This study aimed to describe the frequency of antinuclear antibody (ANA) staining patterns by indirect immunofluorescence assay observed in patients from a tertiary health center in Latin America.
Materials and methodsThis retrospective, descriptive, and observational study evaluated data from all patients undergoing antinuclear antibody indirect immunofluorescence assay from a single-tertiary center (University Hospital Fundación Valle del Lili, Cali-Colombia) in 2020.
ResultsOne thousand and eight patients met the inclusion criteria. The median patient age was 47 (34–59.2) years, and most were female (769, 75.3%). A positive ANA immunofluorescence assay was observed in approximately two-thirds of patients (664, 65.8%). ANA test results were primarily used to exclude a suspected diagnosis in approximately half of the patients (466, 46.2%). Thirty-seven percent (250/664) of the cohort with ANA-positive titers had a systemic autoimmune rheumatic disease (SARD). The most prevalent SARDs included rheumatoid arthritis (RA) (55, 8.2%) followed by systemic lupus erythematosus (SLE) (37, 5.5%). The vast majority of ANA-positive patients had a reported speckled pattern (anti-cell [AC]-2,4,5; 269; 40.5%) followed by homogenous (AC-1; 266; 40%), nucleolar (AC-8,9,10; 46; 6.9%), and centromere (AC-3; 16; 2.4%). The most frequent patterns observed among SLE patients included homogenous (AC-1) patterns in 17 (45.9%) patients, speckled (AC-2,4,5) nuclear patterns in 11 (29.7%) patients, mixed patterns in 7 (18.9%) patients, and reticular/anti-mitochondrial antibody (AMA, AC-21) cytoplasmic patterns in 2 (5.4%) patients.
ConclusionThis study is the first to describe ANA patterns in a Colombian population. Speckled and homogenous patterns were predominant in patients with SARDs.
Este estudio tuvo como objetivo describir la frecuencia de los patrones de tinción de anticuerpos antinucleares (ANA) por ensayo de inmunofluorescencia indirecta, observados en pacientes de un centro de salud de tercer nivel en América Latina.
Materiales y métodosEste estudio retrospectivo, descriptivo y observacional evaluó los datos de todos los pacientes sometidos a un ensayo de inmunofluorescencia indirecta de anticuerpos antinucleares de un único centro terciario (Hospital Universitario Fundación Valle del Lili, Cali-Colombia) durante el 2020.
ResultadosMil ocho pacientes cumplieron los criterios de inclusión. La mediana de edad de los pacientes fue de 47 (34-59,2) años, y la mayoría eran mujeres (769, 75,3%). Se observó un ensayo de inmunofluorescencia ANA positivo en aproximadamente dos tercios de los pacientes (664, 65,8%). Los resultados de la prueba ANA se utilizaron principalmente para excluir un diagnóstico de sospecha en aproximadamente la mitad de los pacientes (466, 46,2%). El 37% (250/664) de la cohorte con títulos positivos para ANA tenía una enfermedad autoinmune sistémica (EAS). Las EAS más prevalentes incluyeron la artritis reumatoide (AR) (55, 8,2%) seguida del lupus eritematoso sistémico (LES) (37, 5,5%). La gran mayoría de los pacientes positivos para ANA presentaron un patrón moteado (anticélula [AC]-2,4,5; 269; 40,5%) seguido de homogéneo (AC-1; 266; 40%), nucleolar (AC- 8,9,10; 46; 6,9%) y patrón nuclear centrómero (AC-3; 16; 2,4%). Los patrones más frecuentes observados entre los pacientes con LES incluyeron patrones homogéneos (AC-1) en 17 (45,9%) pacientes, patrones nucleares moteados (AC-2,4,5) en 11 (29,7%) pacientes, patrones mixtos en 7 (18,9%) pacientes, y patrones citoplasmáticos de anticuerpos reticulares/antimitocondriales (AMA, AC-21) en 2 (5,4%) pacientes.
ConclusiónEste estudio es el primero en describir los patrones de ANA en una población colombiana utilizando las nuevas clasificaciones. Los patrones moteados y homogéneos predominaron en los pacientes con EAS.
Autoimmune diseases encompass a set of conditions characterized by dysregulation of the immune system resulting in the loss of tolerance to self-antigens, cells, tissues, or organs, leading to inflammation and damage.1 These diseases are mainly classified into tissue- or organ-specific and systemic autoimmune rheumatic diseases (SARDs). Examples of SARDs include systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), systemic sclerosis, Sjogren's syndrome (SS), idiopathic inflammatory myopathies, and mixed connective tissue disease.2 These diseases have multiple risk factors, including genetic risk factors,3,4 interferon-induced gene expression,5,6 and environmental triggers, such as viral infections.7 In SARDs, antibodies are produced against self-antigens, such as antinuclear antibodies (ANA)8 and immunoglobulins that recognize autologous cellular components.9
Serum ANA levels are measured as a diagnostic test for multiple SARDs, including SLE.10 The detection of ANA is a serological hallmark in these diseases, and ANA detection can occur years before the initiation of clinical symptoms in what is known as a preclinical phase.11,12 The indirect immunofluorescence assay (IIF) is the preliminary standard for detecting ANA using the human cell line HEp-2 as a substrate.13 Approximately 20–30% of the general population has positive or detectable ANA; however, increased titers are generally associated with connective tissue disorders.14 Moreover, patients with ANA-positive titers without SARDs exhibit increased proportions of activated B and T cells. This condition is expected in the early stages of SARDs.15
Although they are termed “nuclear” antibodies, three categories of ANA staining patterns have been reported: nuclear, cytoplasmic, and cell cycle-associated. Given these different categories, some authors have proposed to change their name to “anticellular antibodies”.16 To provide a standardization instrument that allows professionals to understand the nomenclature and morphological patterns observed by IIF in HEp-2 cells, the International Consensus on ANA Staining Patterns (ICAP) was held in Brazil in 2014. Members of the ICAP reached the consensus that in addition to the positive or negative result, the test has to be titrated (the titer refers to the highest dilution of serum that produces fluorescence intensity), and the fluorescence pattern should be reported. In total, 29 staining HEp-2 cell IIF patterns of clinical significance have been reported, and these patterns are labeled AC-1 to AC-29. Additionally, this consensus recommended two report levels. The first report level must be submitted by all laboratories that perform the test (competent-level) and include 11 staining patterns. The second report level should be reported by bacteriologists with a higher level of expertise (expert-level) and includes 18 additional staining patterns.17
Characteristic ANA patterns observed in a Colombian cohort have not been reported to date. Thus, this study aimed to describe the most common ANA patterns observed in patients at Cali, Colombia's highly complex referral health center.
Patients and methodsStudy design and patientsThis retrospective, descriptive, and observational study evaluated data from all patients who underwent an antinuclear antibody indirect immunofluorescence assay in a single-tertiary center (University Hospital Fundación Valle del Lili-UHFVL-Cali-Colombia) during 2020. Data were obtained by evaluating the electronic clinical records of the patients mentioned above. The clinical records, irrespective of positive or negative results, were analyzed. One thousand eight patients met the inclusion criteria. ANA patterns were described according to the Report of the First International Consensus on Standardized Nomenclature of Antinuclear Antibody HEp-2 Cell Patterns 2014–2015.17 A positive ANA titer was considered as 1:80 dilutions or greater.
Statistical analysisStatistical analysis was performed using Stata® version 14 (StataCorp, College Station, TX, USA). The Kolmogorov–Smirnov test or the Shapiro–Wilk test was used to assess the normality of the numeric variable based on the sample size. Numerical variables are presented as the means with standard deviations or medians with interquartile ranges. Categorical variables are expressed as frequencies. A comparison was made between the utility of the diagnostic test stratified by medical specialty using the chi-squared test with a p-value<0.05 considered statistically significant.
Ethical considerationsThis study complies with the Declaration of Helsinki and complies with the current regulations on bioethical research and since the diagnosis, follow-up or treatment of the patients was not modified and the total anonymity of the subjects was preserved. The institutional ethics committee of UHFVL was requested to exempt informed consent, with the approval of protocol number 1736.
ResultsGeneral patient characteristicsOne thousand and eight patients met the inclusion criteria, and their data were analyzed. The median patient age when the ANA test was performed was 47 (34–59.2) years, and most of the patients were female (769, 75.3%). Two hundred thirty-nine (23.7%) patients were male. A positive ANA immunofluorescence assay result was observed in approximately two-thirds of the patients (664, 65.8%) (Table 1).
Patient's general characteristics.
| Characteristics, n (%) | N=1008, 100% |
|---|---|
| Age | 47 (34–59.2)a |
| Female | 769 (75.3) |
| Male | 239 (23.7) |
| Positive ANA | 664 (65.8) |
| Negative ANA | 344 (34.1) |
| Main reason for laboratory testing | |
| Diagnostic approach | 618 (61.3) |
| Routine check-up | 201 (19.9) |
| Disease activity monitoring | 24 (2.3) |
| ND | 165 (16.3) |
| Utility of diagnostic test | |
| A diagnosis is made | 139 (13.7) |
| A diagnosis is excluded | 466 (46.2) |
| A relapse is identified | 33 (3.2) |
| A relapse is excluded | 101 (10) |
| Medical specialty that requested the laboratory test | |
| Rheumatology | 339 (33.6) |
| Internal Medicine | 107 (10.6) |
| Dermatology | 82 (8.1) |
| Hepatology | 41 (4) |
| Orthopedic Department | 40 (3.9) |
| Pediatrics | 34 (3.3) |
| Endocrinology | 32 (3.1) |
| General Medicine | 23 (2.2) |
| Neurology | 23 (2.2) |
| Infectious Diseases | 18 (1.7) |
| Hematology | 18 (1.7) |
| Allergology | 14 (1.3) |
| Gastroenterology | 14 (1.3) |
| Physiatry | 14 (1.3) |
| Nephrology | 12 (1.1) |
| Cardiology | 8 (0.7) |
| Gynecology | 6 (0.5) |
| Oncology | 5 (0.4) |
| Pneumology | 4 (0.3) |
| Urology | 2 (0.1) |
| Other medical specialties | 6 (0.5) |
| ND | 166 (16.4) |
The leading cause of laboratory testing was to diagnose the patient's signs and symptoms (618, 61.3%) followed by routine check-up (201, 19.9%) and disease activity monitoring (24, 2.3%). Regarding the utility of the diagnostic test, the ANA test result was primarily used to exclude a suspected diagnosis in approximately half of the patients (466, 46.2%). A diagnosis was achieved in 139 patients (13.7%). Disease relapse or flare was excluded in 101 (10%) patients, and relapse was identified in 33 (3.2%) patients. The medical specialty that most often requested the laboratory test was rheumatology (339, 33.6%) followed by internal medicine (107, 10.6%), dermatology (82, 8.1%), hepatology (41, 4%), and orthopedics (40, 3.9%). Other medical specialties that most frequently requested an ANA test are shown in Table 1.
ANA tests solicited by rheumatologists were more likely to be positive (252, 74.3%) than those solicited by other physicians (p<0.00005) and they were more likely solicited as part of a routine check-up (1127, 37.4%) (p<0.00001). When the test was solicited by rheumatologists a diagnosis was more likely made (74, 21.8%) in comparison to the test being solicited by other physicians (65, 9.7%) (p<0.00001) in which the test was more likely used to exclude a diagnosis (345, 51.5%) (p<0.00001). Other characteristics are seen in Table 2.
ANAs by medical specialties.
| Characteristics, n (%) | Rheumatology N=339 | Other specialties N=669 | p-Value |
|---|---|---|---|
| Positive ANAS | 252 (74.3) | 412 (61.5) | .000055. |
| Main reason for laboratory testing | |||
| Diagnostic approach | 199 (58.7) | 419 (62.6) | .226297. |
| Routine check-up | 127 (37.4) | 74 (11) | .00001. |
| Disease activity monitoring | 12 (3.5) | 12 (1.7) | .085807 |
| ND | 1 (0.2) | 164 (24.5) | .00001. |
| Utility of diagnostic test | |||
| A diagnosis is made | 74 (21.8) | 65 (9.7) | .00001. |
| A diagnosis is excluded | 121 (35.6) | 345 (51.5) | .00001. |
| A relapse is identified | 23 (6.7) | 10 (1.4) | .00001. |
| A relapse is excluded | 74 (21.8) | 27 (4) | .00001. |
ANA, antinuclear antibodies; ND, no data.
Thirty-seven percent (250/664) of the cohort with ANA-positive titers had an SARD diagnosis. The most prevalent SARD was RA (55, 8.2%) followed by SLE (37, 5.5%), SS (34, 5.1%), and undifferentiated systemic rheumatic diseases (19, 2.8%). Immunological evaluation of ANA-positive individuals showed that 23 (3.4%) patients had positive anti-Ro antibodies, 16 (2.4%) had positive anti-dsDNA antibodies based on indirect immunofluorescence assay at a median dilution of 1:80 (1:10–1:320), and 12 (1.8%) patients had anti-dsDNA antibodies detected by enzyme-linked immunosorbent assay (ELISA) with a median value of 199IU/mL (39.5–287.2). All of the patients with positive anti-dsDNA antibodies had SLE. Nine (1.3%) patients had positive anti-La antibodies, six (0.9%) had anti-RNP antibodies, and five (0.7%) patients had anti-Sm antibodies (Table 3).
Rheumatological and immune-mediated diseases in ANA-positive patients.
| Rheumatic disease, n (%) | N=664, 100% |
|---|---|
| RA | 55 (8.2) |
| SLE | 37 (5.5) |
| SS | 34 (5.1) |
| Undifferentiated systemic rheumatic (connective tissue) diseases | 19 (2.8) |
| Autoimmune hepatitis | 13 (1.9) |
| Autoimmune thyroiditis | 8 (1.2) |
| Fibromyalgia | 8 (1.2) |
| Thrombocytopenia | 6 (0.9) |
| APS | 4 (0.6) |
| Graves’ disease | 4 (0.6) |
| IIM | 4 (0.6) |
| Psoriasis | 4 (0.6) |
| Polymyalgia rheumatica | 4 (0.6) |
| ANCA-associated vasculitis | 3 (0.4) |
| MCTD | 3 (0.4) |
| AIHA | 3 (0.4) |
| Sarcoidosis | 3 (0.4) |
| CIDP | 3 (0.4) |
| Myasthenia gravis | 3 (0.4) |
| Urticaria | 3 (0.4) |
| SSc | 3 (0.4) |
| Ankylosing spondylitis | 2 (0.3) |
| Discoid lupus | 2 (0.3) |
| Celiac disease | 1 (0.1) |
| Chondromalacia patella | 1 (0.1) |
| Bruton agammaglobulinemia | 1 (0.1) |
| Hypogammaglobulinemia IgG | 1 (0.1) |
| Hypogammaglobulinemia IgA | 1 (0.1) |
| Sacroiliitis | 1 (0.1) |
| Osteoporosis | 1 (0.1) |
| Esophagitis | 1 (0.1) |
| Allergic rhinitis | 1 (0.1) |
RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; SS, Sjogren's syndrome; APS, antiphospholipid syndrome; IIM, inflammatory myopathies; MCTD, mixed connective tissue disease; AIHA, autoimmune hemolytic anemia; CIDP, chronic inflammatory demyelinating polyneuropathy; SSc, systemic sclerosis.
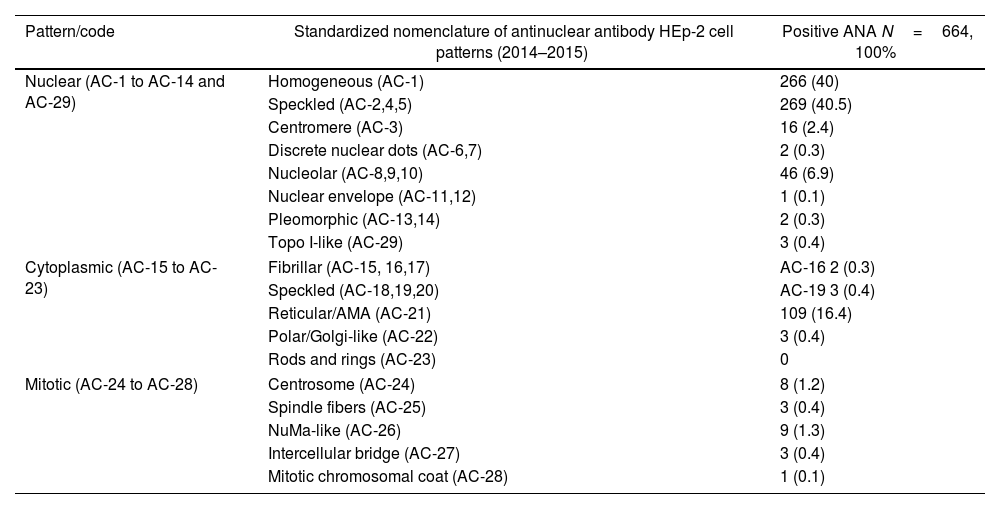
The vast majority of ANA-positive patients exhibited a speckled (anti-cell [AC]-2,4,5; 269; 40.5%) pattern followed by homogenous (AC-1; 266; 40%), nucleolar (AC-8,9,10; 46; 6.9%), and centromere (AC-3; 16; 2.4%) nuclear HEp-2 IIF patterns. Fewer results reported other nuclear patterns, such as topo I-like (AC-29; 3; 0.4%), discrete nuclear dot (AC-6; 2; 0.3%), pleomorphic (PCNA-like) (AC-13, 14; 2; 0.3%) and nuclear envelope (AC-11; 1; 0.1%) patterns. Of the cytoplasmic patterns, the most common reported patterns were reticular/anti-mitochondrial antibody (AMA, AC-21; 109; 16.4%), speckled (AC-18, 19, 20; 3; 0.4%), polar/Golgi (AC-22; 0.4%), and fibrillar (AC-15, 16, 0.4%) cytoplasmic patterns. Regarding the mitotic patterns, NuMa-like (AC-26, 9; 1.3%), centrosome (AC-24; 8; 1.2%), spindle fiber (AC-25, 3; 0.4%), and intercellular bridge (AC-27; 3; 0.4%) patterns were the most common patterns reported in this population (Table 4).
ANA patterns.
| Pattern/code | Standardized nomenclature of antinuclear antibody HEp-2 cell patterns (2014–2015) | Positive ANA N=664, 100% |
|---|---|---|
| Nuclear (AC-1 to AC-14 and AC-29) | Homogeneous (AC-1) | 266 (40) |
| Speckled (AC-2,4,5) | 269 (40.5) | |
| Centromere (AC-3) | 16 (2.4) | |
| Discrete nuclear dots (AC-6,7) | 2 (0.3) | |
| Nucleolar (AC-8,9,10) | 46 (6.9) | |
| Nuclear envelope (AC-11,12) | 1 (0.1) | |
| Pleomorphic (AC-13,14) | 2 (0.3) | |
| Topo I-like (AC-29) | 3 (0.4) | |
| Cytoplasmic (AC-15 to AC-23) | Fibrillar (AC-15, 16,17) | AC-16 2 (0.3) |
| Speckled (AC-18,19,20) | AC-19 3 (0.4) | |
| Reticular/AMA (AC-21) | 109 (16.4) | |
| Polar/Golgi-like (AC-22) | 3 (0.4) | |
| Rods and rings (AC-23) | 0 | |
| Mitotic (AC-24 to AC-28) | Centrosome (AC-24) | 8 (1.2) |
| Spindle fibers (AC-25) | 3 (0.4) | |
| NuMa-like (AC-26) | 9 (1.3) | |
| Intercellular bridge (AC-27) | 3 (0.4) | |
| Mitotic chromosomal coat (AC-28) | 1 (0.1) | |
Concerning staining patterns observed in each AID, the most common nuclear HEp-2 IIF patterns among patients with RA were homogenous (AC-1; 31; 56.3%) and speckled patterns (AC-2,4,5; 15; 27.2%). Three (5.4%) patients had a reticular/anti-mitochondrial antibody (AMA, AC-21) cytoplasmic pattern, and one (1.8%) patient had a positive intercellular bridge (AC-27) mitotic pattern. The most frequent patterns noted among SLE patients included a homogenous pattern (AC-1) in 17 (45.9%) patients followed by speckled (AC-2,4,5) nuclear patterns in 11 (29.7%) patients, mixed patterns in 7 (18.9%) patients, and reticular/anti-mitochondrial antibody (AMA, AC-21) cytoplasmic patterns in 2 patients (5.4%). The most common mixed pattern observed among SLE patients was the combination of AC-1+AC-21 in 4 (57.1%) patients. Other common staining patterns noted among patients with other AIDs are shown in Table 5.
ANA patterns in patients with autoimmune diseases.
| Pattern/code | Standardized nomenclature of antinuclear antibody Hep 2 cell patterns (2014–2015) | RA N=55 | SLE N=37 | SS N=34 | USRD N=19 | AH N=13 |
|---|---|---|---|---|---|---|
| Nuclear (AC-1 to AC-14 and AC-29) | Homogeneous (AC-1) | 31 (56.3) | 17 (45.9) | 14 (41.1) | 13 (68.4) | 1 (7.6) |
| Speckled (AC-2,4,5) | 15 (27.2) | 11 (29.7) | 14 (41.1) | 5 (26.3) | 2 (15.3) | |
| Centromere (AC-3) | 2 (3.6) | 0 | 3 (8.8) | 1 (5.2) | 3 (23) | |
| Discrete nuclear dots (AC-6,7) | 0 | 0 | 0 | 0 | 0 | |
| Nucleolar (AC-8,9,10) | 2 (3.6) | 1 (2.7) | 1 (2.9) | 0 | 0 | |
| Nuclear envelope (AC-11,12) | 0 | 0 | 0 | 0 | 1 (7.6) | |
| Pleomorphic (AC-13,14) | 0 | 0 | 0 | 0 | 0 | |
| Topo I-like (AC-29) | 1 (1.8) | 0 | 0 | 0 | 0 | |
| Cytopla smic (AC-15 to AC-23) | Fibrillar (AC-15, 16,17) | 0 | 1 (2.7) | 0 | 0 | 0 |
| Speckled (AC-18,19,20) | 0 | 0 | 0 | 0 | 1 (7.6) | |
| Reticular/AMA (AC-21) | 3 (5.4) | 2 (5.4) | 3 (8.8) | 2 (10.5) | 5 (38.4) | |
| Polar/Golgi-like (AC-22) | 0 | 0 | 0 | 0 | 0 | |
| Rods and rings (AC-23) | 0 | 0 | 0 | 0 | 0 | |
| Mitotic (AC-24 to AC28) | Centrosome (AC-24) | 0 | 0 | 0 | 0 | 0 |
| Spindle fibers (AC-25) | 0 | 0 | 0 | 0 | 0 | |
| NuMa-like (AC-26) | 0 | 0 | 0 | 0 | 0 | |
| Intercellular bridge (AC-27) | 1 (1.8) | 0 | 0 | 0 | 0 | |
| Mitotic chromosomal coat (AC-28) | 0 | 0 | 0 | 0 | 0 | |
RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; SS, Sjogren's Syndrome; USRD, Undifferentiated systemic rheumatic (connective tissue) diseases; AH, autoimmune hepatitis.
The present study investigated the most frequent ANA patterns observed in patients attending a Colombian university hospital. Thirty-seven percent of the cohort had RA or SLE, representing the most prevalent SARDs. The vast majority of ANA-positive patients in this population had speckled and homogeneous patterns.
These results are similar to other studies evaluating the prevalence of different ANA patterns among patients with SARDs. One study in Saudi Arabia analyzing data from 453 patients reported the speckled pattern as the most common in 32% of their cohort followed by the homogenous pattern, which is consistent with our results.18
The pathogenic role of ANA patterns is controversial, and these patterns have been associated with certain SARDs to some degree. Patients with mixed connective tissue disease typically exhibit a speckled nuclear pattern. In this pattern, different types of speckles are observed all over the nucleus, and common antibodies that may produce this pattern include anti-U1 RNP, anti-Sm, and anti-La.19 Patients with systemic sclerosis SSc predominantly exhibit speckled and nucleolar staining.20 The latter pattern appears as homogeneous or speckled staining in the nucleolus and is produced by autoantibodies, such as RNA helicase, fibrillarin, RNA polymerase I and III, and PM-Scl.21
On the other hand, the centromere staining pattern is predominant in patients with limited systemic sclerosis.22 Patients with SS and SLE are more likely to present homogenous and speckled patterns. In the former pattern, the nucleus is diffusely stained, and the antibodies that produce this pattern include anti-histone proteins, DNA, and DNA-histone complexes.23 However, ANA patterns are not specific for individual SARDs.
The most common ANA patterns in patients with RA were homogenous (AC-1) and speckled patterns (AC-2,4,5), and those in SLE patients included homogenous (AC-1), speckled (AC-2,4,5), and reticular/anti-mitochondrial antibody (AMA, AC-21) patterns. ANA positivity is a mandatory serologic criterion for classifying patients with SLE, as it is believed that these patients invariably express ANA.24 Among patients with SLE, the most common staining patterns observed in a Swedish study included homogeneous patterns followed by the speckled pattern.25 In a Saudi Arabian study, SLE patients exhibited results similar to those noted in our study population. The predominant ANA pattern in that study was speckled in 52% of the patients, and 35% had peripheral patterns.26 In a previous study, speckled patterns were associated with higher ANA titers, and peripheral patterns were associated with elevated acute phase reactants and the highest levels of anti-ds-DNA.26 Other lupus cohorts worldwide have yielded similar results regarding the speckled pattern as the primary pattern, such as the Egyptian cohort of 300 patients27 and the Polish cross-sectional study.28 However, ANA patterns in SLE patients vary based on clinical and ethnic differences noted across nationalities, thus explaining the international heterogeneity that is evidenced in the medical literature.
As mentioned, the IIF HEP-2 cell assay is the recommended technique for measuring ANA. However, this technique possesses some disadvantages, including the need for a long period of time to interpret the results, the need for personnel training and operator-related subjectivity.29 The IIF assay method has other limitations, such as intra- and interlaboratory variability, low specificity, and the subjectivity associated with the determination of both titers and patterns. These limitations have led to discrepancies in test results, even causing differences of greater than two dilutions in the antibody titer results obtained between two expert observers.30 Thus, multiple strategies have been developed to adopt automated determinations of ANA, and computer-aided algorithms and artificial intelligence have been employed to evaluate ANA.31 Although ANAS positivity is a hallmark for the diagnosis of certain SARDs, the knowledge regarding interpretation continues to change constantly along with the techniques available to detect SARDs.
ConclusionsThis study is the first to describe ANA patterns in a large Colombian cohort. Patients who underwent an ANA HEp2 IIF assay were primarily female, and the test was primarily requested by physicians to obtain a diagnosis. The ANA test result was mainly used to exclude a suspected diagnosis. In patients with SARDs, speckled and homogenous patterns represented the predominant patterns observed in this population.
Impact statementIn a tertiary health center in Latin America, ANA test results are mainly used to exclude a suspected autoimmune disorder diagnosis and the most common staining patterns among positive-ANA patients are the speckled and the homogeneous patterns.
FundingNo funding was received.
Conflicts of interestWe have no conflicts of interest to disclose.