Inhalant users may develop toluene leukoencephalopathy, a devastating neuropsychiatric disorder. We present a case of toluene-induced damage to the corticospinal and the corticonuclear tracts, which presented with involuntary emotional expression disorder.
MethodsCase study of a 20-year-old man with a 3-year history of frequent solvent abuse was admitted to the Neuropsychiatry Unit of the National Institute of Neurology and Neurosurgery because “he could not speak or walk” but would keep “laughing and crying without reason”.
ResultsNeuropsychiatric examination revealed pathological laughter and crying, facial and speech apraxia, a bilateral pyramidal syndrome, and lack of control of urinary sphincter. Magnetic resonance imaging revealed a highly selective bilateral damage to the pyramidal system and the somatosensory pathway. SPECT imaging showed left fronto-parietal hypoperfusion.
ConclusionsThis document provides support for the understanding of involuntary emotional expression disorders as a differential diagnosis in the clinical practice of psychiatrists, as well as the functional anatomy of these conditions.
Los usuarios de inhalantes pueden contraer leucoencefalopatía por tolueno, un trastorno neuropsiquiátrico devastador. Se presenta un caso de daño inducido por tolueno en el tracto corticoespinal y corticonuclear, que se manifestó con un trastorno involuntario de la expresión emocional.
MétodosUn varón de 20 años con antecedente de 3 años de abuso de solventes ingresó en la Unidad de Neuropsiquiatría del Instituto Nacional de Neurología y Neurocirugía porque «no podía hablar ni caminar» y presentaba episodios súbitos de risa y llanto sin razón aparente.
ResultadosLa valoración neuropsiquiátrica reveló risa y llanto patológicos, apraxia facial y fonatoria, síndrome piramidal bilateral y ausencia de control del esfínter urinario. La resonancia magnética cerebral mostró un daño bilateral muy selectivo del sistema piramidal y la vía somatosensorial. La imagen de tomografía computarizada por emisión monofotónica mostró hipoperfusión frontoparietal izquierda.
ConclusionesEste documento proporciona apoyo para la comprensión de los trastornos de la expresión emocional involuntaria como diagnóstico diferencial en la práctica clínica de los psiquiatras, así como de la anatomía funcional de estas condiciones.
It is known that inhalant users, a frequent problem in developing countries, may be in higher risk of having psychiatric disorders, HIV infection, and suicidality.1 The chronic use of the volatile organic solvent known as “thinner”, which contains toluene as main component in many countries (including Mexico and Colombia), is related to many neurologic and psychiatric disturbances, including: cerebellar ataxia, decreased visual acuity, tremor, opsoclonus, dysarthria, gait inability, apathy and dementia.2–4Syndromes of involuntary laughing and crying have long been recognized. Darwin is thought to have offered their first description in 1872.5 As now identified, involuntary emotional expression disorders (IEED) are distressing and debilitating conditions characterized by uncontrollable episodes of laughing and/or crying, that causes extensive social and occupational dysfunction amongst patients.6 However, IEED are often overlooked or misdiagnosed for a psychiatric illness in the clinical practice.7,8
We present a case of toluene induced damage to the pyramidal system, both the corticospinal and the corticonuclear components, which manifested as involuntary emotional expression disorder among other neurological and behavioral disturbances. The patient was evaluated in the National Institute of Neurology and Neurosurgery of Mexico.
Case reportA 20-year-old man with a 3 year history of frequent solvent abuse was admitted to the Neuropsychiatry Unit of the National Institute of Neurology and Neurosurgery of Mexico because “he could not speak nor walk” but would keep laughing and crying. During his childhood, the patient lived with family violence. For variable periods, he was homeless, living on the street, without formal social support. He frequently abused paint thinner for at least 3 years. His relatives estimate a daily use pattern. He also had a history of daily tobacco use since he was 14 years old (4 cigarettes/day), and weekly alcohol use to the point of intoxication since age 17.
First hospitalizationIn April 2008 he experienced spastic paraparesis with drowsiness and dizziness that lead to hospitalization. A diagnosis of incomplete medullar and cerebellar syndrome was first made, probably secondary to the toluene abuse. The MRI was normal. After discharge he was treated with B12 vitamin and folic acid; the symptoms partially subsided. A few months later he resumed solvent abuse.
Second hospitalizationSeven months before admission he developed severe dysartria, complete loss of sphincter control, and the need of complete assistance for daily activities due to generalized weakness, as well as laughing and crying spells. He had continued to abuse paint thinner after his first hospitalization. On admission (December, 2009) he was awake, alert, partially oriented, with difficulty maintaining attention; he was able to obey simple orders and he communicated by means of mimic language with his right hand; he had scant speech with oral and facial apraxia. Stereotyped episodes of involuntary laughter and crying were present most of the time. These were inappropiate to the inciting stimulus and ocurred independent of the underlying mood. In fact, he denied being sad when he cried or happy when he laughed, and responses were usually trigger by tactile stimulation, including laughing when receiving a painful stimuli. Neurologic examination revealed decreased visual acuity, impaired hearing, weakness and spasticity of the four limbs, bilateral hypereflexia, Babinski sign and spontaneous clonus, predominantly in the left side of the body.
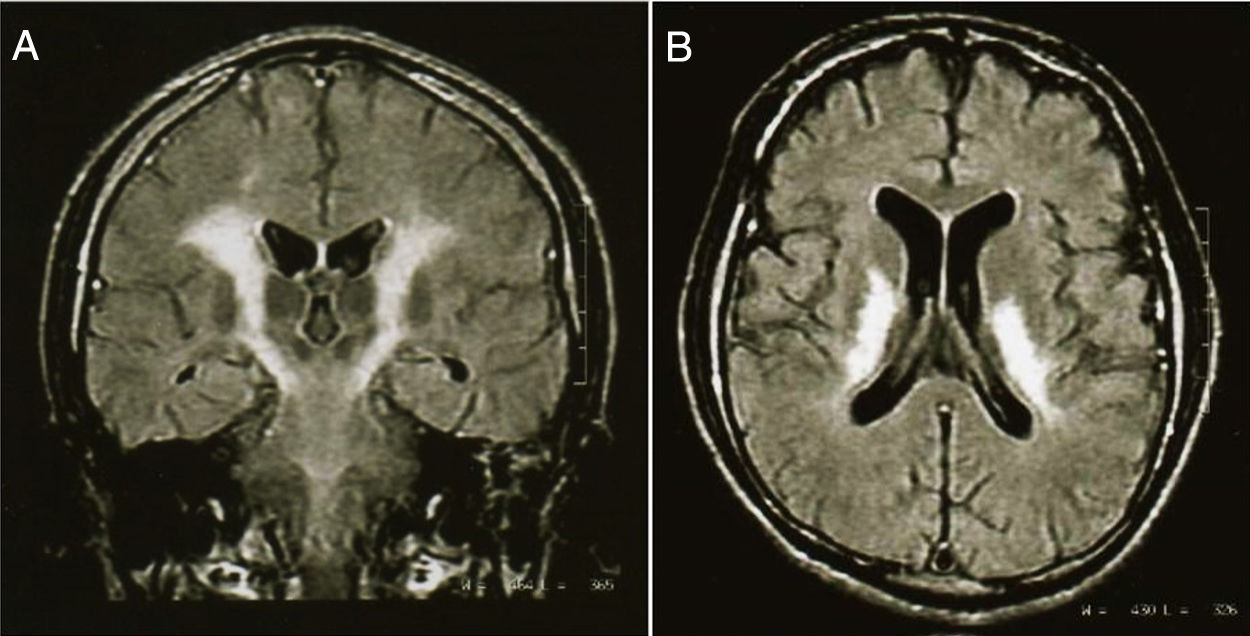
Complementary testsLaboratory tests were unremarkable (including CSF analysis) except for high levels of serum CPK (1171 UI/L; normal, 38-174 UI/L). Vitamin blood levels (folates and B12) were normal. Electroencephalogram showed generalized dysfunction without epileptic activity. Nerve conduction studies and electromyography showed bilateral and symmetric dysfunction of the propioceptive pathways in the four extremities with absence of response on the cervical posterior cord and the sensory cortex. T1-weighted brain magnetic resonance imaging (MRI) revealed low-signal-intensity changes, flair and T2-weightened shows high-signal-intensity changes of white matter; specifically in the pyramidal tract from the frontal cortex to the pons, in the posterior arm of the internal capsule, bilaterally (fig. 1). Also, high-signal-intensity changes were detected in parietal and frontal white matter, bilaterally. Single-photon emission computed tomography (SPECT) imaging showed left fronto-parietal hypoperfusion.
Coronal projection (A) of a magnetic resonance imaging scan in T2 sequence, of a 20-year old male with chronic inhalant abuse, which shows a selective damage of the pyramidal pathway. The axial projection (B) shows severe abnormalities of the posterior internal capsule. The patient had cuadriparesis, oral and facial apraxia, and involuntary emotional expression disorder.
After 1 month of hospitalization, pathological laughing and crying improved by means of sertraline treatment. No improvement was observed in speech and cuadriparesis. Two months later, the symptoms worsened. He is now severely disabled.
DiscussionToluene leucoencephalopathyTwo decades ago, an influential article stated that “in the light of present knowledge, the possibility that permanent structural brain damage, with accompanying psychiatric manifestations, results from solvent abuse remains inconclusive”.2 Since then, several pieces of evidence have shown the existence of structural brain lesions induced by inhalants, and particularly by toluene. Chronic exposure to toluene vapors in humans may have a severe impact on central nervous myelin, with leads to toluene leucoencephalopathy, a devastating neuropsychiatric disorder characterized by dementia and positive neurological signs, including tremor, opsoclonus, dysarthria and gait inability.4,9
Most of what we know about the mechanism of toxic demyelination has been drawn from morphologic observations, rather than biochemical studies, and from of animal models research, rather than human tissues. One of the most striking features in this case is the selective damage to pyramidal tract. This is an opportunity to review some aspects related with myelination process. Myelination is a highly controlled phenomenon. After sufficient myelin has been deposited, a plateau is reached, at which time active synthesis of myelin ceases.10 It would appear that during myelinogenesis, a number of developmental milestones exist:
An initial period during which the tissue acquires a state conductive to myelination onset.
The inspissations. During maturity, since maintenance and repair of myelin are frequently necessary, it is possible that a state of biological surveillance persists.10–12 CNS myelin is produced by oligodendrocytes in a process comprising at least initiation, specialization, and stabilization of glia-axonal interactions at the nodes, paranodes an internodes, and longitudinal expansion of myelin segments coinciding with axonal elongation during body growth. Then, a variety of specific and nonspecific metabolic insults may inhibit, alter or retard myelination. A morphological correlate of such studies is a decrease in myelin staining material and, when ultrastructural investigations are conducted, a decrease in the number of myelin lamellae surrounding axons of a given size, probably contributing to susceptibility to toxic damage.11
In the case of acquired toxic myelin disease, lesions are noninflammatory, as revealed in this case by the normality of the CSF studies; when myelin is broken down, phagocytosis is usually accomplished by cells of local origin, with severe dilatation of myelin sheaths by splits occurring at the intraperiod line and the filling of the vacuoles with fluid as consequence of altered synthesis of lipids and proteins in some susceptibility brain areas, like in other toxic patterns.13 The extension and selectivity of damage may depend on time and extent of exposition.
Involuntary emotional expression disorderThe present case shows the existence of selective damage of pyramidal and somatosensory tracts, induced by toluene, which presented a bilateral pyramidal syndrome, associated to the following neuropsychiatric data: a) oral and facial apraxia, probably related to the left fronto-parietal anatomical and functional deficit, and b) pathological laughing and crying as part of a corticonuclear disconnection syndrome, associated with pseudobulbar palsy.
As known, the occurrence of stereotyped and sudden episodes of crying and/or laughing, without feelings of sadness or happiness has been recognized in neuropsychiatric patients since the nineteenth century,14 and have been conceptualized in many ways, including “facial dissociation” “emotional lability”, “pseudobulbar affect”, “emotional incontinence”, “pathological crying and laughing”.7,15–17 The boundaries between this concepts are imprecise, but they all describe patients with frequent and intense episodes of laughter and crying, which are excessive regarding the social context and the subjective emotional experience, and with a lack of voluntary control. This phenomenon has been observed in patients with motor neuron disorders,18 stroke,15,19 as well as white matter disorders.20,21
According to the nomenclature proposed by Lauterbach et al.,6 involuntary emotional expression disorder (IEED) includes the syndromes of pathological laughing and crying (PLC) and emotional lability (EL). Key features of PLC, include stereotyped episodes of involuntary laughing and/or crying that are inappropriate or disportionate to the inciting stimulus and occur independent of underlying mood. On the other hand, EL includes disproportionate non-stereotyped episodes of involuntary laughing and/or crying that are consistent to the stimulus and are congruent with mood. The term of pseudobulbar affect is reserved for cases showing features of pseudobulbar palsy (dysartric bulbar speech, dysphagia or disinhibited facial and gag reflexes). In consonance with this model, the patient fulfilled criteria for an IEED, within the pathological and laughing subtype, with a pseudobulbar affect specifier. A variety of structures have been provided as controlling emotional expression. However, the view of an emotionally-driven “involuntary” pathway that is inhibited by a consciously-driven “voluntary” pathway proposed by Wilson22 in 1924 is still the most compelling and time-tested concept. The “voluntary” pathway travels from neocortical structures to the pons through corticonuclear fibers. Damage to this system produces disinhibition of the periacueductal gray matter, which is influenced mainly by paralimbic cortices and limbic nuclei. Hence, a loss of voluntary control of emotions appears.6
ConclusionsChronic exposure to toluene vapors can lead to multiple and devasting neuropsychiatric manifestations, including pathological laughing and crying. As in the presented case, toxic demyelination induced by toluene can compromise corticonuclerar fibers producing the disinhibition of emotionally-driven “involuntary” pathways wich contributes to the develoment of IEED. Psychiatris must be aware of the organic basis of these behaviors and not presume that they are only a manifestation of psychiatryc illness, even when psychiatric treatments may be effective in this conditions.
Conflicto de interesesLos autores declaran no tener ningún conflicto de intereses.