug has no effect on the membrane-bound BAFF or on other members of the family of the TNF ligand.89,90
Its selectivity leads to the decrease of the number of CD20+ B lymphocytes, virgin B cells, activated B cells and plasmablasts, without reducing the number of plasma cells. In contrast, it increases the number of memory B cells by day 28, but they return to their baseline count by week 52. This finding is related to the fact that these cells express higher levels of TACI.89,90 The treatment with belimumab also significantly decreased the levels of different Ig, of relevance the IgG, and increased the levels of complement, findings suggestive of a biologically active product with a response profile.91,92 The drug has a half-life of 13–17 days, with a clearance of 4±1.56ml/day/kg, reaching at 8 months a complete elimination and a Vss (baseline) of 68±20.8ml/kg, with a dose-dependent pharmacokinetic profile.93
Its efficacy in the treatment of SLE was documented in 2 pivotal phase iii, double-blind, randomized, placebo controlled, multicenter studies in patients with active SLE (SLEDAI>6), which led to its approval by the FDA as a biological therapy for the treatment of SLE.94,95
Data of its safety and sustained efficacy at 7 years have been reported with a follow-up of 1745patients/year, in whom a reduction in the level and the presence of antibodies has been documented, in addition to a steroid sparing effect.94,95
In RA, to date there is a published phase ii, multicenter, double-blind, placebo controlled, study of dosing in patients with moderate to severe RA with failure of previous treatments of 24 weeks of duration, with an open-label extension phase, in which was demonstrated an adequate tolerance with improvement in the ACR 20 scale (criterion of improvement of 20% of the American College of Rheumatology) at 24 weeks, especially in patients with high disease activity, positive RF, virgins to anti-TNF treatment and in whom methotrexate had previously failed. However, it failed to demonstrate a significant improvement in ACR50 and ACR70 responses without a dose/response relationship.96,97
In pSS the efficacy and safety of belimumab (n=30) at a dose of 10mg/kg in weeks 0, 2 and 4, with subsequent monthly application until week 24, was evaluated in a one-year open prospective study with primary outcomes of improvement in dry symptoms, systemic activity, or improvement in biomarkers of B cells. The primary outcome was reached in 60% of patients with a statistically significant improvement of the disease activity index (ESSDAI: EULAR Sjögren syndrome disease activity index) from 8.8 to 6.3 (p=0.0015) and a particular improvement in the glandular domain of the disease and the non-malignant parotid swelling, but without changes in salivary flow or Schirmer's test, promising results that justify the performance of further controlled studies.98 In an analysis of 10 patients of the same cohort, between the 10 and 52 weeks of treatment, the subtypes of B cell and the expression of BAFF and BAFF-R were assessed, and it was found that they had an increase of the circulating BL with expansion of the virgin and transitional subtypes (similar to those of healthy controls), higher levels of BAFF, but with lower expression of BAFF-R, decreased levels of Ig, RF, antinuclear antibodies and increase of the C4 complement fraction, which evidenced a normalization of the frequency, phenotype and function of the B cells.99
AtaciceptIt is a recombinant chimeric fusion protein conformed by the extracellular portion of the TACI receptor linked to the Fc domain of the human IgG1, with affinity for BAFF and APRIL.35,100 When it binds to the heterotrimers and homotrimers of BAFF and APRIL it causes a depletion of mature B cells, plasma cells and some serum antibodies.101,102 It reaches a serum peak 16 hours after the first administration, its concentration vs. time increases proportionally to the dose, without having a greater clinical effect, but with a good safety profile, with only a slight increase of 1.6% of the infections in some patients.103,104 In autoimmunity, atacicept is associated with a rapid reduction in the circulating levels of Ig, especially IgM, after the first dose, which persists with the subsequent administration.105,106 Unlike belimumab, by inhibiting APRIL there is a greater inhibition of the Ig levels.104
Its safety in SLE has been evaluated in phase Ib studies where the levels of Ig (IgA, IgM, IgG) and the number of B cells decreased in a dose-dependent manner, with promising findings.106 But this could not be revalidated in further randomized phase II/III studies of efficacy, with concomitant initiation of steroids and mycophenolate mofetil in patients with active lupus nephritis. These studies had to be discontinued due to an increase in the rate of infections and a pronounced decrease of Ig levels.107,108
In AR, the AUGUST study evaluated the effectiveness of atacicept in patients with failure of the treatment with methotrexate or anti-TNFα biological therapy. In this study was demonstrated a reduction in serum levels of Ig: IgM, IgA, IgG, the 3 types of RF, erythrocyte sedimentation rate, C-reactive protein, mature BL and plasma cells, without changes in anti-CCP. No effects on the T cells or natural killer cells were documented.103,104 After week 16, the plasma cells increased in number, without observing new changes in the mature B cells, possibly due to a selective mobilization of plasma cells or to a rapid transition of mature B cells into plasma cells, which is independent of the BAFF/APRIL system.104 The titles of Ig and RF initially decrease, but they return to normal levels 13 weeks after stopping the medication. It was observed in particular a greater reduction of the IgG and IgA fractions of the RF compared with the levels of the same isotype of serum Ig, which could be explained by a greater sensitivity of the antibodies to the BAFF/APRIL inhibition.103
Despite these findings, a better response of the ACR20-C-reactive protein (ACR20-CRP) has not been observed in the first 26 weeks, compared with placebo, since atacicept does not modulate enough the humoral response at the site of inflammation to achieve a clinical benefit.103,104
In RA, atacicept did not reach any primary efficacy outcome in this or in other phase iistudies, despite the acceptable safety profile and the significant biological activity. This was, probably, due to the inability to reach adequate concentrations in the synovial fluid compared with the serum levels, which requires, in addition, a local suppression necessary for a clinical effect.66,103–105,109,110 There are more ongoing studies to demonstrate its effect in the different diseases.
TabalumabIt is a type IgG4 human monoclonal anti-BAFF antibody that neutralizes the active BAFF both in its soluble and membrane-bound forms. It has a non-linear pharmacokinetics, with a half-life of ∼25 days.109,110 It causes an initial increase of the total and mature B cells, with a progressive decrease of these cells starting from the week 16, as the serum concentration of tabalumab decreases, and is maintained until week 24 on average.111 It is more selective for the virgin BL in approximately 60%, with a relative preservation of the mature BL. It also decreases IgM significantly in comparison with IgG and IgA, with no significant changes in the C-reactive protein and the erythrocyte sedimentation rate.110
Currently, there are ongoing clinical studies for RA, SLE, multiple myeloma and end-stage kidney disease.112,113
In RA it did not reach the primary outcome at week 16, a clinical response was observed at week 9, associated with a peak of the drug, by decreasing the disease activity score 28-C-reactive protein (DAS28-CRP),111 with subsequent decrease in its efficacy and serum concentration, without having a dose–response relationship. The effectiveness in comparison with placebo varies, from being equal or slightly better in those who have had previously failure with DMARDs o anti-TNF, respectively, after week 16.110,111
BlisibimodIt is a polypeptide fusion protein produced using E. coli that targets the soluble and membrane BAFF. It is made up of a novel binding domain linked to the N-terminal end of the constant fraction of human Ig.114 The efficacy of blisibimod was evaluated in patients with active SLE (SLEDAI greater than 6), in the randomized, double blind phase IIb clinical study, called PEARL-SC study (n=547), with the primary outcome measure at 24 weeks of an improvement in the systemic lupus response index-5 greater than 5. While the primary outcome did not improve significantly with regard to the placebo in the total group, the patients with the highest dose reached greater responses in comparison with placebo, with statistical significance at week 20 (p=0.02); it was also observed a significant decrease in proteinuria (p>0.01), in anti-DNA and improvement in the complement. The realization of phase iii studies is expected.115
Effects of the anti-CD20 depletion on BAFF levelsRituximabIt is a chimeric monoclonal antibody, which incorporates the heavy chain of the human IgG1κ and the variable regions of the murine Ig, directed against the human CD20.116 The CD20 is expressed from the pre-B cells until the memory cells, but not in the mature plasma cells. It is approved for the treatment of the B-cell lymphoma.117 In AR it has the approval for the use in patients who experience failure to biological therapy with anti-TNF.117 In SLE the evidence for its efficacy is anecdotal, particularly in patients who are refractory to treatment.117 Rituximab has been evaluated in clinical trials phase ii (known as EXPLORER) and phase iii (known as LUNAR) with disappointing results, where the primary outcomes were not reached, which could be attributed in part to methodological failures: the measurement of the disease activity differed from the one used in the studies with belimumab, the presence of heterogeneous patient populations, and the concomitant recruitment and immunotherapy that could mask its effect.117
In the majority of patients treated with rituximab the BL population kinetics shows 2 phases:
The first, in which it can be observed a complete depletion of BL, mainly memory cells at low titers (decrease of proinflammatory, auto-antigen specific memory BL: producers of lymphotoxin and TNF) associated with a progressive increase in the number of virgin and transitional B cells, secondary to the reactive increase in the BAFF levels in response to the decrease in the number of BAFF receptors in the B cells, and to the delay in the regulation of the BAFF mRNA.118,119 The clinical efficacy of belimumab is based on the decrease of the transitional virgin, activated and short-lived plasma cells.33 This first phase has a variable duration determined by the levels of BAFF and the reconstitution of the number of B cells, which could last between 6 and 12 months.33
The second phase or reconstitution phase: is characterized by the reappearance or memory B cells, that translated to the clinic can be indicative or relapse and retreatment of patients with SLE.33
Therefore, it is suggested that a depletion of long-lived plasma cells (rituximab) combined with a therapy that results in the loss of emerging autoimmune B cells (belimumab) could prevent the repopulation of long-lived cells of the B-cell compartment with autoimmune plasma cells, which lead us to think that the combination therapy would have complementary mechanisms of action, but in theory they could be mutually exclusive because of the increased risk of infection in a patient with complete B cell depletion.33 This hypothesis must be evaluated in clinical studies.
Conflict of interestThe authors declare that they have no conflict of interest.
Please cite this article as: Ospina FE, Betancur JF, Suso JP, Muñoz-Buitron E, Cañas CA, Tobón GJ. Papel de la citocina BAFF en las enfermedades autoinmunes: rol fisiopatológico y estrategias terapéuticas. Rev Colomb Reumatol. 2016;23:177–194.





![Variants of human APRIL (a proliferation-inducing ligand) and TWEAK (tumor necrosis factor [TNF]-like weak inducer of apoptosis). Exons are represented in boxes; introns in thick gray lines. THD, TNF homology domain; CRD, cysteine-rich domain; TMD, transmembrane domain; NTR, 5′ and 3′ non-translated regions. Variants of human APRIL (a proliferation-inducing ligand) and TWEAK (tumor necrosis factor [TNF]-like weak inducer of apoptosis). Exons are represented in boxes; introns in thick gray lines. THD, TNF homology domain; CRD, cysteine-rich domain; TMD, transmembrane domain; NTR, 5′ and 3′ non-translated regions.](https://static.elsevier.es/multimedia/24444405/0000002300000003/v1_201701180108/S2444440516300450/v1_201701180108/en/main.assets/thumbnail/gr4.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)