Rheumatoid arthritis (RA) is a chronic, inflammatory, autoimmune multisystemic disease that affects the synovial joints. An appropriate and early management improves prognosis and course of the disease.
ObjectiveTo evaluate the clinical and functional outcomes of patients with RA.
MethodologyObservational study with longitudinal follow up in a cohort of patients with early RA, with less than 12 months of evolution, classified according to the European League Against Rheumatism and American College of Rheumatology (ACR 2010) criteria. Remission criteria were taking into account according to Disease Activity Scale (DAS28-VSG), clinical activity disease index, and functional status according to Modified Health Assessment Questionnaire.
ResultsThe analysis included 99 patients with a mean age of 47.8+15.5 years, and of which 92 (93%) were women. All patients were treated with synthetic disease-modifying antirheumatic drugs. At 3 months of follow-up, a significant decrease was observed in DAS28 scores and clinical activity disease index compared to the value at baseline values (p<.05). No significant differences were found between patients diagnosed before and after 3 months from onset of symptoms (p>.05).
ConclusionsA substantial improvement was observed in patients with early RA treated during first year from onset symptoms. Continuous and periodic monitoring of the pathology is an indispensable tool for evaluating disease progress and making adjustments in the therapeutic management.
La artritis reumatoide (AR) es una enfermedad crónica, inflamatoria, autoinmune y multisistémica, cuyo principal blanco es la membrana sinovial. El manejo adecuado y temprano mejora la evolución y pronóstico de la enfermedad.
ObjetivoEvaluar los resultados clínicos y funcionales en pacientes con AR temprana.
MetodologíaEstudio observacional, con seguimiento longitudinal, de una cohorte de pacientes con AR temprana de menos de 12 meses de evolución, clasificados según los criterios de la Liga Europea Contra el Reumatismo y del Colegio Americano de Reumatología (ACR 2010). Se tuvieron en cuenta los criterios de remisión según la Escala de Actividad de la Enfermedad (DAS28-VSG) y el índice de actividad clínica de la enfermedad. Estado funcional según Cuestionario modificado de Evaluación de la Salud.
ResultadosSe analizaron 99 pacientes. La edad promedio de los pacientes fue de 47.8±15.5 años, el 93% (92) eran mujeres. Todos los pacientes fueron tratados con fármacos antirreumáticos modificadores de la enfermedad sintéticos. Durante el seguimiento a los 3 meses se observó una disminución significativa en los puntajes del DAS28 y actividad clínica de la enfermedad respecto al valor en la visita basal (p<0.05). No se encontraron diferencias significativas en la evolución de pacientes diagnosticados antes y después de 3 meses desde el inicio de los síntomas (p>0.05).
ConclusionesSe evidencia mejoría sustancial de los pacientes con AR temprana tratados durante el primer año de inicio de los síntomas. El seguimiento continuo y periódico de la patología es una herramienta indispensable para evaluar el progreso de la enfermedad y hacer ajustes en el manejo terapéutico.
Rheumatoid arthritis (RA) is a chronic, inflammatory, autoimmune multisystemic disease that affects mainly the synovial membrane. RA is distributed worldwide, with higher prevalence in women1 and in developed countries.2 In Latin America, it has been reported a prevalence close to 0.5%.3 In Colombia, there are no accurate data on prevalence, although some approximations have been made with Afro-Colombian population, with an estimate of 0.4%.4
The definition of early RA varies according to the time of evolution. Some authors set as a time limit less than 3 months from the onset of the symptoms. However, for other authors this time can get to be of up to 3 years.4,5
The current treatment of RA is aimed at the remission, that is, to the absence of inflammatory activity, or at least to a significant reduction or a status of low disease activity.6 In the way to achieving this goal, patients should be closely monitored in their evolution, using different indicators that allow to assess the disease activity in order to determine the necessary modifications in the treatment and reach the expected outcome.
An adequate treatment, with periodic controls of the disease activity, improves the prognosis of RA. This has been focused on the concept of “goal-directed treatment” (T2T) or targeted treatment, developed by a group of international specialists, which recommends having the goal of achieving the remission or low disease activity, through the systematic monitoring.7 The purpose of the present study is to evaluate the clinical and functional behavior of the patients with early RA, treated in the Clinic of Arthritis and Rheumatology of the Imbanaco Medical Center (CAR-CMI) and to characterize demographically and clinically this population.
Patients and methodsType of studyDescriptive, longitudinal, case series study, which included patients between 18 and 65 years, who consulted to the CAR-CMI in the period 2004–2014, diagnosed with RA according to the criteria of the European League Against Rheumatism (EULAR) and the American College of Rheumatology (ACR) EULAR/ACR 2010,8 with a time of evolution of symptoms shorter than 12 months, with at least one follow-up visit during the first year. Patients with diagnosis of fibromyalgia, systemic lupus erythematosus and other concomitant joint diseases such as gout and severe osteoarthritis were excluded, as well as those patients with major depression.
Place of studyThe study was carried out at the CAR-CMI, a high complexity private health care provider institution, in the city of Cali, Colombia.
The evaluations were carried out periodically, as part of the routine clinical follow-up of patients. This study was approved by the Ethics Committee of the institution and all patients signed the informed consent form for the collection of information.
VariablesThe demographic information was collected using a multidimensional questionnaire that included variables such as data of identification, gender, age, educational level marital status and socioeconomic stratum. The analytical variables were: rheumatoid factor, anti-cyclic citrullinated peptide antibody, antinuclear antibodies, erythrocyte sedimentation rate (ESR), C-reactive protein and complete blood count. The clinical follow-up was carried out by means of DAS28-VSG (the Spanish version of DAS28-ESR), CDAI, MHAQ, global assessment of the disease activity by the patient and the physician, and visual analog scale for pain. In addition, the type of treatment used was documented.
The clinical remission was defined based on the DAS28-VSG with a cutoff point <2.6. The EULAR response criteria were taken into account as follows: the good responders were patients who had a decrease in DAS28-VSG by at least 1.2units with regard to the initial assessment: moderate response in those patients with a change between <1.2 and >0.6 units, and non-responders those who had changes ≤0.6 units.9,10 In the CDAI scale, it was considered a low disease activity when it was between 2.8 and 10.
As for the ACR response criteria, it was assigned a percentage of improvement of the number of swollen and painful joints and improvement in 3/5 of the following variables: global assessment of the disease activity by the patient and by the physician, AVS, MHAQ and ESR or C-reactive protein, giving different percentages with response at 20, 50 or 70%.11
The modifications in the treatment of each patient were carried out according to the medical criteria of the rheumatologist, based on the above mentioned parameters. Clinimetrical evaluations of the patients during the medical management were carried out approximately every 3 months. For this study, the evaluations of the patients were analyzed at 3, 6 and 12 months of follow-up. 85% of patients had information at 3 months, 58% at 6 months and 53% at one year.
Statistical analysisA descriptive analysis was performed using frequency tables and measures of central tendency and dispersion, for qualitative and quantitative variables, respectively. Mixed-effect models for repeated measures were used for the comparison of the clinical (laboratory tests, DAS28-VSG, CDAI) and functional (MHAQ scale) outcomes through the follow-up and according to the time of evolution (<3 months). The models were adjusted in the statistical software R.3.1.3, by restricted maximum likelihood estimation (REML).
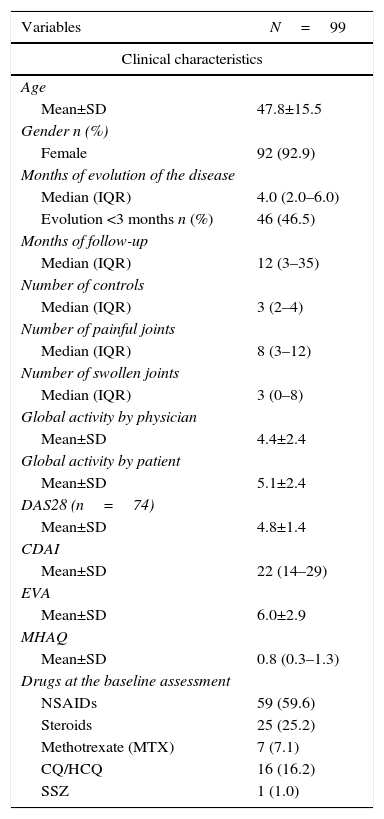
ResultsStudy populationIn the study period, 134 patients with early RA of less than one year of evolution were evaluated; of them, 99 patients who met the selection criteria were included; 35 patients were not included because they did not have a minimum evaluation at one year. The average age of the patients was 47.8±15.5 years and 93% (92) were women. 85% of patients were diagnosed at ≤6 months from the onset of the symptoms and 46.6% of patients had less than 3 months of evolution.
Baseline evaluationAt baseline evaluation it was found that half of patients had between 3 and 12 painful joints. The anti-cyclic citrullinated peptide antibodies and the rheumatoid factor were positive in 77.8 and 77.5% of the subjects, respectively. In the DAS28-VSG scale, it was found that 38.8% had a moderate disease activity and 45.9% a high activity; the CDAI reported 37.3 and 48.4%, respectively. The treatment scheme and other clinical characteristics are summarized in Table 1.
Baseline characteristics of the patients.
| Variables | N=99 |
|---|---|
| Clinical characteristics | |
| Age | |
| Mean±SD | 47.8±15.5 |
| Gender n (%) | |
| Female | 92 (92.9) |
| Months of evolution of the disease | |
| Median (IQR) | 4.0 (2.0–6.0) |
| Evolution <3 months n (%) | 46 (46.5) |
| Months of follow-up | |
| Median (IQR) | 12 (3–35) |
| Number of controls | |
| Median (IQR) | 3 (2–4) |
| Number of painful joints | |
| Median (IQR) | 8 (3–12) |
| Number of swollen joints | |
| Median (IQR) | 3 (0–8) |
| Global activity by physician | |
| Mean±SD | 4.4±2.4 |
| Global activity by patient | |
| Mean±SD | 5.1±2.4 |
| DAS28 (n=74) | |
| Mean±SD | 4.8±1.4 |
| CDAI | |
| Mean±SD | 22 (14–29) |
| EVA | |
| Mean±SD | 6.0±2.9 |
| MHAQ | |
| Mean±SD | 0.8 (0.3–1.3) |
| Drugs at the baseline assessment | |
| NSAIDs | 59 (59.6) |
| Steroids | 25 (25.2) |
| Methotrexate (MTX) | 7 (7.1) |
| CQ/HCQ | 16 (16.2) |
| SSZ | 1 (1.0) |
| Paraclinical characteristics | |
|---|---|
| Hemoglobin (n=84) | |
| Median (IQR) | 13.3 (12.3–14.1) |
| ACCP n (%) | |
| Positive (n=77) | 77 (77.8) |
| Rheumatoid factor n (%) | |
| Positive (n=89) | 69 (77.5) |
| Antinuclear antibodies n (%) | |
| Positive (n=66) | 6 (9.1) |
| CRP (n=67) | |
| Positive, median (IQR) | 41 (61.2), 24.0 (14.4–41.5) |
| ESR | |
| Median (IQR) | 26.5 (10.0–38.7) |
The scheme of treatment was decided in the first visit of confirmation of the diagnosis and the first follow-up assessment was carried out about 3 months later. All patients received synthetic DMARDs, which included combined therapies with MTX, CQ/HCQ and SSZ. We only had knowledge of 2 patients who changed their treatment regime to leflunomide plus MTX after 7 and 18 months of follow-up and one patient who required biological therapy with etanercept at 31 months of evolution.
67% of patients received DMARDs combined with MTX plus CQ/HCQ during the first year. At 3 months of follow-up 27% of the patients received monotherapy with MTX or CQ/HCQ. Only 3% received triple therapy that included MTX, CQ/HCQ and SSZ.
At the first follow-up visit, 65% of patients were treated with steroids at doses lower than 15mg, at one year of follow-up 45% of the patients evaluated continued with steroids at doses less than or equal to 5mg. At the third month, 22% of patients were managed with anti-inflammatory agents, decreasing to 4.7% in the first year.
Follow-upAt the time of the diagnosis, the patients had an average ESR of 29mm/h, with a decrease of 8mm/h at 3 months and one year of follow-up (p<0.05). The evaluation of the global disease activity performed by the physician (4.4±2.4), reported lower values compared with those reported by the patient (5.1±2.4), in the baseline visit. A reduction in the score of the global disease activity was found during the follow-up, based on what was reported by the physician and the patient (p<0.05). However, only the scores determined under medical criterion varied significantly between the patients diagnosed before and after 3 months of evolution, finding a greater disease activity in the patients diagnosed in the first trimester (p<0.05).
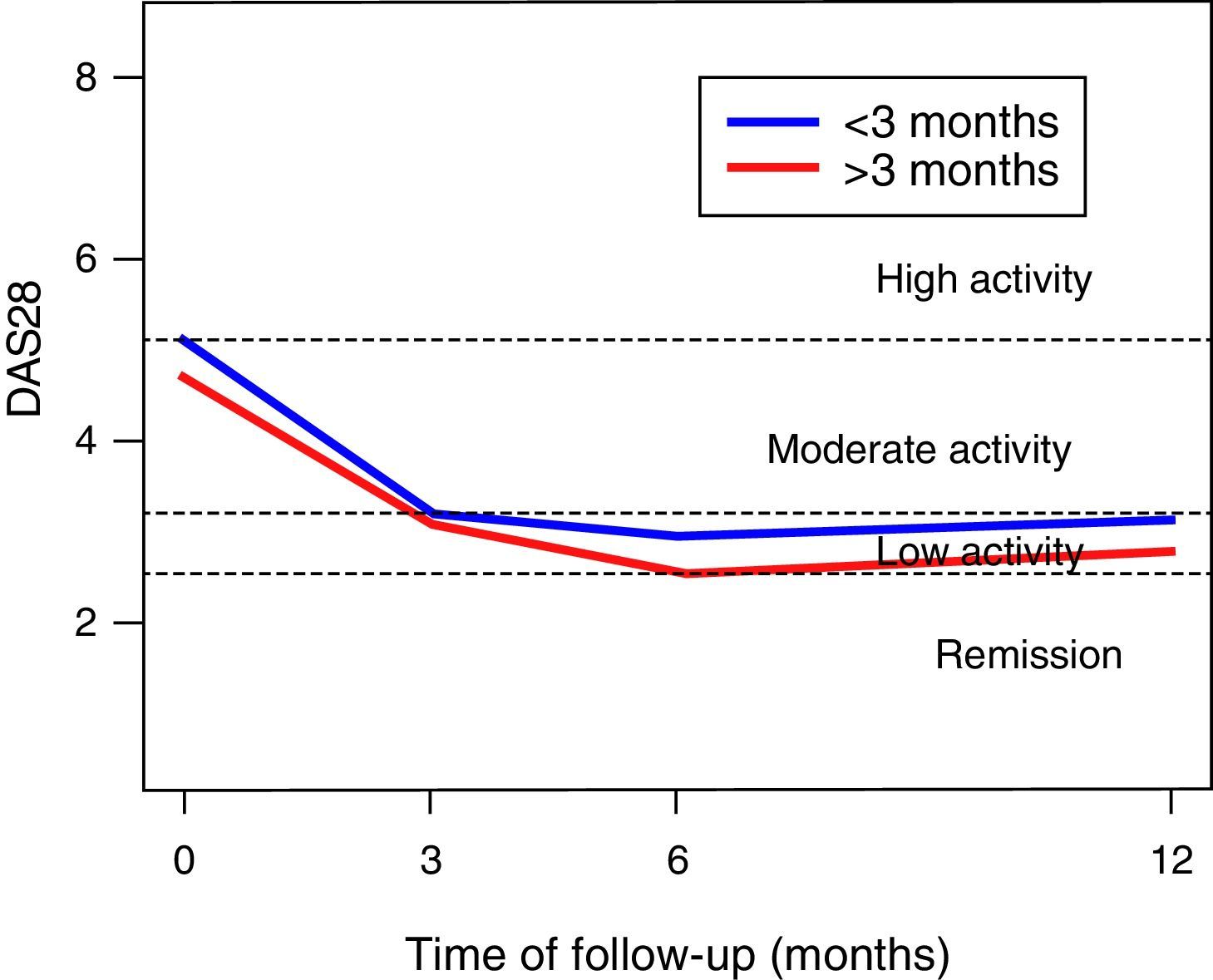
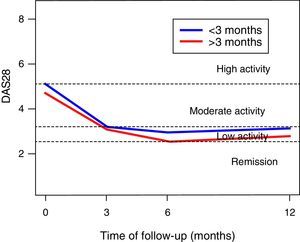
In the DAS28-VSG scale, it can be observed a significant change in the scores during the follow-up (p<0.001), with a reduction in 1.5 points at 3 months, of 1.9 at 6 months, and of 1.6 at one year of follow-up (Fig. 1). The CDAI scale, as well as the DAS28-VSG scale, indicates a significant decrease in the disease activity in the patients (p<0.05).
No differences in the ESR values were found in the global assessment of the disease by the patient and in the DAS28-VSG scale, in the patients diagnosed before 3 months of evolution. The data suggest higher scores on the CDAI scale in the patients diagnosed in the first trimester of evolution of the disease (p: 0.056).
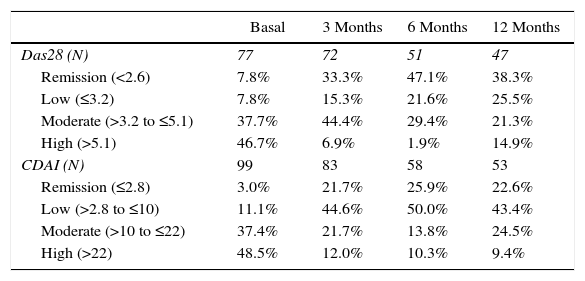
The activity of the disease, classified according to the DAS28-VSG and CDAI scales, is shown in Table 2. In the third month, it was found that one out of 3 patients was in remission of the disease, according to the DAS28-VSG scale. A decrease in the proportion of patients with high disease activity is observed in both scales through the follow-up.
Percentage of remission (DAS28<2.6) and (CDAI≤2.8).
| Basal | 3 Months | 6 Months | 12 Months | |
|---|---|---|---|---|
| Das28 (N) | 77 | 72 | 51 | 47 |
| Remission (<2.6) | 7.8% | 33.3% | 47.1% | 38.3% |
| Low (≤3.2) | 7.8% | 15.3% | 21.6% | 25.5% |
| Moderate (>3.2 to ≤5.1) | 37.7% | 44.4% | 29.4% | 21.3% |
| High (>5.1) | 46.7% | 6.9% | 1.9% | 14.9% |
| CDAI (N) | 99 | 83 | 58 | 53 |
| Remission (≤2.8) | 3.0% | 21.7% | 25.9% | 22.6% |
| Low (>2.8 to ≤10) | 11.1% | 44.6% | 50.0% | 43.4% |
| Moderate (>10 to ≤22) | 37.4% | 21.7% | 13.8% | 24.5% |
| High (>22) | 48.5% | 12.0% | 10.3% | 9.4% |
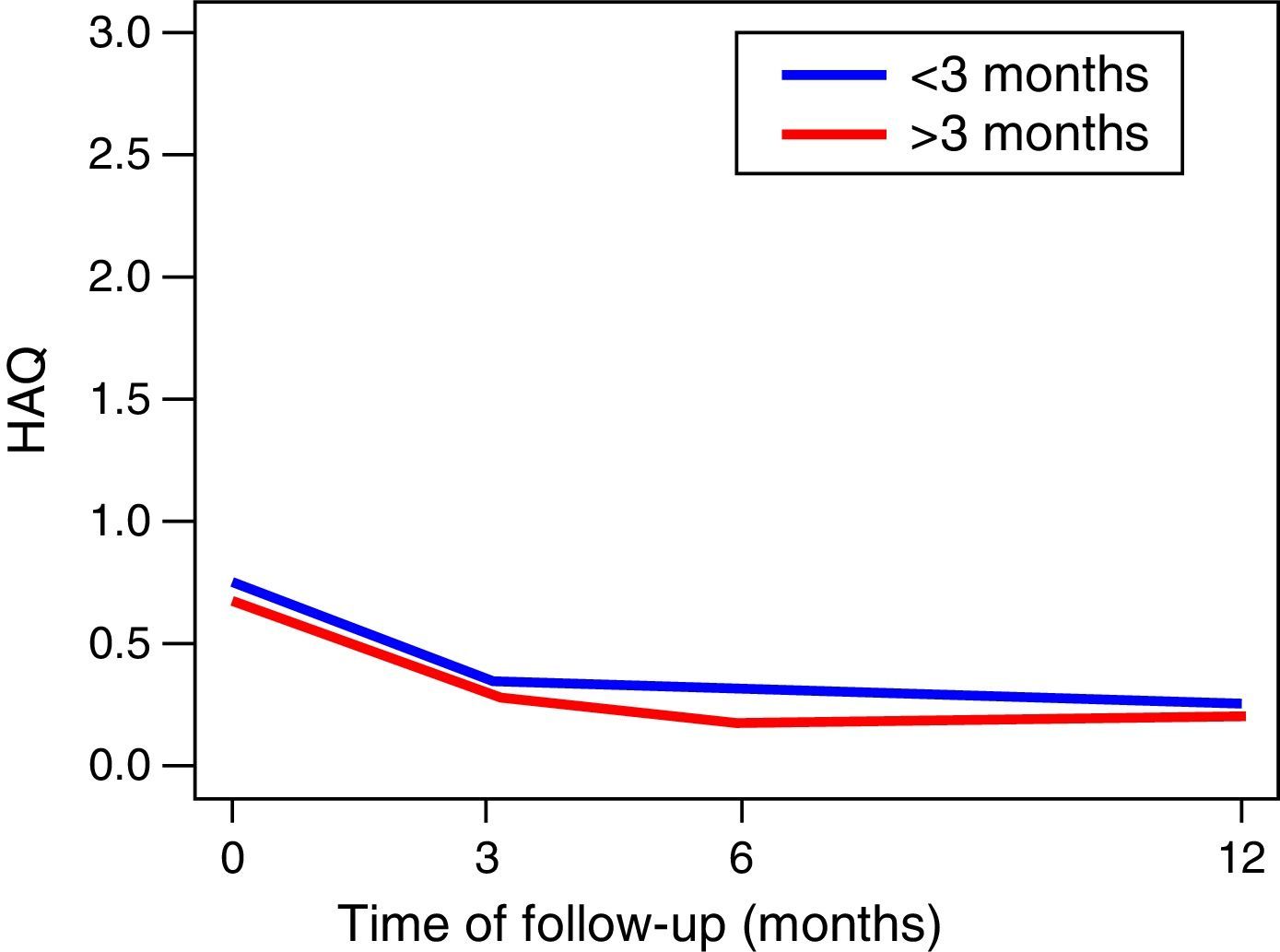
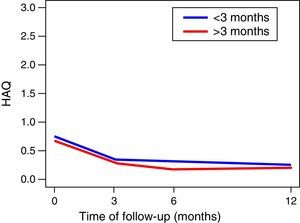
The functionality of the patients, assessed by the MHAQ, showed a statistically significant improvement (p<0.05) in the first year of follow-up. The foregoing suggests that patients intervened for the control of their disease, are able to improve their quality of life in the performance of daily activities, such as getting dressed, eating, walking, among others. No changes in the functionality of the patients were observed in the MHAQ scores over time, in the patients diagnosed before and after 3 months of evolution (Fig. 2).
Response to treatmentFor the evaluation of the response to treatment, the patients were classified according the EULAR response criteria. It was found that at 3 months of follow-up, 32% were good responders, 48% moderate responders and, 20% non-responders to treatment. By the ACR criteria 20, 50 and 70, at 3 months of treatment it was found a response of 72.8, 45.7 and 22.2%, respectively. 35% of the patients who did not respond to treatment withdraw the medications before completing the management, causing relapses and reactivation of the disease.
DiscussionIn this study, it was found that the demographic characteristics of the population with RA do not differ from those reported in previous national and international studies. It was observed a predominance of the disease in women12–14 and in people older than 45 years.1,15
Our data suggest that the remission of the disease is achieved in around 3 months, with similar results at one year of follow-up, as evidenced with the DAS28-VSG and CDAI scales. This is an important finding, since the control of the disease for prolonged periods entails less joint destruction, and an improvement of the functionality and of the quality of life. These findings are similar to those reported by other authors,16–18 but lower than those indicated in the TICORA study.19 We found no differences in the evolution of the patients who were diagnosed during the first trimester from the onset of the symptoms. The foregoing differs from that reported by other authors who have considered that the very early diagnosis and management (<3 months) of the disease is related to better outcomes.16,20
The evaluations carried out demonstrate the effectiveness of the management of the disease before 12 months, according to the therapeutic response as stated by EULAR and ACR. Currently, it is considered that treatment with disease-modifying drugs confers a significant advantage in the management of RA21 and even more, if it has been started early, improving the functional response and the quality of life.17,22,23
The initial treatment of the patients with RA managed in the CAR-CMI, consists in the use of steroids as bridge therapy, starting with intermediate doses, which are progressively reduced over time according to the therapeutic effect of the DMARDs. Although an evaluation of the patient response according to the type of therapy used was not carried out in this study, our results show an improvement of the patients that can be attributed to the initial treatment. Different studies have demonstrated the efficacy of low-dose steroids together with DMARDs in RA of less than 2 years of evolution.24–26 The combination of these drugs has shown to reduce the symptomatology, improve the functional prognosis of the patients, reduce the radiological progression and likewise reduce the need for biological therapy to achieve clinical remission.
The therapeutic management with DMARDs was provided to the patients based on the International27,28 and Latin American4,29 guidelines. These guidelines support the early use of the DMARDs as first-line treatment and of the biological agents as second-line treatment. This is because the biological therapy may entail a greater cost for the patient and is reserved for those who have persistent active disease and do not respond to conventional combinations. We only had information that 1% of patients required management with biological therapy, after 3 years of follow-up. The foregoing suggests a control of the early disease with the conventional DMARDs. However, this percentage may be higher, due to the great number of losses to follow-up. It is important to highlight that the biological medicines are effective. Nevertheless, the need for a long-term treatment and their toxicity can make them less cost-effective and increase the direct costs related to the drugs.30,31 While what it matters is the control of the disease with the use of available therapies, the economic analyses support the concept of therapies with DMARDs and the rapid stepping of treatment when there is an insufficient response, seeking alternatives that generate cost-benefit both for patients and for the healthcare system, this concept goes hand in hand with the current recommendations of EULAR which are consistent with the rational use of the resources of the society.32
It is important to mention that the la CAR-CMI has a special educational program for patients with RA, which contains educational videos, lectures for patients, accompaniment in rehabilitation by physical therapy, occupational therapy and personnel trained for the integral treatment of the pathology, which seeks to guide the patient in the management of his disease and to achieve a greater adherence to the proposed treatments. This is supported by previous studies that evaluated the role of patients’ education, which provides additional benefits for the management and control of the disease.33,34 Despite all patients entered in the education program, one-third had relapses due to treatment interruption.
This study represents the real life in clinical practice. The treatments were planned and adjusted according to the disease activity, count of swollen and painful joints, functionality, comorbidities and tolerability of the treatment. Since the data come from the clinical follow-up carried out during the medical management of the patients in the CAR-CMI and they were not strictly collected for this study, there may exist problems in the quality of the information and in some cases it was not possible to obtain the follow-ups of the clinical and functional variables in the first year of follow-up.
On the other hand, a standardized treatment for the group was not established as in other protocols, however, these data may reflect the management given to patients in clinical practice.
ConclusionsA substantial improvement of the patients with early RA treated during the first year after onset of symptoms is evidenced. The continuous and periodic follow-up of the pathology is an indispensable tool to assess the progress of the disease and make adjustments in the therapeutic management, which leads to alter the course of the disease and improve the quality of life.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
FundingThis work was financed with own resources of the authors.
Conflict of interestThe authors declare they did not have any conflict of interest at the time of the writing of the manuscript.
The authors acknowledge the collaboration provided by the institution source of information, in the support of the collection of data for this project, mainly the students of Medicine of the Universidad del Valle, Alejandro Correa and Diego Escarpetta.
Please cite this article as: González ML, Rueda J, González H, Cantor E, Martínez A. Artritis reumatoide temprana: resultados clínicos y funcionales de una cohorte en un centro de alta complejidad, Cali-Colombia. Rev Colomb Reumatol. 2016;23:148–154.