Polyarteritis nodosa is a rare form of vasculitis which affects medium and small arteries. Gastrointestinal involvement occurs in 14–65% of patients, the most common symptom being abdominal pain; in a few cases, intestinal perforation may occur. Chronic diarrhea at disease onset is, however, very uncommon.
ObjectiveTo present the case of an elderly man with chronic diarrhea as the main manifestation of polyarteritis nodosa that progressed to intestinal perforation.
Material and methodsThe diagnosis of polyarteritis nodosa was made by intestinal biopsy; cyclophosphamide was administered as induction treatment with progressive clinical improvement. Furthermore, a PubMed literature review was conducted.
ResultsThree communications on which diarrhea was reported as a manifestation of polyarteritis nodosa; in one of these patients, a 13-year-old girl, chronic diarrhea was the main manifestation.
ConclusionThis case illustrates the diagnostic complexity of polyarteritis nodosa due to non-specific symptoms and atypical presentation such as diarrhea, underscoring the importance for early recognition and intervention.
La poliarteritis nodosa es una forma rara de vasculitis que afecta a las arterias medianas y pequeñas. La afectación gastrointestinal ocurre entre un 14 y un 65% de los pacientes, siendo el dolor abdominal el síntoma más común. En algunos casos puede ocurrir perforación intestinal, sin embargo, la diarrea crónica al inicio de la enfermedad es muy poco común.
ObjetivoPresentar el caso de un varón adulto con diarrea crónica como manifestación principal de la poliarteritis nodosa, que progresó a perforación intestinal.
Materiales y métodosEl diagnóstico de poliarteritis nodosa se llevó a cabo mediante biopsia intestinal; se administró ciclofosfamida como tratamiento de inducción y se evidenció una mejora clínica progresiva. Además, se hizo una revisión en PubMed.
ResultadosSe encontraron 3 reportes en los cuales la diarrea fue una manifestación de la poliarteritis nodosa; en uno de estos pacientes, una niña de 13 años, la diarrea crónica fue la manifestación principal.
ConclusiónEste caso ilustra la complejidad diagnóstica de la poliarteritis nodosa, debido a su presentación con síntomas no específicos y manifestación atípica como la diarrea, y resalta la importancia de una identificación e intervención temprana.
Polyarteritis nodosa (PAN) is a necrotizing vasculitis of medium and small arteries which represents a challenging diagnosis [1]. Its annual incidence rate is of 0.9–8.0 per million persons in Europe and its prevalence is of 31 per million [2]. In Latin America, the incidence and prevalence of PAN are largely unknown; however, in Peru, an incidence of 0.5 per million has been reported [3]. Gastrointestinal involvement occurs in 14–65% of patients, being the small intestine and gallbladder most commonly affected; the most common presentation is abdominal pain, which could be serious and carry high risk of complications and mortality [4]. However, chronic diarrhea as initial manifestation of PAN is very uncommon. Therefore, we present a case of PAN with this unusual presentation and a review of literature about it.
CaseFour months before his admission, a 65-year-old man developed four to five watery diarrheas per day while also experimenting some weight loss. One week before his admission, he also developed abdominal pain. During his entire clinical course, the patient did not have fever. He was admitted to the hospital because of his persisting symptoms. On physical examination, he was found to have abdominal tenderness over the left abdominal region. During his hospitalization, he presented dysesthesia in the lower limbs and decreased distal muscle strength.
Baseline tests were obtained: Hemoglobin 13.2 (12–18)g/dL, white blood cell 11,800/μL, erythrocyte sedimentation rate (ESR) 15mm/h, C-reactive protein (CRP) 142.69 (0–10)mg/l, albumin 2.7 (3.5–5)g/dL, rheumatoid factor 303.9UI/ml, creatinine 0.5mg/dL, complement C3: 80 (90–180) mg/dL and C4: 5 (10–40) mg/dL. Antinuclear antibody, antineutrophil cytoplasmic antibody, hepatitis B and C virus, and human immunodeficiency virus were negative. The study was complemented with electromyography which showed acute stage of mononeuritis multiplex in both lower limbs.
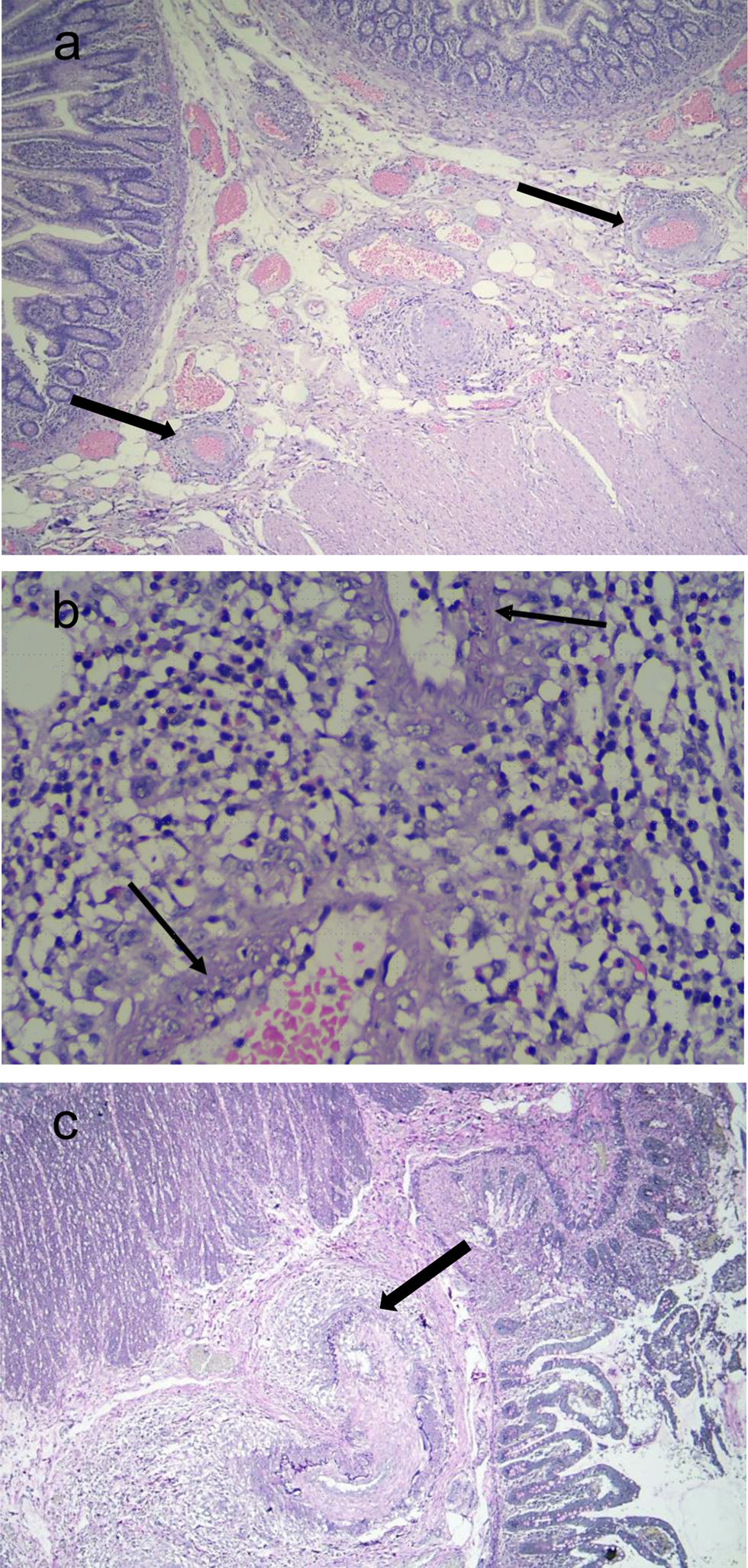
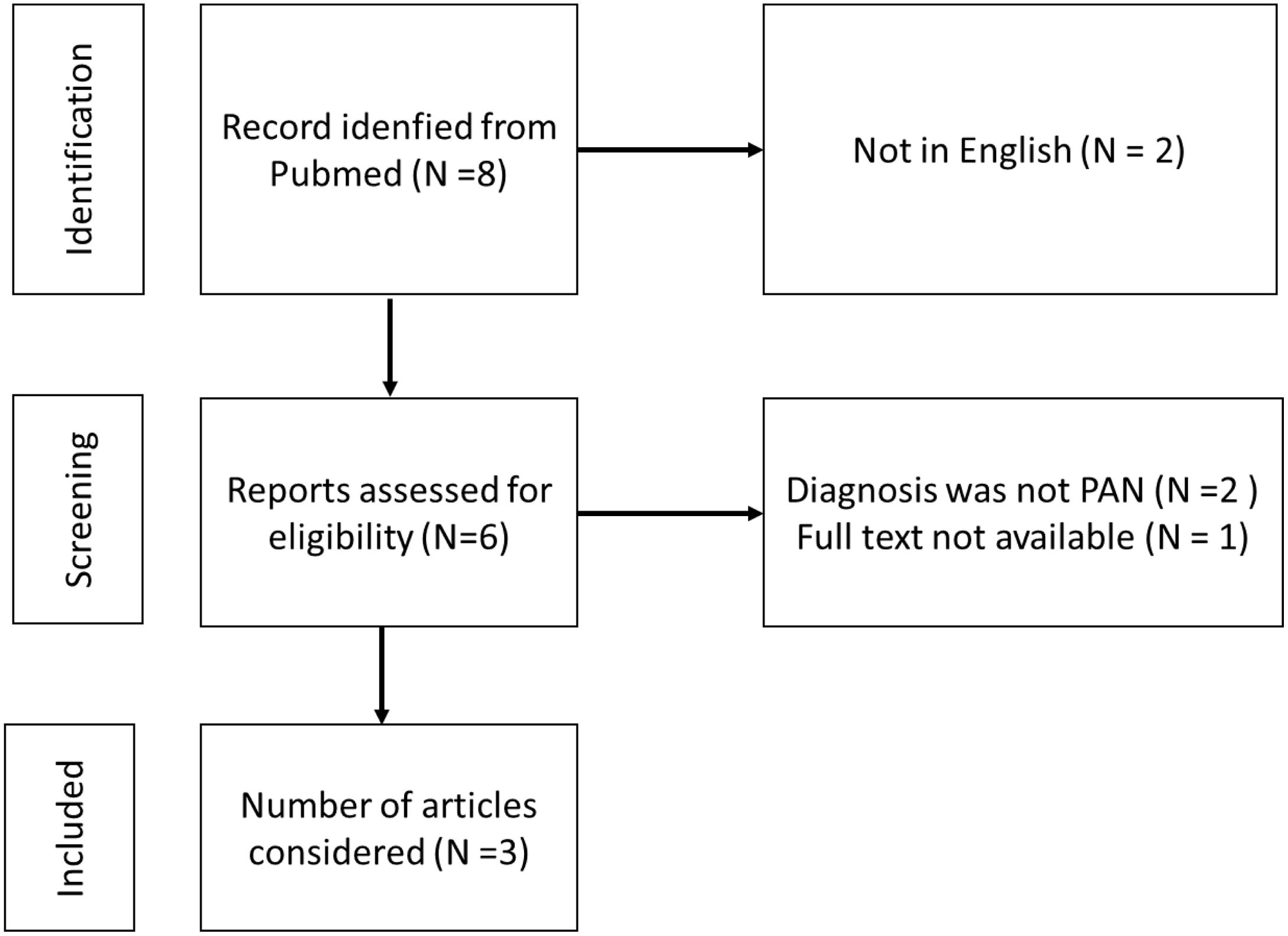
Two weeks after his admission, he developed an intense acute abdominal pain predominantly in the right abdominal and iliac regions. Due to the presence of pneumoperitoneum demonstrated on abdominal X-ray films, he underwent surgery, and an ileus perforation was found; thus, an ileostomy was performed. An abdominal angiotomography and angiography were performed which showed no signs of aneurysms, stenosis, or thrombosis; the Ileus biopsy showed mucosa with areas of ischemic necrosis of multifocal distribution, edema of the submucosa, with small and medium vessels presenting an inflammatory infiltrate around them as well as a transmural inflammatory infiltrate with a predominance of polymorphonuclear cells, karyorrhexis and fibrinoid necrosis (Fig. 1). Thus, based on the clinical and anatomopathological findings, the patient was diagnosed of PAN. He received methylprednisolone pulses (500mg/d for three consecutive days), continuing with prednisone 50mg/d and intravenous cyclophosphamide (CYC) pulse 650mg/m2. He received twelve CYC pulse and tapering of corticosteroids (CS) was performed, with a favorable response; in fact, the patient was able to return to work. Currently, the patient is receiving maintenance therapy with azathioprine 75mg/d and prednisone 5mg/d; however, he still has the ileostomy.
Histopathological findings. (a) Hematoxylin–eosin stain (4×): In submucosa, it was observed blood vessels with transmural inflammatory infiltrate, vascular congestion, and edema (black arrow). (b) Hematoxylin–eosin stain (40×): Polymorphonuclear cells and karyorrhexis were observed in the vessel wall. Furthermore, a dense mixed inflammatory infiltrate comprising polymorphonuclear cells and eosinophils was noted perivascular and intramural (black arrow). (c) Verhoeff staining: Intramural inflammatory infiltrate involving the elastica (black arrow).
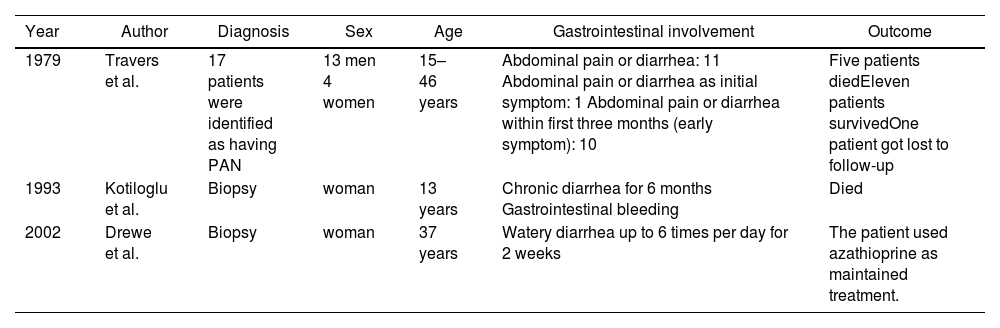
A literature review was performed in MEDLINE. The search strategy included the following terms: “Diarrhea”[Mesh] AND “Polyarteritis Nodosa”[Mesh] spanning 40 years (1963–2023). Eight articles were retrieved (Fig. 2). Two articles were excluded because they were not in English, one article did not have full text, and in two articles the patient diagnosis was not PAN. In three articles diarrhea as gastrointestinal involvement of PAN was reported; in one of these patients, it lasted six months (Table 1).
Cases of PAN with gastrointestinal involvement in which diarrhea was reported.
| Year | Author | Diagnosis | Sex | Age | Gastrointestinal involvement | Outcome |
|---|---|---|---|---|---|---|
| 1979 | Travers et al. | 17 patients were identified as having PAN | 13 men 4 women | 15–46 years | Abdominal pain or diarrhea: 11 Abdominal pain or diarrhea as initial symptom: 1 Abdominal pain or diarrhea within first three months (early symptom): 10 | Five patients diedEleven patients survivedOne patient got lost to follow-up |
| 1993 | Kotiloglu et al. | Biopsy | woman | 13 years | Chronic diarrhea for 6 months Gastrointestinal bleeding | Died |
| 2002 | Drewe et al. | Biopsy | woman | 37 years | Watery diarrhea up to 6 times per day for 2 weeks | The patient used azathioprine as maintained treatment. |
PAN: polyarteritis nodos.
In the case we are presenting, the clinical manifestations occurred gradually and were non-specific which probably accounted for a delayed diagnosis. Gastrointestinal involvement as diarrhea in PAN is rare with only three cases having been reported in the literature reviewed. The first case is the one reported by Travers et al [5]. within a case-series of seventeen patients with clinical diagnosis of PAN; of them, only one had diarrhea or abdominal pain as the initial manifestation; ten patients had such manifestations as early symptoms, within first three months. The second case reported by Kotiloglu et al [6]. is of a 13-year-old adolescent that had chronic diarrhea for six months; unfortunately, she developed gastrointestinal bleeding in two occasions, which led to her death; the diagnosis of PAN was done post-mortem. Finally, the third case [7] was reported by Drewe et al.; in this case the diagnosis of PAN was done in the setting of a complex multiple autoimmune disease in a 33 year-old woman with insulin dependent diabetes mellitus, hypothyroidism and idiopathic thrombocytopenic purpura (ITP). Six years before presentation she developed watery diarrhea which was controlled with loperamide and remitted when the dose of CS for her ITP was increased. Subsequently, she developed cholecystitis; the diagnosis of PAN was done from the biliary histology. Although in this case the diarrhea was not the main manifestation, it highlights a possible initial manifestation of PAN that was probably partially suppressed with CS.
The diarrhea that developed in our patient was atypical for intestinal ischemia; there are some reports about painless diarrhea, secondary to chronic intestinal ischemia but the mechanism is unknown [8,9]. The diarrhea could be explained as a complex interaction between autocrine, luminal, paracrine, neural, and endocrine factors. It is also been thought that changes in the microbiome could be involved [10]; the microbiome has been recently reported as altered in patients with small, medium and large vessels vasculitis [11].
During his clinical course, our patient experienced an ileus perforation; in the literature, both, small and large bowel perforations have been reported. Pagnoux et al., for example, reported six out of thirty-eight patients with gastrointestinal PAN that develop intestinal perforation [12]. In other case series, eight patients with either bowel infarction or perforation (4 large bowel and 4 small bowel) of twenty-four patients with PAN and gastrointestinal involvement have been reported [13]. Furthermore, Buldukoglu et al. reported the first case of jejunum perforation as the initial presentation of PAN in an older adult [14].
In our patient, peripheral nervous system involvement was also identified. Levine et al. reported a case-series of twenty-four patients with gastrointestinal involvement in which ten of them had peripheral nervous system involvement on presentation [13]. Moreover, Sanchez-Cubias et al. described thirty-one Mexican patients with PAN who had neurologic and gastrointestinal manifestation present at diagnosis in 52% and 29% of them, respectively [15].
PAN can present as a single episode; however, this can be life-threatening when a proper treatment is not initiated. Advances in the understanding of the pathogenesis of this condition and of its treatment, have had a positive impact on these patients’ survival, In fact, Jardel et al. from France have shown a gradual decrease in mortality starting in 1980 [16]. Furthermore, Pagnoux et al. have reported a survival rate of 83.4% at five years for PAN and 64.4% at five years for those patients older than 65 years of age [17].
The management of PAN is defined on a case-by-case basis according to its severity; when there is life or organ threatening manifestations, the condition is considered severe [18]. In our case, the patient had intestinal perforation and mononeuritis multiplex; for this reason, methylprednisolone pulses and CYC were chosen. In relation to the doses of methylprednisolone that are used in these settings, there is no consensus. The French suggest to use methylprednisolone at a dose of 7.5–15mg/kg/day [19]. There is evidence about better survival in patients with CS plus CYC versus CS alone from a study which included 278 patients with PAN, microscopic polyangiitis, and eosinophilic granulomatosis with polyangiitis [20]. Furthermore, the randomized controlled trial, CORTAGE (CORTicosteroid and cyclophosphamide-base induction therapy for systemic necrotizing vasculitis patients AGEd≥65 years), suggest that fixes doses (CYC 500mg every two weeks for the first three pulses and then every three weeks until remission) in comparison with CYC 500mg/m2 had lower serious adverse events at three years with similar remission rates. Indeed, the hazard ratio for serious adverse events was 0.61 for the fixes doses versus conventional arm [21].
Finally, we suggest that in chronic diarrhea accompanied by systemic symptoms as dysesthesia or decreased muscle strength we could regard PAN as differential diagnosis. In conclusion, the case presented in this review represents a challenging diagnosis because of the non-specific clinical manifestations and atypical presentation of diarrhea as a manifestation of intestinal ischemia. It also highlights the importance of providing early treatment.
Authors contributionIdeation of manuscript was done by VRPQ and PJTL; drafting of manuscript was done by PJTL. Critical revisions were done VRPQ, MFUG, JSU and GSA. Final approval of version was done by all the authors.
Ethical considerationsThe authors obtained written informed consent from the patient. Also, all information is protected by confidentiality. Ethics Committee approval was not required as no experimental intervention was conducted.
FundingNone.
Conflict of interestNone.