Psoriatic arthritis is a chronic inflammatory arthropathy characterized by subtypes with distinct phenotypes and variable clinical course, which requires optimal management of joint manifestations and skin involvement.
ObjectivesThis study aims to provide recommendations for the pharmacological treatment of psoriatic arthritis based on evidence and applicability to the Colombian health system.
Methodology and methodsThese guidelines were developed according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology. Clinical experts, rheumatologists, and dermatologists formulated the recommendations based on clinical evidence, the disease's impact, patient values and preferences, and the resource availability in the country.
ResultsThe guideline includes 12 recommendations related to the use of anti-inflammatory drugs, glucocorticoids, and disease-modifying antirheumatic drugs (DMARDs), including conventional synthetics, biologics, and targeted synthetic DMARD, for the PsA domains: peripheral disease, axial disease, enthesitis, and dactylitis.
ConclusionsThis document presents a set of updated and evidence-based recommendations that guide decision-making about pharmacological management of psoriatic arthritis in adults in Colombia. They constitute general guidelines that should be interpreted and implemented according to the individualized evaluation of the patient and the medical criteria in the different clinical settings.
La artritis psoriásica es una artropatía inflamatoria crónica con distintos fenotipos y curso clínico variable, que requiere un manejo óptimo de las manifestaciones articulares y del compromiso cutáneo.
ObjetivosEste documento pretende hacer recomendaciones para el tratamiento farmacológico de la artritis psoriásica basadas en la evidencia y la aplicabilidad al sistema de salud colombiano.
Metodología y métodosEstas guías fueron desarrolladas de acuerdo con la metodología Grading of Recommendations Assessment, Development, and Evaluation (GRADE). Expertos clínicos, reumatólogos y dermatólogos formularon las recomendaciones con base en la evidencia clínica, el impacto de la enfermedad, los valores y las preferencias de los pacientes y la disponibilidad de recursos en el país.
ResultadosLa guía incluye 12 recomendaciones relacionadas con el uso de antiinflamatorios, glucocorticoides y fármacos antirreumáticos modificadores de la enfermedad (FAME), incluidos los sintéticos convencionales, los biológicos y los sintéticos dirigidos, para los dominios de la artritis psoriásica: enfermedad periférica, enfermedad axial, entesitis y dactilitis.
ConclusionesEste documento presenta un conjunto de recomendaciones actualizadas y basadas en la evidencia que orientan la toma de decisiones sobre el manejo farmacológico de la artritis psoriásica en adultos en Colombia. Estas recomendaciones constituyen lineamientos generales que deben ser interpretados e implementados de acuerdo con la evaluación individualizada del paciente y el criterio médico en los diferentes escenarios clínicos.
Psoriatic arthritis (PsA) is a chronic inflammatory arthropathy in patients with psoriatic disease. It is characterized by involvement of different domains (peripheral arthritis, axial disease, enthesitis, and dactylitis) and a variable clinical course.1 In most patients, PsA manifests with skin involvement before the onset of arthritis, although 20% of patients experience the onset of arthritis before signs of psoriasis.2 Prevalence of PsA varies from 0.04% to 0.2% in the general population and from 6% to 41% in patients with psoriasis.2,3 Reports for Latin America are variable between countries; however, the prevalence of psoriatic disease is higher in countries where Caucasian ethnicity predominates.4 A cross-sectional study, based on data from the official registry of the Ministry of Health between 2012 and 2018 estimated 13.5 cases of PsA per 100,000 inhabitants over 20 years of age, with a prevalence of 5.8% of psoriasis patients. In this study, PsA was reported with a slight predominance in the female population (53%), and higher frequency in the age group 55–59 years old.5
In patients with psoriasis, the development of PsA is associated with worse functional status and greater disability compared to patients without PsA. In Colombia, in particular, it has been reported that patients with PsA have significantly greater severity in measures of quality of life, absenteeism and work impact.6 However, in the Latin American context, the optimal management of patients continues to be a great challenge and the need for a better approach and speed of treatment is highlighted.7 A rheumatologist should perform diagnosis and classification of articular involvement in a patient with psoriatic disease to integrate strategies and therapeutic goals for control of the inflammatory process and management of skin lesions. Joint damage prevention and intervention reduces the impact of PsA on daily activities and quality of life.
To provide an adequate level of care to PsA patients, it is crucial to keep an updated clinical practice with the best available evidence from scientific and biotechnological advances.4 The increasing availability of pharmacological options for treating PsA challenges optimal selection of treatment modalities. The objective of this document is to provide up-to-date, evidence-based guidelines on pharmacological treatment of PsA that is applicable in the Colombian healthcare system. These guidelines are aimed at dermatology and rheumatology specialists, helping them manage adults with a confirmed diagnosis of PsA in any healthcare service. This document complements the Colombian Association of Dermatology and Dermatologic Surgery's (Asocolderma) 2022 Clinical Practice Guide for the Treatment of Psoriasis in Colombia.
MethodsThe guideline updating process followed the Colombian Ministry of Health and Social Protection standards.8,9
The development group consisted of methodological experts and specialists in dermatology and rheumatology. It also included a patient representative. The members of the development group (clinical experts and methodologists) filled out the conflict-of-interest form proposed by the Methodological Guide for the Development of Clinical Practice Guidelines. The analysis of the information was performed by two dermatology specialists and a methodologist, using the criteria defined in the methodological manual. No participant presented conflicts that limited their participation in the guide (seesupplementary material).
The authors selected topics to update by reviewing PsA questions in the Asocolderma 2018 Psoriasis Guideline. The authors prioritized questions by relevance to current clinical practice, availability of new evidence of effectiveness and safety, and contextual changes in drug use.
The authors identified five clinical questions of interest for this guideline, four to be updated and one de novo. Question formulation used the PICO structure (P: question. I: intervention. C: comparison. O: outcome). Clinical experts prioritized outcomes (Likert scale rating from 1 to 9) according to their importance in decision making.
Search, selection, and evaluation of evidenceThe authors updated the literature search with the same strategies as for the 2018 Psoriasis Guidelines. They also created new strategies for de novo searches, with no date limits. The sources for systematic reviews and randomized clinical trials (RCT) were Medline and EMBASE, using systematic review filters developed by SIGN8 and RCT filters developed by McMaster University HIRU-Hedges.10
Two methodological experts (LI and SM) screened references from the searches results by title and abstract, according to the inclusion criteria for evidence selection. Disagreements among peer reviewers were resolved by consensus. The relevant references underwent full-text analysis (see PRISMA11flowchart insupplementary material).
Evaluation and evidence synthesisThe AMSTAR instrument12 prioritized and assessed systematic reviews and meta-analyses. Cochrane Collaboration criteria complemented selection and evaluation of RCT.13
Evaluation of the evidence was based on Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology.13 Two methodologists extracted data from the selected studies. Elaboration of the GRADE evidence profiles relied on GDT software for comparisons and outcomes of study results (seesupplementary material).
Formulation of recommendationsTo formulate recommendations, experts discussed the evidence using the evidence to decision (EtD) framework. The discussion included: priority of the problem, magnitude and balance of intervention effects, overall quality of evidence, patient values and preferences, costs and resources requirements, impact on health equity, and feasibility of the intervention. The experts expressed their level of agreement (Likert scale from 1 to 9) on each recommendation. All recommendations obtained consensus and approval. The final recommendations used the GRADE methodology for direction and strength, and all the recommendations were justified.
Further specifications of these processes are available in the Asocolderma's 2022 Clinical Practice Guide for the Treatment of Psoriasis in Colombia.
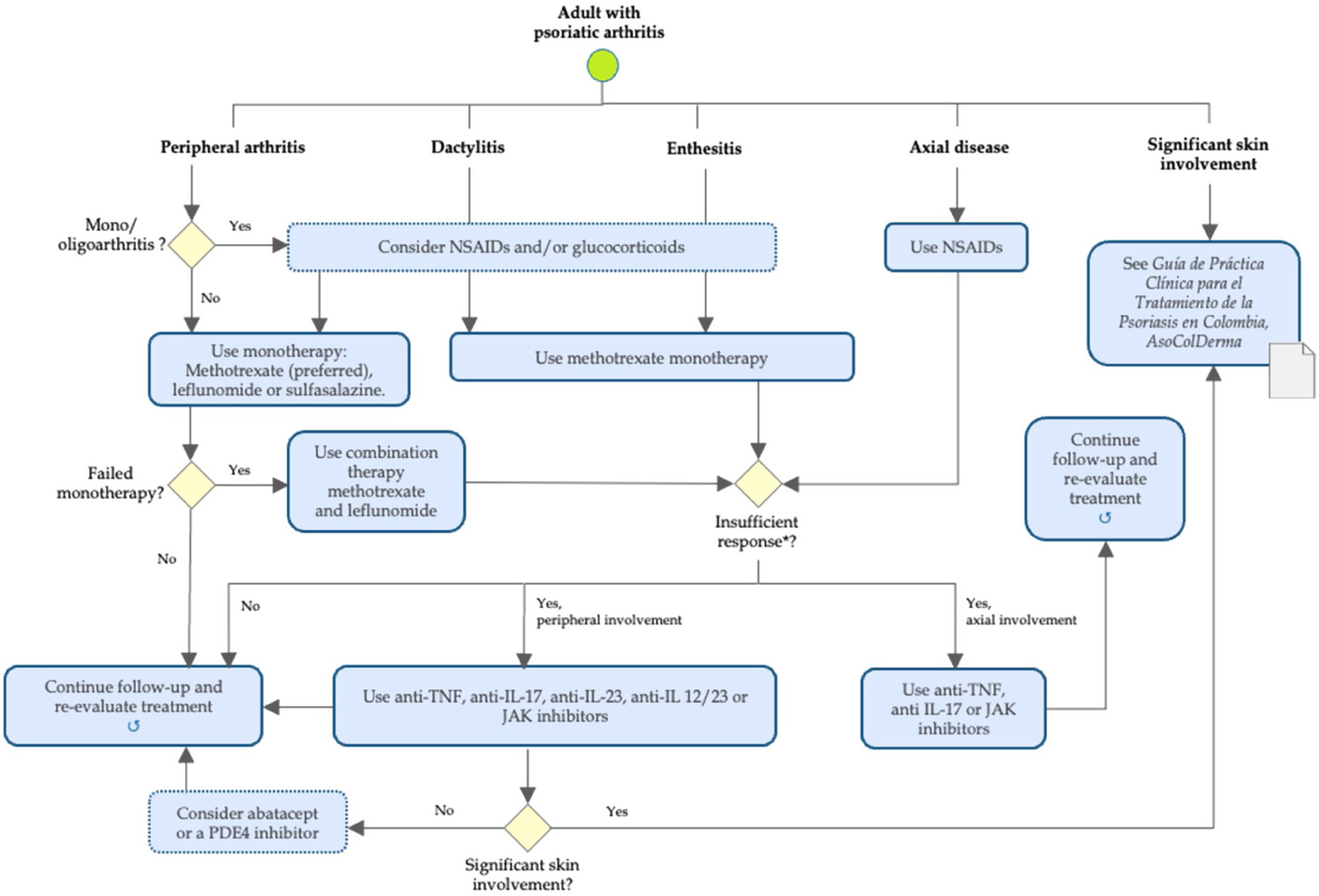
ResultsAs a general principle of PsA management in adults, therapeutic decisions should consider the characteristics of the disease, previous therapies, comorbidities, prognosis, and patient expectations. Table 1 and Fig. 1 summarize recommendations for pharmacological management in adults with PsA in different disease domains: Axial disease, peripheral arthritis, dactylitis, and enthesitis.
Recommendations for the pharmacological management of psoriatic arthritis.
| 1 | Use of NSAIDs (COX-2 selective or non-selective COX inhibitors) is recommended for management of musculoskeletal signs and symptoms in PsA patients with axial or peripheral involvement. Strong recommendation in favor, moderate-quality evidence. |
| 2 | Use of NSAIDs (COX-2 selective or non-selective COX inhibitors) is recommended for initial management of patients with axial PsA. Strong recommendation in favor, moderate-quality evidence. |
| 3 | In PsA patients with arthritis (mono/oligoarthritis), enthesitis, or dactylitis, local injection of glucocorticoids can be considered. Weak recommendation in favor, very low-quality evidence. |
| 4 | Use of systemic glucocorticoids at more than 10mg/day of prednisolone (or equivalent doses) is not recommended, nor is administration for more than three weeks. Avoid use of depot glucocorticoids. Strong recommendation against, low-quality evidence. |
| 5 | In patients with peripheral arthritis, preferential use of methotrexate monotherapy is recommended. Other options are leflunomide and sulfasalazine. Strong recommendation in favor, low-quality evidence. |
| 6 | In patients with peripheral arthritis and failure of conventional DMARD – monotherapy, combination therapy with methotrexate and leflunomide can be considered. Strong recommendation in favor, low-quality evidence. |
| 7 | In patients with dactylitis, the use of methotrexate monotherapy is recommended. Strong recommendation in favor, low-quality evidence. |
| 8 | In patients with enthesitis, the use of methotrexate monotherapy is recommended. Strong recommendation in favor, low-quality evidence. |
| 9 | The use of conventional DMARDs is not recommended for the management of axial PsA. Strong recommendation against, low-quality evidence. |
| 10 | In patients with axial PsA and insufficient response or contraindication for NSAIDs, the use of anti-tumor necrosis factor (TNF), anti-IL-17, or janus kinase (JAK) inhibitors is recommended. Strong recommendation in favor, low-quality evidence. |
| 11 | In patients with PsA and peripheral involvement (peripheral arthritis, enthesitis, or dactylitis) and insufficient response or contraindication for conventional DMARDs, the use of anti-TNF, anti-IL-17, anti-IL-23, anti-IL 12/23, or JAK inhibitors is recommended. Strong recommendation in favor, low-quality evidence. |
| 12 | In patients with PsA and peripheral involvement (peripheral arthritis, enthesitis, or dactylitis) without significant skin involvement or with contraindication to biologic DMARDs or JAK inhibitors, abatacept or a PDE4 inhibitor may be considered. Strong recommendation in favor, low-quality evidence. |
Algorithm of pharmacological management of psoriatic arthritis in adults. *Intolerance or contraindication to defined treatment. NSAIDs: nonsteroidal anti-inflammatory drugs (COX-2 selective or nonselective); TNF: tumor necrosis factor; IL: interleukin; JAK: janus kinase; PDE4: phosphodiesterase 4.
- 1.
Use of NSAIDs (COX-2 selective or non-selective COX inhibitors) is recommended for management of musculoskeletal signs and symptoms in PsA patients with axial or peripheral involvement. Strong recommendation in favor, moderate-quality evidence.
- 2.
Use of NSAIDs (COX-2 selective or non-selective COX inhibitors) is recommended for initial management of patients with axial PsA. Strong recommendation in favor, moderate-quality evidence.
Good practice point: The NSAID selection should use clinical criteria based on individualized evaluation, since evidence does not support preferential use of a NSAID.
Evidence synthesisTwo RCT in patients with PsA (n=80) reported significant reduction in pain, morning stiffness, tenderness, and joint swelling after 4 weeks of nimesulide (200mg and 400mg/day) compared to placebo.14 Another RCT in active PsA reported efficacy of celecoxib (400mg and 200mg) at 2 and 6 weeks compared to placebo. There was no difference among groups, due to a high placebo response.15 Comparative studies among NSAIDs found no differences in efficacy.16,17 Though use of NSAIDs has not been associated with increased cardiovascular risk,18 it is advisable to consider the individual risk-benefit ratio of these drugs.
Current PsA management guidelines19 recommend the use of NSAIDs to relieve non-synovial musculoskeletal symptoms and signs. In peripheral arthritis with persistent disease activity, NSAID monotherapy should be used for a maximum of 4 weeks. Treatment can be extended up to 12 weeks for predominant axial disease or enthesitis.
Glucocorticoids in psoriatic arthritis- 1.
In PsA patients with arthritis (mono/oligoarthritis), enthesitis, or dactylitis, local injection of glucocorticoids can be considered. Weak recommendation in favor, very low-quality evidence.
- 2.
Use of systemic glucocorticoids at more than 10mg/day of prednisolone (or equivalent doses) is not recommended, nor is administration for more than three weeks. Avoid use of depot glucocorticoids. Strong recommendation against, low-quality evidence.
Though there are no RCT data on the use of systemic glucocorticoids for PsA, experts in Latin America use them in select patients.20,21 A recent multicenter observational study of 46 subjects with PsA compared oral anti-inflammatory drugs to steroid injection in 73 fingers of patients with dactylitis. The study also showed the effectiveness of steroid injection from months 1 to 3 for reducing pain, functional impairment, and scores on the Leeds Dactylitis Index (LDI-b).22
Local or systemic administration of glucocorticoids may be useful in some patients with PsA. That administration includes sacroiliac injections for axial involvement and psoriasis,23 and intra-articular injections for mono/oligoarthritis, dactylitis, and enthesitis.24–26 According to current recommendations, glucocorticoids in PsA should be used at low doses for short periods of time, considering their long-term safety profile. The recommendations also include use of oral corticosteroids for maintenance.19,27–30
Conventional disease-modifying antirheumatic drugs (DMARDs) in psoriatic arthritis- 3.
In patients with peripheral arthritis, preferential use of methotrexate monotherapy is recommended. Other options are leflunomide and sulfasalazine. Strong recommendation in favor, low-quality evidence.
- 4.
In patients with peripheral arthritis and failure of conventional DMARD – monotherapy, combination therapy with methotrexate and leflunomide can be considered. Strong recommendation in favor, low-quality evidence.
- 5.
In patients with dactylitis, the use of methotrexate monotherapy is recommended. Strong recommendation in favor, low-quality evidence.
- 6.
In patients with enthesitis, the use of methotrexate monotherapy is recommended. Strong recommendation in favor, low-quality evidence.
- 7.
The use of conventional DMARDs is not recommended for the management of axial PsA. Strong recommendation against, low-quality evidence.
Good practice points: Use of parenteral methotrexate is an option in case of gastrointestinal intolerance.
All patients on methotrexate treatment should receive concomitant folic acid supplementation.
Evidence synthesisThere is limited and low-quality evidence on the efficacy of conventional DMARDs for PsA.31,32 The Methotrexate In Psoriatic Arthritis (MIPA) study is a placebo-controlled clinical trial that included 221 patients. It reported a clinically significant benefit of methotrexate for pain, function, and also for patient and physician assessments.33 A prospective34 open-label study of 73 patients with dactylitis and enthesitis reported significant and sustained improvement at 9 months with an oral methotrexate regimen. Also, the trial TIght COntrol of Psoriatic Arthritis (TICOPA), along with other trials, have demonstrated the efficacy of methotrexate monotherapy for disease control (significant reduction in LDI scores) in patients with PsA, peripheral disease, dactylitis, and enthesitis.35,36
In patients with PsA, leflunomide demonstrated at 24 weeks a significant positive response versus placebo.37 In the Observational Study of Psoriatic Arthritis Treated with Leflunomide (OSPAL) trial, leflunomide was effective and well tolerated.38 Though of very low quality, evidence has shown that leflunomide and methotrexate (20mg daily orally) have similar efficacy, superior to the efficacy of cyclosporine.39–41 Sulfasalazine (2000mg/day) demonstrated superiority over symptomatic treatment (NSAIDs and/or glucocorticoids) in the spondylitis functional index and with the American College of Rheumatology (ACR) inflammation and response outcome instruments ACR50 and ACR70.42
Combination therapyCombination of methotrexate and leflunomide demonstrated efficacy in clinical trials: A two-center, controlled, open-label study compared monotherapy with combination therapy. It showed at 24 weeks significant improvement in both treatment arms.41 The improvement rate, however, was higher with the combination therapy (methotrexate 75%, leflunomide 68.8%, in combination 83.3%). Incidence of treatment-related adverse events was lower for the combination therapy (methotrexate 38.5%, leflunomide 38.9%, in combination 35%).41 The combination also achieved better pain reduction and better Health Assessment Questionnaire (HAQ) scoring versus leflunomide. The clinical trial COmparing Methotrexate monotherapy with methotrexate Plus LEflunomide combination Therapy in Psoriatic Arthritis (COMPLETE-PsA) also reported that combination therapy achieved greater improvement against disease activity versus monotherapy. The COMPLETE-PsA assessed disease activity by using the Psoriatic Arthritis Disease Activity Score (PASDAS). Tolerability, however, was better for methotrexate monotherapy versus combination therapy.43
As indirect evidence, the TICOPA trial44 also supports the efficacy of combined methotrexate and leflunomide. The ongoing study Severe Psoriatic Arthritis – Early intervEntion to Control Disease (SPEED), will increase evidence for this combination in PsA.45
Biologic DMARDs and synthetic targeted DMARDs in psoriatic arthritis- 8.
In patients with axial PsA and insufficient response or contraindication for NSAIDs, the use of anti-tumor necrosis factor (TNF), anti-IL-17, or janus kinase (JAK) inhibitors is recommended. Strong recommendation in favor, low-quality evidence. For significant skin involvement, see recommendations for psoriasis. (https://revista.asocolderma.org.co/index.php/asocolderma).
- 9.
In patients with PsA and peripheral involvement (peripheral arthritis, enthesitis, or dactylitis) and insufficient response or contraindication for conventional DMARDs, the use of anti-TNF, anti-IL-17, anti-IL-23, anti-IL 12/23, or JAK inhibitors is recommended. Strong recommendation in favor, low-quality evidence. For significant skin involvement, see recommendations for psoriasis. (https://revista.asocolderma.org.co/index.php/asocolderma).
- 10.
In patients with PsA and peripheral involvement (peripheral arthritis, enthesitis, or dactylitis) without significant skin involvement or with contraindication to biologic DMARDs or JAK inhibitors, abatacept or a PDE4 inhibitor may be considered. Strong recommendation in favor, low-quality evidence.
Biologics in PsA domains. The clinical trials SPIRIT P1 (Study of Ixekizumab in Participants With Active Psoriatic Arthritis),46 SPIRIT H2H (Study of Ixekizumab in Participants With Active Psoriatic Arthritis, head-to-head),47 and EXCEED (Efficacy of Secukinumab Compared to Adalimumab in Patients With Psoriatic Arthritis)48 evaluated ixekizumab versus adalimumab, and secukinumab versus adalimumab. This was done in PsA patients with no prior biologic DMARDs use. There were no significant differences in responses for peripheral arthritis, dactylitis, and enthesitis among the compared drugs.
In peripheral arthritis, the meta-analysis by McInnes et al.,49 showed that some anti-TNF may be numerically better, though not significantly, than interleukin (IL) inhibitors in ACR response. The meta-analysis by Torres et al.50 identified no differences between comparators for this PsA domain.
Several studies have investigated biological treatments for enthesitis and dactylitis.50 McInnes et al.49 analyzed 14 clinical trials with evidence of effect on enthesitis at 12–16 weeks. Those authors also reviewed 10 clinical trials with evidence of effect on dactylitis at 24 weeks. All biological treatments were more effective than placebo, except for ustekinumab 45mg in enthesitis and abatacept in enthesitis/dactylitis. In terms of median effect, anti-IL-17, and anti-IL-23 ranked best. The classic meta-analysis by Simons et al.51 compared anti-IL17, anti-TNF, and placebo for resolution of enthesitis and dactylitis. The meta-analysis found a better response for anti-IL17 (enthesitis RR 2.31, 95% CI 1.60–3.34. Dactylitis RR 2.65, 95% CI 1.79–3.94) and for anti-TNF (enthesitis RR 1.99, 95% CI 1.36–2.90. Dactylitis RR 2.07, 95% CI 1.38–3.12) compared to placebo. Moreover, the study Enthesial CLearance In PSoriatic Arthritis (ECLIPSA)52 found favorable results in ustekinumab versus anti-TNF in patients with PsA and enthesitis.
The Managing AXIal Manifestations in psorIatic arthritis with SEcukinumab (MAXIMIZE) study53 evaluated efficacy and safety of secukinumab (150mg and 300mg) in axial manifestations of patients with PsA and insufficient response to NSAIDs (minimum two NSAIDs for 4 weeks). The study demonstrated significant improvement with secukinumab compared to placebo in signs and symptoms of axial disease.
Janus Kinase Inhibitors in PsA. The number of studies on the effect of tofacitinib in PsA is limited.54,55 A recent systematic review56 reported early and sustained effectiveness of tofacitinib versus placebo for treatment of all PsA domains, including peripheral involvement, axial disease, enthesitis, and dactylitis. A network meta-analysis57 compared efficacy of DMARDs in patients with insufficient response to anti-TNF and without prior anti-TNF use. Tofacitinib versus most biologic DMARDs and apremilast had similar efficacy improving joint symptoms (ACR20). Tofacitinib versus some biologic DMARDs showed similar efficacy improving skin symptoms (severity index and psoriasis area). Improvements in dactylitis severity score and Leeds enthesitis index were comparable among treatments.
The efficacy of upadacitinib compared to placebo was demonstrated in patients with axial PsA with inadequate response or intolerance to at least one biologic DMARD.58 Similarly, subgroups of patients in the trials SELECT-PsAi 1 and SELECT-PsA 2 showed improved axial symptoms (measured with Bath Ankylosing Spondylitis Disease Activity Index – BASDAI and C-reactive protein (CRP)-based Ankylosing Spondylitis Disease Activity Score ASDAS-CRP) without increased rates of uveitis.59 A RCT60 compared the efficacy of upadacitinib (15mg or 30mg) placebo, or subcutaneous adalimumab (40mg every other week). Upadacitinib 30-mg dose was superior to adalimumab at week 12, but a 15-mg dose was not. Regarding filgotinib, the Efficacy and Safety of Filgotinib in Active Psoriatic Arthritis (EQUATOR) phase 2 placebo-controlled trial in patients with active PsA demonstrated efficacy compared to placebo (ACR20) at week 16, and it improved health-related quality of life.61,62
All studies with JAK inhibitors have shown the efficacy of this group of drugs compared to placebo in peripheral arthritis outcomes. Similarly, tofacitinib 5mg and upadacitinib 15mg have shown efficacy for enthesitis and dactylitis. Reported data showed, however, a non-statistically significant increased risk of serious adverse events with JAK inhibitors. Longer-term follow-up is required.63
Phosphodiesterase-4 inhibitors in PsA. Apremilast demonstrated superior efficacy (ACR20) versus placebo at 12 months.64–70 Some studies have suggested a beneficial effect of apremilast in enthesitis65–67 and a cohort study of 150 patients with PsA/spondyloarthritis demonstrated its effectiveness in oligoarticular disease.71 The Efficacy and Safety Study of Apremilast to Treat Active Psoriatic Arthritis (PALACE 4) clinical trial assessed appremilast monotherapy at 5 years in 527 PsA patients with no prior DMARD treatment. At week 260, the study found 65.8% of patients with apremilast 30mg achieved ACR20 responses, with improved signs and symptoms and tolerability.69 Efficacy results for apremilast were reported at week 16 in patients receiving other concomitant conventional DMARDs.67,68 Although the magnitude of the effect is small, comparative data between apremilast and tofacitinib favor tofacitinib.72
In terms of safety, apremilast has shown a favorable profile, with mostly mild events, low discontinuation rates (<7.5%), and tolerability for as long as 156 weeks.73
DiscussionThis document presents recommendations for the pharmacological management of adults with PsA, based on updating the 2018 Asocolderma guidelines. The resulting document provides the best available evidence for clinical practice and interdisciplinary medical management of patients with psoriatic disease. Recommendations in these guidelines are limited to drugs commonly used in the pharmacological management of adults diagnosed with PsA, including NSAIDs, glucocorticoids, conventional DMARDs, biologic DMARDs, and targeted synthetic DMARDs. These recommendations are a general guideline for common patient care. Their interpretation and implementation should consider individual characteristics through an assessment that includes the patient's values and preferences, and through shared decision-making.
Colombia has almost universal coverage of the health system (99.01% according to the last official measurement of October 2022),74 and there is coverage through the health system of all drugs approved in the world for the treatment of psoriatic arthritis (methotrexate, leflunomide, five anti-TNF, two IL-17 inhibitors, one IL-23 inhibitor, one IL 12–23 inhibitor, and one janius kinase inhibitor). In this context of a wide range of drugs approved for PsA, it is of great importance to be able to prioritize therapies for patients who really need them, and to achieve this, clinical practice guidelines become a fundamental tool to guide professionals in choosing the best therapeutic option in each particular case, based on the best available clinical evidence.
Limitations of these guidelines include the low certainty of evidence for several pharmacological interventions in the population with PsA. Periodic review is necessary to update information as scientific knowledge grows. Subsequent up-to-date information can be consulted in the Asocolderma full document 2022 Clinical Practice Guideline for the Treatment of Psoriasis in Colombia.
This document is part of the Psoriasis guideline update developed by Asocolderma with the financial support of Abbvie, Janssen, Lilly, Novartis and Pfizer. The entire evidence review process, the development of the recommendations and the manuscript preparation were developed independently by Asocolderma. The sponsors had no involvement in the preparation, development, and the content of this guideline.
The guideline update did not address costs, but the authors did consider the economic feasibility of their recommendations, in an effort to contribute to the accessibility and practical use of drugs in Colombia. Also, these general guidelines provide scientific evidence for the development of health policies that favor the care of patients with PsA by facilitating the adequate management of the disease, improving quality of life, and optimizing health resources.
Ethical approvalThis work did not involve the use of human subjects. This guideline is based on systematic reviews of literature and all the information included in this article have been referenced.
Authors’ contributionsAll authors have contributed substantially to the article.
FundingThis clinical practice guideline update was developed by Asocolderma with support from, in alphabetical order, Abbvie, Janssen, Lilly, Novartis, and Pfizer. The entire process of the evidence review, construction of the recommendations, and preparation of the manuscript were developed independently by Asocolderma. The funding entities had no involvement in the conduct, development and content of this guideline.
Conflict of interestThe authors declare that they have no conflicts of interest.
The group of experts of this paper want to thank Dr. Adela Rosa Castro, who participated in the translation of the final version of the manuscript.