ToPAS-2 is a questionnaire designed to detect cases of psoriatic arthritis (PsA) in patients with psoriasis (PsO).
ObjectiveTo validate the ToPAS-2 questionnaire in patients with psoriasis in Argentina.
Materials and methodsPatients ≥18 years old with a diagnosis of PsO. As a control group, patients diagnosed with PsA (CASPAR criteria) and osteoarthritis (OA) (ACR criteria) were included. All patients completed the ToPAS-2 questionnaire and were then evaluated by a rheumatologist. Blood tests were performed to determine Rheumatoid Factor (RF) and erythrocyte sedimentation rate (ERS). Radiographs of the hands, feet, panoramic view of the pelvis, Ferguson, and lumbar and cervical spine were performed.
Statistical analysisDescriptive statistics. ROC curves for determination of sensitivity, specificity, and cut-off points of the questionnaire. A p<.05 was considered significant.
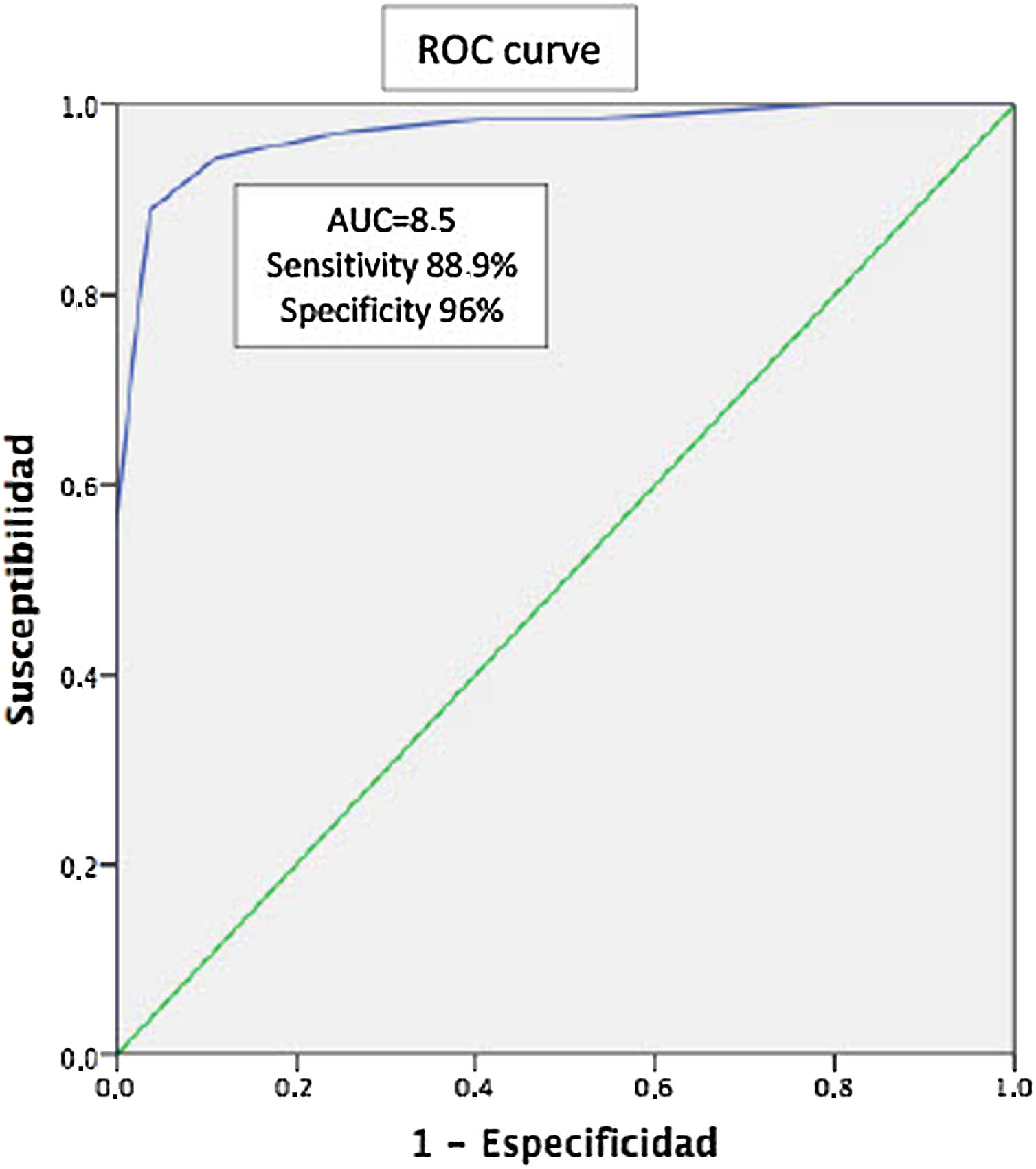
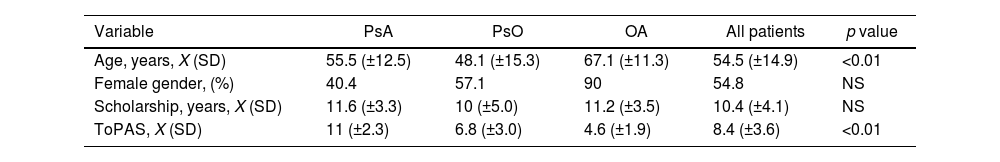
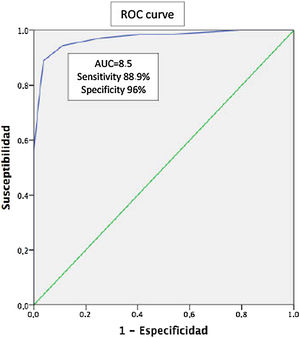
Results126 patients were evaluated, 54.8% female with a mean age of 54.5 (±14.9) years. The mean schooling was 10.4 (±4.1) years. A total of 45.2% had a diagnosis of PsA, 38.9% of PsO, and 15.9% of OA. The ToPAS-2 result was X 8.4 (±3.6). The mean age according to the groups was 55.5 (±12.5), 48.1 (±15.3), and 67.1 (±11.3) years, with 40.4% female, 57.1%, and 90% respectively. The mean of the ToPAS-2 according to the different groups was 11 (±2.3), 6.8 (±3.0), and 4.6 (±1.9) (p<.01 between all groups) for APs, PsO, and OA, respectively. Of the 49 patients with PsO, 16 patients were diagnosed as PsA, after evaluation by the rheumatologist. The mean ToPAS-2 in these patients was significantly higher than those not diagnosed as PsA [10.1 (±1.8) vs 5.2 (±2)] (p<.001). In the ROC curve analysis, the area under the curve was .97 (95% CI .94–.97). A TOPAS cut-off value of 8.5 had a sensitivity of 88.9% and a specificity of 96% for the diagnosis of PsA.
ConclusionThe ToPAS-2 questionnaire proved to be a valid, sensitive, and specific tool for the detection of PsA in patients with psoriasis.
ToPAS-2 es un cuestionario diseñado con el fin de detectar casos de artritis psoriásica (APs) en pacientes con psoriasis.
ObjetivoValidar el cuestionario ToPAS-2 en pacientes con psoriasis en Argentina.
Materiales y métodosSe incluyeron pacientes ≥18 años con diagnóstico de psoriasis. Como grupo control se incluyeron pacientes con diagnóstico de APs (criterios CASPAR) y osteoartritis (criterios ACR). Todos los pacientes completaron el cuestionario ToPAS-2 y luego fueron evaluados por un reumatólogo. Se hizo análisis de sangre para la determinación de factor reumatoide (FR) y eritrosedimentación (ERS). Se realizaron radiografías de manos, pies, panorámica de pelvis, Ferguson y columna lumbar y cervical.
Análisis estadísticoEstadística descriptiva; curvas ROC para determinación de la sensibilidad, la especificidad y los puntos de corte del cuestionario. Se consideró significativa una p<0,05.
ResultadosSe evaluaron 126 pacientes, 54,8% de sexo femenino, con una edad media de 54,5 (±14,9) años. La media de escolaridad fue de 10,4 (±4,1) años. El 45,2% presentaban diagnóstico de APs, el 38,9% de psoriasis y el 15,9% de osteoartritis. El resultado del ToPAS-2 fue X 8,4 (±3,6). La media de edad según los grupos fue de 55,5 (±12,5), de 48,1 (±15,3) y de 67,1 (±11,3) años, siendo de sexo femenino el 40,4%, el 57,1% y el 90%, respectivamente. La media del ToPAS-2 según los diferentes grupos fue de 11 (±2,3), de 6,8 (±3,0) y de 4,6 (±1,9) (p<0,01 entre todos los grupos) para APs, psoriasis y osteoartritis, respectivamente. De los 49 pacientes con psoriasis, 16 fueron diagnosticados como APs luego de la evaluación del reumatólogo. La media del ToPAS-2 en estos pacientes fue significativamente mayor a los no diagnosticados como APs (10,1 [±1,8] vs 5,2 [±2]) (p<0,001). En el análisis por curvas ROC, el área bajo la curva fue de 0,97 (IC95%: 0,94-0,97). Un valor de corte del ToPAS de 8,5 tuvo una sensibilidad del 88,9% y una especificidad del 96% para el diagnóstico de APs.
ConclusiónEl cuestionario ToPAS-2 demostró ser una herramienta válida, sensible y específica para la detección de APs en pacientes que presentan psoriasis.
Psoriatic arthritis (PsA) has been defined as an inflammatory arthritis associated with psoriasis (PsO), usually diagnosed based on criteria proposed by Moll and Wright.1 The clinical manifestations of the disease include arthritis, enthesitis, dactylitis, axial disease and skin or nail involvement. The majority of people with this condition have pre-existing PsO.2 The prevalence of PsA in PsO patients has been reported between 6 and 42%.3,4
A period of preclinical disease may occur and cases of established disease may remain unidentified for some time. The reasons why cases of established PsA remain unidentified have not been elucidated but one possible cause is the lack of musculoskeletal expertise among primary care physicians and treating dermatologists.5,6 Since the CASPAR classification criteria have a high sensitivity and specificity for PsA, they might perform well as diagnostic criteria. However, since the CASPAR criteria are applied only for patients with inflammatory musculoskeletal disease, an assessment by a physician with expertise looking for the presence of inflammatory musculoskeletal disease in the joints, spine or entheses is crucial. Therefore, it may be difficult to use these criteria in the context of epidemiological or family investigations when it is difficult to have all patients reviewed by a doctor.
Several screening tools have been developed as referral tools to help dermatologists and general practitioners to identify suitable individuals for referral to rheumatologists. Most are self-questionnaires, although their method of development and purpose are slightly different. The Psoriatic Arthritis Screening Evaluation (PASE) was developed in a hospital dermatology setting with a sensitivity of 82% and specificity of 73%. PASE assess the presence of articular symptoms and, in addition, the impact in terms of disability.7 The Psoriasis Epidemiology Screening Tool (PEST) has been developed in a primary care-based population with psoriasis.8 The PEST had a sensitivity of 94% and specificity of 78%.
The original version of The Toronto Psoriatic Arthritis Screening (ToPAS) is a tool developed by Gladman et al., used for screening of PsA in PsO patients, which has been validated in both dermatology and rheumatology clinics. It allows to identify patients with PsA in the general population, psoriasis and other rheumatic and dermatological diseases. It consists of 12 questions that include domains of skin, nails, peripheral and axial involvement. In the original paper, a cut-off value of 8 has demonstrated a good sensitivity (87%) and specificity (93%).9 Later, the modified version of the original ToPAS (ToPAS-2) was validated by the same group.10 This version has included images to assess skin involvement (psoriasis), dactylitis and the presence of arthritis, making it easier for the patient to understand. In addition, it also includes questions about axial involvement, such as lumbar and cervical pain, stiffness and exercise.
The aim of this study was to validate the ToPAS-2 questionnaire in an Argentinian PsO cohort.
MethodsPatient selectionPatients with previous PsO diagnosis by a dermatologist were recruited consecutively during a period of 6 months, from different Argentinian rheumatology hospitals. PsA patients (fulfilling CASPAR criteria) and patients with hand osteoarthritis (OA) diagnosed by rheumatologist were included as control group. The Spanish translation and cross-cultural adaptation of ToPAS-2 was performed. All patients completed ToPAS-2 questionnaire without the help of any assistant and were referred for rheumatologist assessment, according to standard protocol.
Ethical considerationsThe local ethical committee approved the study and written informed consent according to the Declaration of Helsinki was obtained from all patients.
Clinical evaluationAll patients underwent a complete evaluation, including demographic (age and gender) and clinical data (family history of PsO and PsA, disease duration, peripheral and axial disease, nail involvement, dactylitis and enthesitis).
Plain radiographs of hands, feet, Ferguson, hips, cervical and lumbar spine were performed in all patients at initial presentation. Presence of rheumatoid factor (RF) and levels of erythrosedimentation (ERS) rate was determined.
Statistical analysisComparisons for categorical variables were made by Fisher's exact test and χ2 when appropriate. Continuous variables were compared by Student's t test. Sensitivity, specificity and cut-off points for ToPAS-2 questionnaire were assessed by ROC analysis. A value of p<0.05 was considered significant.
ResultsOne hundred and twenty-six patients (n=126) were recruited in the study. Sixty-nine patients (54.8%) were females with a mean age of 54.5 (±14.9) years. Mean of scholarship was 10.4 (±4.1) years. In our cohort, 45.2% of patients had a previous diagnosis of PsA, 38.9% PsO and 15.9% OA diagnosis, with a mean age of 55.5 (±12.5), 48.1 (±15.3) and 67.1 (±11.3) years, respectively.
For the entire cohort, a mean ToPAS-2 score of 8.4 (±3.6) was reported. According to diagnosis, the mean ToPAS-2 score was 11 (±2.3) in PsA patients, 6.8 (±3.0) in PsO and 4.6 (±1.9) in OA patients (p<0.01 between all groups) (Table 1).
Taking into account only PsO patients (n=49), sixteen (n=16) of them were diagnosed having PsA after a thorough rheumatologist evaluation. The mean ToPAS-2 score in PsA patients were significantly higher 10.1 (±1.8) as compared with those with PsO without arthritis: 5.2 (±2) (p<0.001) (Table 2). When ROC was analyzed, area under the curve (AUC) was 0.97 (IC95% 0.94–0.97). The ToPAS-2 cut-off value of 8.5 had a sensitivity of 88.9% and specificity of 96% for PsA diagnosis (Fig. 1).
In our cohort, including patients with PsO, OA and PsA, the ToPAS-2 questionnaire has proved to be a valid, sensitive and specific tool for the detection of PsA in an Argentinian cohort of PsO patients.
It is noteworthy that the values of the ToPAS-2 questionnaire were significantly higher in patients with PsA when compared with patients with psoriasis and OA. Interestingly, a cut-off value of 8.5 was found to perform well, with an AUC of 0.97 (88.9% of sensitivity and 96% of specificity).
One of the main limitations in the spondyloarthritis group and in PsA in particular is that most are underdiagnosed in the primary care setting, with a considerable delay in diagnosis. The use of screening tools (such as self-questionnaires) in patients with psoriasis has been shown to improve the optimal referral and the diagnosis of PsA.10,11 In our study, among 49 patients with psoriasis, 16 patients (32%) were diagnosed as having PsA, demonstrating the need for a correct screening in these patients.
Patients with hand OA were included in the study as control group, in order to test the performance of the test in this group. Patients with OA diagnosis, as expected, were older than the other groups, which might influence the results of the test. Interestingly in this group, the mean TOPAS-2 score was lower than patients with PsO and PsA.
In our study, the ToPAS-2 questionnaire was found to be an useful referral tool for PsA diagnosis. As previously noted, this new version has included images to assess psoriasis, dactylitis and the presence of arthritis, making it easier for the patient to understanding. In addition, it also includes other items not included in the original ToPAS, such as questions about axial involvement (lumbar and cervical pain), stiffness and exercise.
Recently, a validation of the TOPAS-2 questionnaire was also reported in a Turkish population and Brazilian population, with a good sensitivity and specificity as compared to the original questionnaire.12,13
This study has some limitations. The reproducibility of the questionnaire was not evaluated in this study, also the time it took patients to complete the questionnaire was not recorded.
In summary, we report the validation of the ToPAS-2 questionnaire in an Argentinian cohort, having shown that is a valid, sensitive and specific tool for the diagnosis of PsA in patients with psoriasis.
Conflict of interestsThe authors declare they have no conflict of interest.