Clostridioides difficile is an opportunistic spore-forming pathogen responsible for antibiotic-associated diarrhea in humans. C. difficile produces two main toxins: TcdA and TcdB as well as a third toxin named binary toxin (CDT) that is also involved in virulence. The present study aimed at characterizing the C. difficile isolate ALCD3 involved in a relapse episode of nosocomial infection. Molecular characterization showed that isolate ALCD3 belongs to toxinotype 0/v and the MLST analysis demonstrated allelic profile adk:91, atpA:1, dxr:2, glyA: 1, recA:27, sodA: 1 and tpi:1 which corresponds to ST293 (MLST clade: 1). During growth, isolate ALCD3 showed an early increase in the sporulation ratio as well as maximal values of heat resistant forms after 2 days of incubation. Both sporulation kinetics and production of heat resistant forms were faster for isolate ALCD3 than for the reference strain VPI 10463. Germination in the presence of the natural germinant taurocholate was faster for isolate ALCD3 than for strain VPI 10463, which indicates that isolate ALCD3 starts cortex hydrolysis earlier than strain VPI 10463. Furthermore, the co-germinant glycine, induces rapid release of dipicolinic acid (DPA) in isolate ALCD3. These findings indicate that isolate ALCD3 is particularly efficient in both sporulation and germination. The present work represents the first report of the circulation of C. difficile ST293 in Argentina. The ability of isolate ALCD3 to produce toxins and its high sporulation/germination capacity are key features compatible with a microorganism with high dissemination potential and the possibility of inducing recurrent infections.
Clostridioidesdifficile es un patógeno esporulado oportunista responsable de diarrea asociada a antibióticos en humanos. C. difficile produce 2 toxinas principales: TcdA y TcdB, además de la toxina binaria (CDT), también asociada a la virulencia. Este estudio buscó caracterizar el aislamiento ALCD3, involucrado en un episodio de recurrencia de una infección nosocomial. La caracterización molecular mostró que dicho aislamiento pertenece al toxinotipo 0/v y el análisis por MLST demostró un perfil alélico adk:91, atpA:1, dxr:2, glyA: 1, recA:27, sodA: 1 y tpi:1, lo cual corresponde al ST293 (MLST clado 1). Durante el crecimiento, el aislamiento ALCD3 mostró un incremento temprano de la tasa de esporulación y valores máximos de formas termorresistentes luego de 2 días de incubación. Tanto la cinética de esporulación como la producción de formas termorresistentes fueron más rápidas en el aislamiento ALCD3 que en la cepa de referencia VPI 10463. La germinación en presencia del germinante natural taurocolato fue más rápida en el aislamiento ALCD3 que en la cepa VPI 10463, lo que indica que aquel comienza la hidrólisis del córtex antes. También, el co-germinante glicina indujo una rápida liberación de ácido dipicolínico en ALCD3. Estos hallazgos indican que el aislamiento ALCD3 es particularmente eficiente en la esporulación y en la germinación. El presente trabajo representa el primer informe de la circulación de C. difficile ST293 en Argentina. La habilidad del aislamiento ALCD3 para producir toxinas y su alta capacidad de esporulación/germinación son características claves compatibles con un alto potencial de diseminación e inducción de infecciones recurrentes.
Clostridioides difficile is a spore-forming strict anaerobe that colonizes the intestinal tract of approximately 5% of adults and 15–70% of infants. These percentages could be higher in hospitalized patients and nursing home residents35. This pathogen is the main etiological agent responsible for antibiotic-associated diarrhea26, and was believed to be almost exclusively associated with nosocomial infections. However, there is increasing evidence of a shift in the epidemiology of this pathogen with the occurrence of young healthy carriers without a history of antibiotic use15,28 and the presence of alternative ways of spore transmission such as food, grass, compost, manure, animals, and other environmental sources9,14. Clinical manifestations of C. difficile infection (CDI) include asymptomatic, mild, and self-limiting disease to severe, life-threatening pseudomembranous colitis leading to toxic megacolon, sepsis, and death3,20,39. Virulence of C. difficile is mainly related to the production of two large protein toxins, i.e., toxin A (TcdA), toxin B (TcdB). These toxins glycosylate proteins of the Rho GTPase family thus leading to the disruption of the actin cytoskeleton, cell death and a strong inflammatory response22. A third toxin, the binary toxin (CDT), can be produced by C. difficile and is composed of two separate components: CDTa and CDTb45. While CDTa presents ADP-ribosyltransferase activity that modifies actin, CDTb is responsible for the binding of the toxin complex to the host cell surface16. In a murine model, CDT expression along with TcdA and TcdB induce an exacerbated inflammatory response6. It has been suggested that the production of CDT is related to high fatality rates in patients infected with CDT-producing strains compared to those infected with CDT-negative strains2,24,34.
The infective cycle of C. difficile relies on the ability to sporulate/germinate. The spores are ingested and then they germinate giving rise to vegetative cells that in turn produce virulence factors in the host's intestine. Afterwards, sporulation favors the elimination of infective forms with feces and further spreading. Since the anaerobic nature of C. difficile makes it impossible for vegetative forms of bacteria to survive in aerobic environments21, the impaired ability to sporulate limits drastically its potential to persist in the host and further transmission8 and the correlation between increased sporulation ability and disease severity has been demonstrated5.
The ability of C. difficile spores to resist physical and chemical stress (e.g. heat, desiccation and disinfectants) lead to the possibility of survival in the environment thus favoring the transmission by the fecal–oral route8,31. Spore germination allows for vegetative growth and toxin production25. In this context, sporulation and germination are key events in the cell cycle of C. difficile and are crucial for virulence.
Antibiotic treatment favours C. difficile overgrowth through disruption of the intestinal microbiota and subsequent changes in bile salt metabolism thus leading to the increase of germinant concentrations (e.g. cholate, taurocholate) and further spore germination17,43.
The present study aimed to characterize a C. difficile isolate involved in a relapse episode of nosocomial infection and to gain insight into its sporulation/germination ability.
Materials and methodsBacterial strains and culture conditionsC. difficile ALCD3 was isolated from a recurrent episode of CDI. The patient, an 89-year-old woman received antibiotic therapy after a surgical resection of the right ureter due to a malignant tumor. In the post-surgery period, CDI was diagnosed by toxin detection in feces and treatment with vancomycin was prescribed (14 days, 4 doses of 125mg per day). Ten days after recovery, she was re-admitted with symptoms compatible with CDI (confirmed by toxin detection in feces). She recovered after treatment with metronidazole–vancomycin and no further relapses were reported.
Isolation was done as follows: the fecal sample was treated with ethanol (1:1) for 30min at room temperature. Then the material was homogenized with sterile phosphate buffered saline (PBS: 0.144g/l KH2PO4, 9g/l NaCl, 0.795g/l Na2HPO4, pH 7.5). Afterwards, suspensions were streaked on Differential Clostridia Medium – (DCM) agar (Laboratorios Britania S.A., Argentina) supplemented with 0.1% w/v sodium taurocholate (Santa Cruz Biotechnology, Dallas, Texas, USA). Plates were incubated for 48h at 37°C in anaerobic conditions (AnaeroPak; Mitsubishi Gas Chemical Co, Inc.). Colonies were selected based on morphology and Gram staining and genetically characterized as indicated below.
The isolate and reference strain VPI 10463 were stored at −80°C with 20% v/v of glycerol as cryoprotectant. Before the experiments, bacterial suspensions were thawed, inoculated (1%, v/v) in Brain Heart Infusion (BHI: Biokar Diagnostic, Beauvais, France) containing 0.05% w/v l-cysteine hydrochloride (BHIC) and incubated in anaerobic conditions at 37°C for 22h (AnaeroPack™anaerobic system, Mitsubishi Gas Chemical America, Inc., New York, USA).
ToxinotypingSpent culture supernatants (SCS) were obtained from a 72-h-old culture of ALCD3 in BHIC by centrifugation and further filter sterilization (0.45μm). Presence of TcdA and TcdB in SCS was assessed by the dot blot assay by using mouse anti-TcdA (1/1000) or anti-TcdB (1/500) monoclonal antibodies (Meridian Life Science Inc., USA) respectively as previously described44. Biological activity of SCS (mainly associated to TcdB) was determined in vitro by using cultured Vero cells44. The coding genes for TcdA (tcdA), TcdB (tcdB) and components of the binary toxin (cdtA and cdtB) were detected according to Stubbs et al.42 and Rupnik et al.37. To analyze the PaLoc region, the 3′-end of tcdA (A3) and 5′-end of tcdB (B1) were amplified by PCR. Next, A3 and B1 fragments were digested with EcoRI (Biolabsinc, New England) or HincII/AccI (Biolabsinc), respectively. An algorithm considering restriction fragment length polymorphism profile (PCR-RFLP), toxin production and presence of the CDT gen allowed to allocate the isolate to one of the 34 existing toxinotypes38 (http://www.mf.um.si/mf/tox/profile.html). Details on primer sequences and PCR conditions are given as supplementary material (Tables S1 and S2).
Molecular typingTo perform the DNA extraction, C. difficile ALCD3 was grown in BHI broth supplemented with 0.05% w/v l-cysteine for 48h at 37°C in anaerobic conditions. After incubation, 1ml of the culture was centrifuged (16000g, 3min). Pellet was washed with 1ml of 0.1M NaCl, suspended in 300μl of 6% w/v CHELEX (BIO-RAD, USA) and incubated at 60°C for 20min. After vortexing, the sample was heated at 100°C for 8min, centrifuged at 16000g for 3min, aliquoted and stored at −20°C until use.
Multilocus sequence typing (MLST) analysis was conducted by amplification and sequencing of the housekeeping genes: adk, atpA, dxr, glyA, recA, sodA and tpi as previously described by Griffiths et al.18 (details in supplementary material). Amplicons sequences were compared with the MLST database (https://pubmlst.org/cdifficile/) to identify the allelic profiles and the corresponding sequence type (ST).
Isolate ALCD3 was able to produce TcdA and TcdB, was positive for the cdt gene (binary toxin) and belonged to toxinotype 0/v. Strain VPI 10463 was used for comparison purposes.
Growth kineticsA series of replicate cultures (one culture per planned timepoint) were done in BHIC at 37°C under anaerobic conditions. At different timepoints, cell density was assessed by OD600 readings from individual cultures (Thermo electron Co, HEλiosy spectrophotometer).
To evaluate viable counts at 24h incubation, serial dilutions of samples in NaCl 0.9% w/v were plated onto DCM supplemented with 0.1% w/v of sodium taurocholate. Plates were incubated for 24h at 37°C under anaerobic conditions as indicated above.
Spore production and purificationPlates of solid DCM were inoculated with 100μl of 22h cultures in BHIC and incubated for 7 days at 37°C under anaerobic conditions (see “Molecular typing” section). Next, spores were recovered and purified as described by Sorg and Sonenshein41. Briefly, cells were harvested from agar plates with ice-cold distilled water (1.5ml, twice) and spore suspensions were stored for 72h at 4°C and washed 5 times with 1ml sterile ice-cold water (7000g for 5min). Suspensions in distilled water (1ml) were layered on top of 10ml of 50% w/v sucrose in water and centrifuged in a swinging-bucket rotor at 3200g for 20min at 4°C. After centrifugation, pellets containing mature spores were washed 5-times as described above, suspended in sterile distilled water and stored at −20°C until use17,41.
Sporulation kineticsMicroscopic evaluation of morphotypesOne-hundred microliters of an overnight culture, approximately 1.5×107CFU/ml, (corresponding to OD600nm=1) of C. difficile were inoculated on DCM agar plates and incubated for 1, 2, 5 or 7 days at 37°C under anaerobic conditions. After incubation, cells were harvested with NaCl 0.9% w/v as described in “Bacterial strains and culture conditions” section and analyzed immediately by bright-field microscopy at 1000× magnification. Vegetative forms (Vg) and three spore morphotypes were detected, i.e., phase dark (D), phase bright (B) and free (F) spores corresponding to different sporulation stages10. At least 300 total forms (TF=Vg+D+B+F) were evaluated for each microorganism under study. Results were expressed as sporulation ratio (SR) calculated as the ratio between total sporulated cells (TSC=D+B+F) and total forms (TF). The ratio of each morphotype (MR) was calculated as the number of cells belonging to a determined morphotype divided by TSC.
Heat-resistanceOne hundred and fifty microliters of spore suspensions harvested at different timepoints were heat-treated at 65°C for 20min. Ten-fold serial dilutions of both heat-treated and non-treated suspensions were plated on DCM supplemented with sodium taurocholate 0.1% w/v (DCM-TA). Plates were incubated at 37°C for 24h and colonies were counted. The heat resistance ratio (HRr) was calculated as the ratio between counts of heat-treated and non-treated samples at each time-point32.
Spore germinationAssessment of changes in optical densitySuspensions of purified spores (OD620=0.8–1) were incubated in flat-bottom 96 wells plates (Jet Biofil, DKSH Australia) in the presence of different concentrations (0, 50, 100 and 150mM) of sodium taurocholate in PBS buffer (pH=7.2–7.4). Decrease of OD620nm, that evidences changes in spore refringence and correlate with early steps in germination, was monitored at 1min intervals (TECAN microplate spectrophotometer Infinite F50)33. Ratios between OD620 values at each timepoint and the initial value (t=0) were defined as relative OD620 and were plotted against time. Germination rates (VOD620nm) were determined by calculating the slopes in the initial linear region of the relative OD620nm vs t plots.
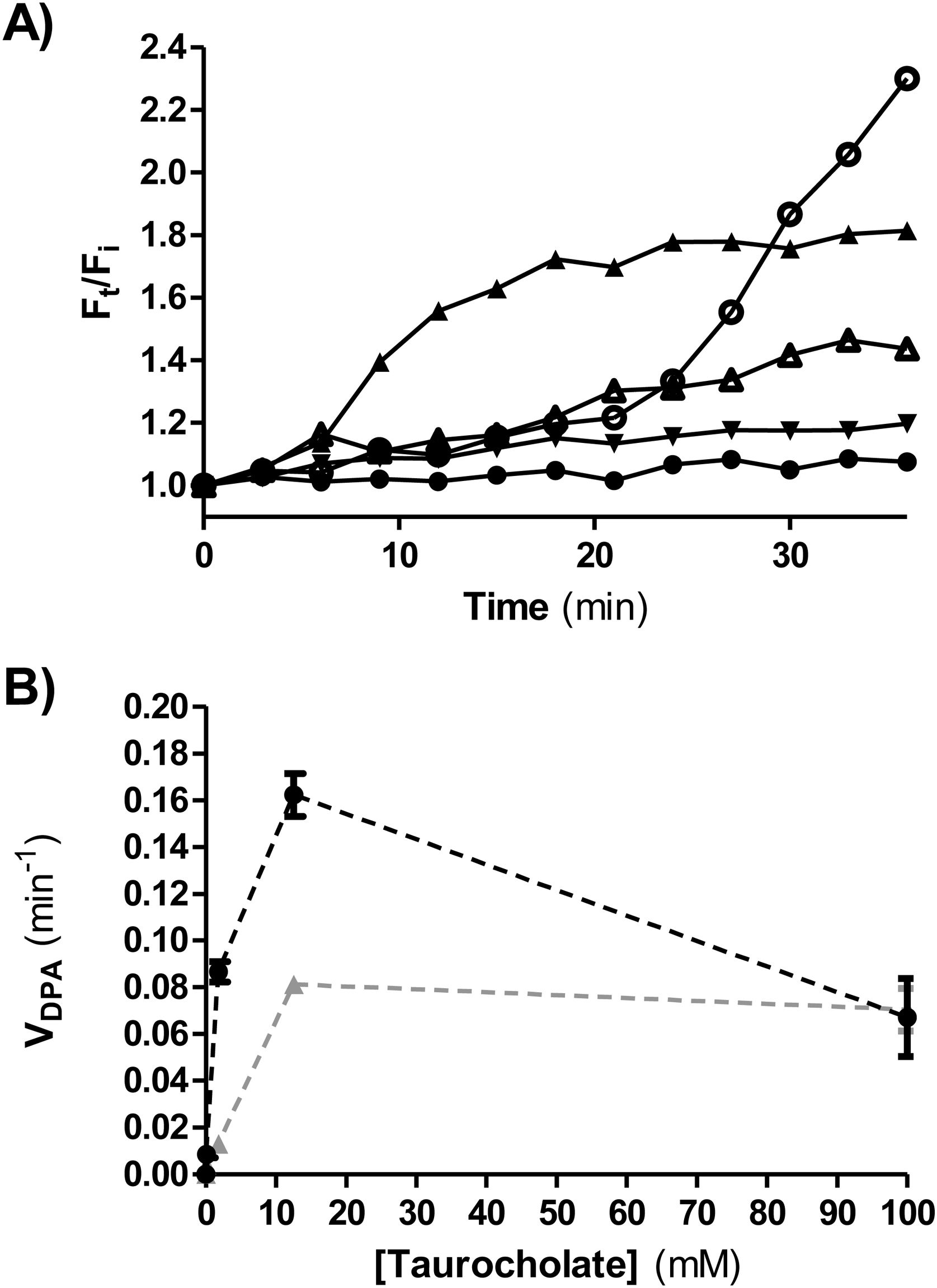
Ca dipicolinate release (CaDPA)CaDPA release is one of the early steps in germination of C. difficile. It was monitored in real time by measuring fluorescence in the presence of terbium (III) chloride4. An opaque flat-bottom 96-well plate (Greiner Bio-One) was prepared with 125μl of 10mM Tris (Sigma-Aldrich, St. Louis, USA) (pH 7.5), 150mM NaCl (Cicarelli, Santa Fe, Argentina), 800μM TbCl3 (Sigma-Aldrich) and different sodium taurocholate concentrations (0, 0.1, 1.8, 12.5 and 100mM). Two experimental conditions were tested. Condition 1:1.5μl of concentrated spore suspensions (OD600nm=40) were added per well; condition 2, glycine (Sigma-Aldrich) was used as co-germinant (final concentration 100mM) and 5μl of a heat-activated spore suspension (at 65°C for 20min) were added per well. Fluorescence was monitored for 80min at 37°C (Infinite 200 PRO TECAN fluorescence plate reader) using the following wavelengths: excitation=270nm; emission=545nm; cutoff=420nm. The ratio Ft/Fi was plotted versus time (min), where Ft is the fluorescence after t min and Fi is the initial fluorescence. To determine DPA release rate (VDPA) the slopes of the linear region of Ft/Fi kinetics were calculated and plotted at each taurocholate concentration tested.
Statistical analysisGrowth kinetics and sporulation assays were performed in duplicate. Slopes were analyzed using the GraphPad slope comparison test that compares the simple models obtained of each dataset with a global model using an F-test. A one-way ANOVA with Tukey's multiple comparison test was used to analyze HR, VOD620nm and VDPA. Ratios between different morphotypes were compared by means of the exact Fisher's test. Statistical analysis was performed by using InfoStat software (InfoStat, version 2020 for Windows, FCA-UNC Córdoba, Argentina) and GraphPad Prism version 5.00 for Windows, GraphPad Software, San Diego California USA, www.graphpad.com.
ResultsCharacterization of isolate ALCD3C. difficile ALCD3 was able to produce TcdA and TcdB. In addition, sequences of the genes associated to the binary toxin (cdt) and RFLP analysis, showed that this isolate belongs to toxinotype 0/v. MLST showed the allelic profile adk:91, atpA:1, dxr:2, glyA: 1, recA:27, sodA: 1 and tpi:1, which corresponds to ST293 (MLST clade: 1).
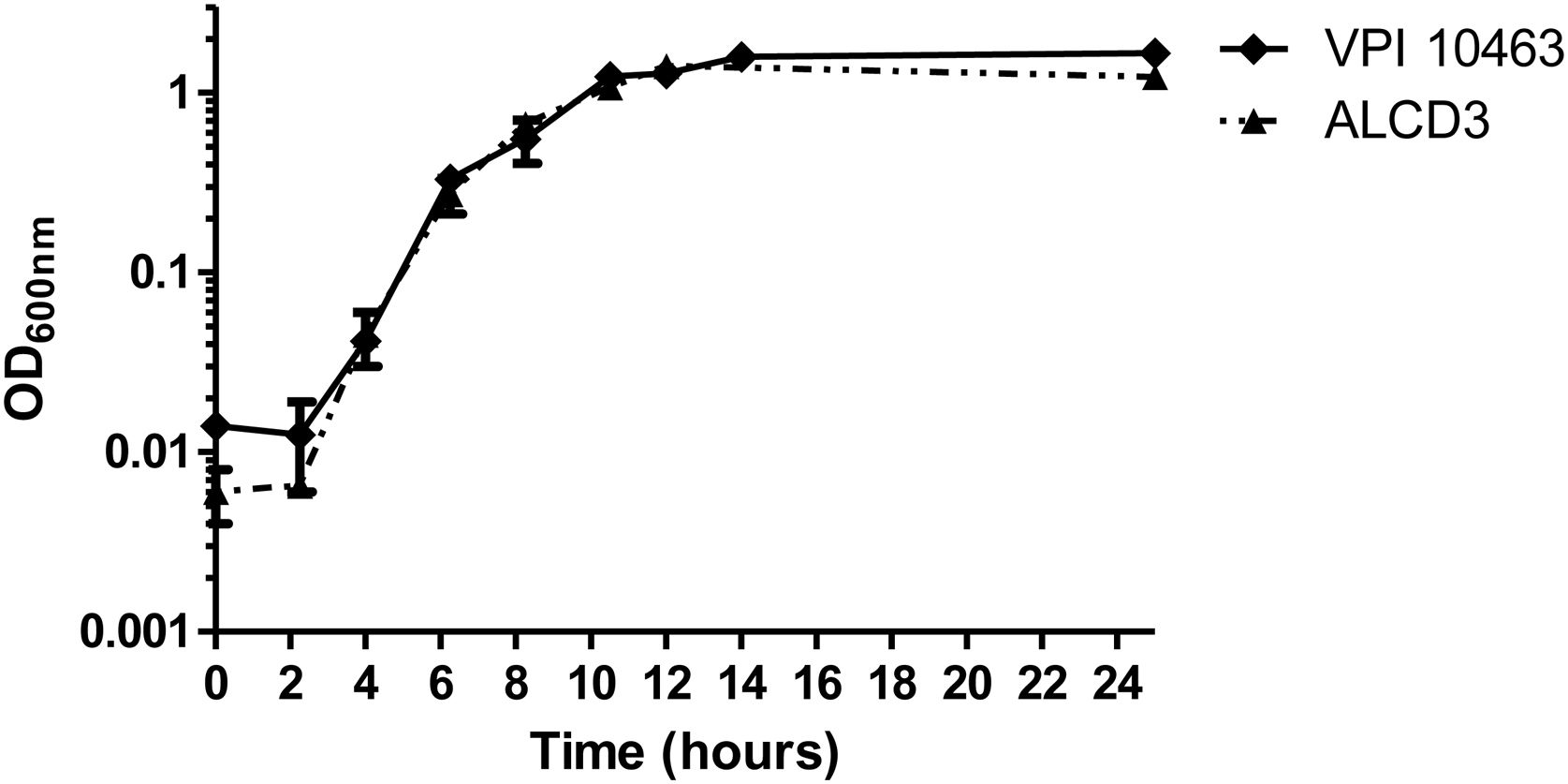
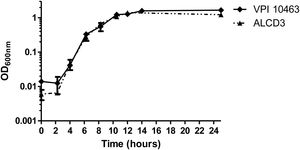
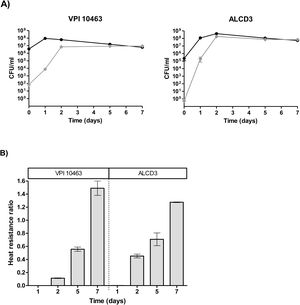
Growth kineticsGrowth kinetics in BHIC medium are shown in Figure 1. Growth rates were 0.43±0.1 (1/h) for ALCD3 and 0.66±0.1 (1/h) for VPI 10463 and lag periods were around 2h. After 12–14h, cultures reached stationary phase with OD600 values ranging from 1.2 to 1.6 units. Viable counts in 24-h-old cultures were 5.6×106 (VPI 10463) and 1.4×107 (ALCD3) CFU/ml.
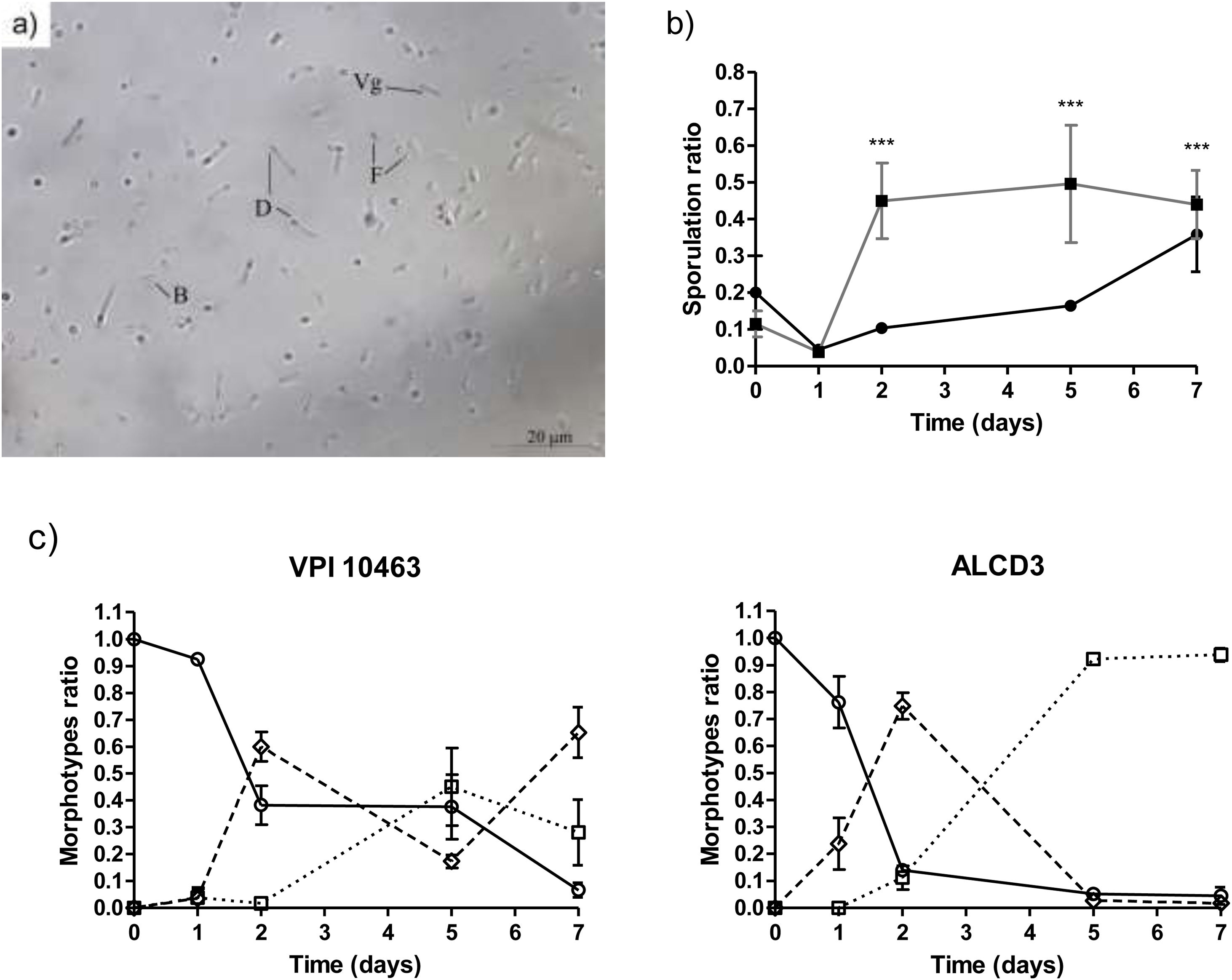
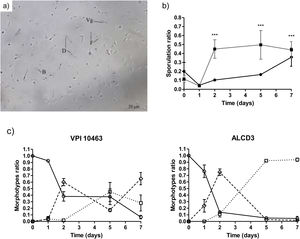
Sporulation kinetics and morphotype evaluationMorphological changes during sporogenesis can be assessed by microscopic examination. As shown in Figure 2a, different morphotypes were evidenced, i.e., vegetative forms (Vg) and three morphotypes: phase-dark (D), phase-bright (B) and free spores (F).
(a) Bright field micrography showing different morphotypes (1000×): Vg (vegetative cell), D (dark phase sporulated form), B (bright phase sporulated form) and F (free spore). (b) Kinetics of sporulation ratios for C. difficile strains in DCM medium at 37°C. (■) ALCD3 and (●) VPI 10463. (c) Kinetics of morphotype ratios. (○) Dark phase, (◊) bright phase and (□) free spores. Ratios were compared with the Fisher exact test (p<0.05).
Kinetics of sporulation in DCM agar is shown in Figure 2b. At t=0 sporulation rates (ranging from 0.1 to 0.2) are due to the carry-over of spores from the initial inoculum. After 1-day incubation, sporulation ratios (SR) were low for strain VPI 10463 (0.045±0.002) and isolate ALCD3 (0.040±0.01). This represents, in absolute values, 4–13 sporulated forms respectively, per 300 total forms.
C. difficile ALCD3 showed an early increase in the sporulation ratio (reaching maximal values after 2 days of incubation (SR: 0.46±0.03; Fig. 2b)). In contrast, sporulation ratios of C. difficile VPI 10463 increased until day 7 but were always below those of ALCD3 isolate. These results represent, in absolute values, numbers of total sporulated forms per 300 total forms of 107±43 (VPI 10463) and 132±40 (ALCD3).
Quantification of the different morphotypes (expressed as morphotype ratio, MR) revealed strain-dependent sporulation patterns (Fig. 2c). At time zero, the spores were in the early stages of the cycle (MRD=1). These spores arise from the inoculum carryover. As expected, on day 1, high ratios of dark phase forms were observed for both microorganisms under study (ratios ranged from 0.75 to 0.95). On day 2, ratios of dark phase forms decreased, and bright phase forms were observed. Interestingly, free spores (MRF=0.11) were observed on day 2 for isolate ALCD3 that also showed high ratios of bright phase spores (MRB=0.75) at this timepoint (Fig. 2c). These findings are in agreement with the sporulation kinetics for C. difficile ALCD3 strain shown in Figure 2b.
The maximal ratios of free spores (MRF) were found on day 5: 0.92 for ALCD3 and 0.45 for VPI 10463. These values were significantly different (p<0.001) and remained stable on day 7 (Fig. 2c).
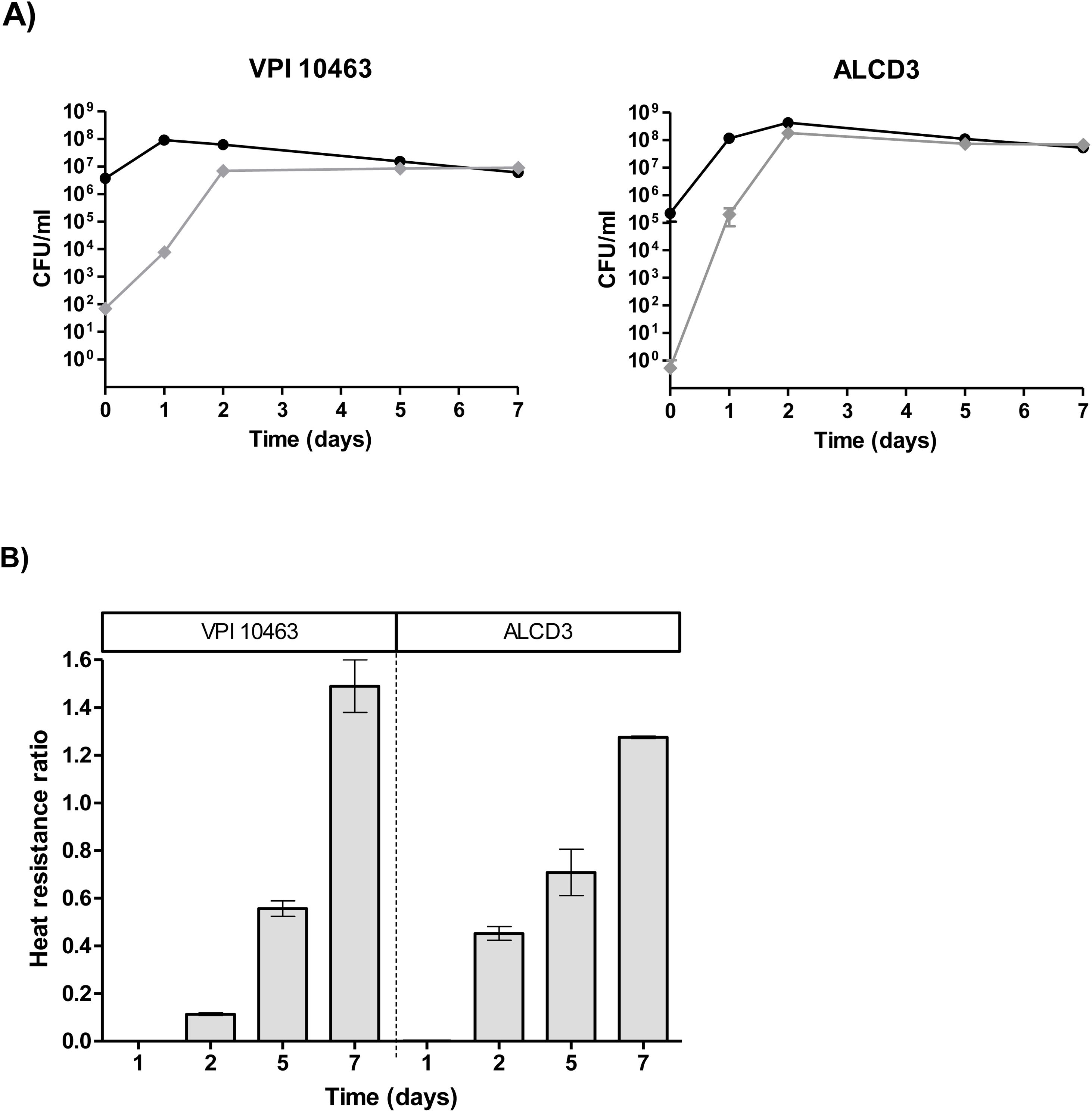
Heat resistanceProgress of the sporulation process was monitored by assessing the ratio of thermo-resistant forms. On day 1, cultures were in stationary phase and total bacteria counts (before heating) were around 108CFU/ml (Fig. 3A) with ratios of heat resistant forms (HRr=heat resistant/total counts) lower than 0.002 (Fig. 3B). Both microorganisms under study reached maximum values of heat resistant forms at 2 days of incubation. In addition, the ratio of heat-resistant forms steadily increased and HRr>1 were found at 7 days of incubation for both strains (Fig. 3B).
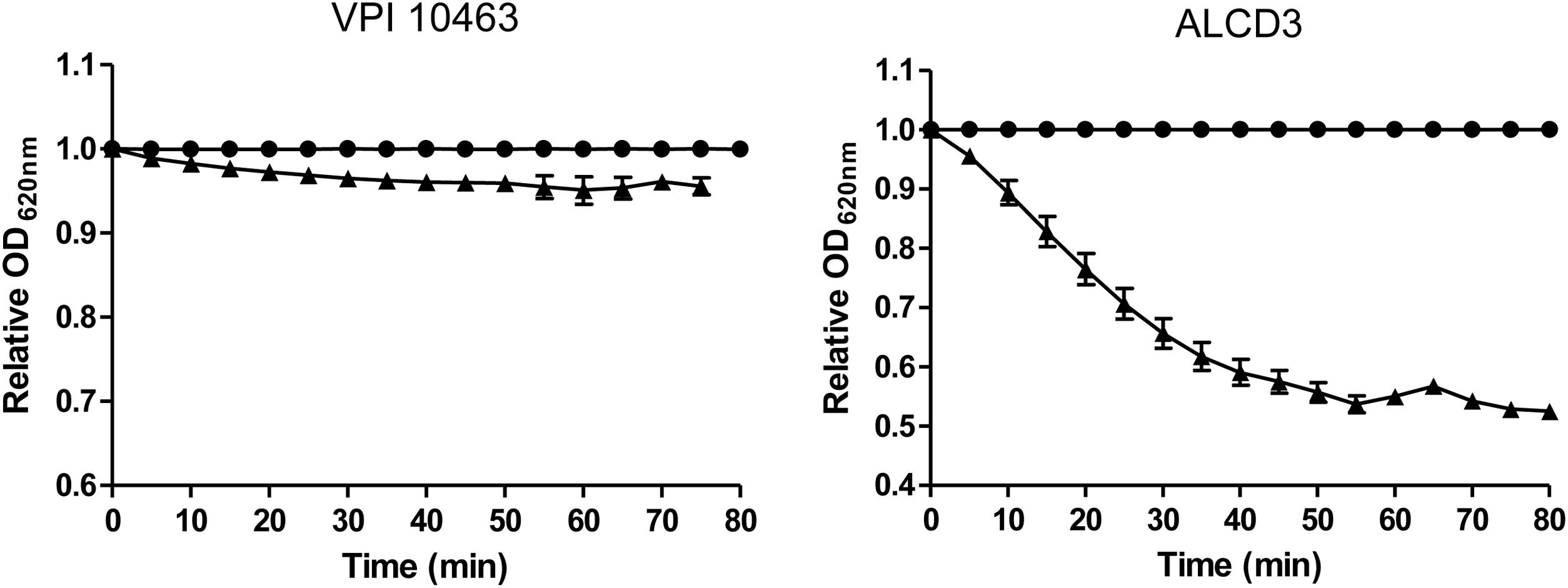
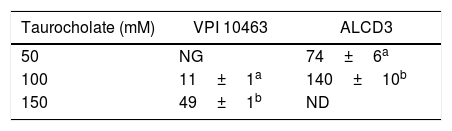
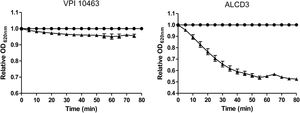
Spore germinationAs shown in Figure 4, isolate ALCD3 showed the most noticeable changes in the values of relative OD620 when spores were exposed to 100mM of taurocholate. As shown in Table 1, isolate ALCD3 showed the highest germination rates even at the lowest taurocholate concentration tested (50mM). Furthermore, this microorganism responds to the increase of taurocholate from 50 to 100mM by doubling the germination rate. No detectable germination was observed at 50mM taurocholate concentrations for isolate VPI 10463, but it increased 5 times the germination rate in the presence of 150mM taurocholate.
Germination rates* for C. difficile strains in the presence of different taurocholate concentrations.
| Taurocholate (mM) | VPI 10463 | ALCD3 |
|---|---|---|
| 50 | NG | 74±6a |
| 100 | 11±1a | 140±10b |
| 150 | 49±1b | ND |
NG: no germination was detected; ND: not determined.
Different letters in the same column indicate significant differences (p<0.001).
Germination rates were defined as the slopes of the linear region of ODt/ODi plots (as per Fig. 4).
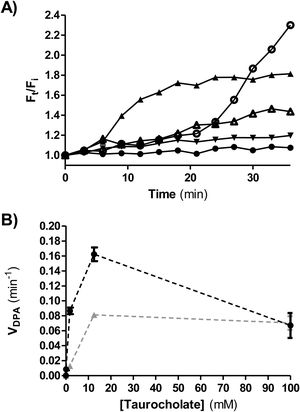
Figure 5A shows a representative plot of DPA release from isolate ALCD3 in condition 2 (with glycine as co-germinant). As expected, the rate of DPA release depends on the germinant concentration. High Ft/Fi values were detected after 30min incubation in the presence of 12.5 and 100mM taurocholate.
(A) Kinetics of CaDPA release from heat-activated spores of the ALCD3 strain in the presence of 100mM glycine and different concentrations of sodium taurocholate. (▴) 100mM, (○) 12.5mM, (Δ) 1.8mM, (▾) 0.1mM and (●) 0mM. (B) Initial rate of CaDPA release from spores of strain ALCD3 in the presence of different concentrations of sodium taurocholate with neither glycine nor heat-activation (▴) or with glycine 100mM and heat-activation for 20min at 65°C (●). Results show a representative experiment and bars are the standard deviation of the linear regression.
Rates of DPA release (VDPA) were calculated from the linear region of Ft/Fi vs t plots and were plotted for different taurocholate concentrations (Fig. 5B). As expected, the presence of the co-germinant significantly increased the rate of DPA release from heat-activated spores of the ALCD3 strain as compared with germination without glycine (Fig. 5B).
DiscussionThis study found that C. difficile ALCD3 ST293 isolated from a recurrent infection presents high sporulation/germination efficiency. The deposit of ST293 in https://pubmlst.org/cdifficile/ database corresponded to an isolate recovered in China in 2015; however, to the best of our knowledge there are no previous publications in the indexed literature reporting the relationship of this ST to CDI. Interestingly, ST293 is a single locus variant of ST3, which only differs in the allele of recA. ST3 was one of the prevalent ST in China associated with CDI and community-acquired infections. It is worth noting that ST3 is associated to PCR ribotypes 001, 009, 072 and 115 that are not related to typical hypervirulent strains18,27.
It has been proposed that enhanced sporulation and toxin production correlate with an apparent increase in virulence in the isolates called “hypervirulent” associated with specific genotypes1. High nosocomial spread of strains belonging to ribotype 027 was related to an increased resistance of its spores to environmental factors and disinfectants20. It is evident that isolate ALCD3 was successful in spreading, infecting and producing toxins. However, although the ability to produce a high number of spores correlates with the dissemination/infectious potential, it is not directly associated to the ability to release toxins. In this context, strain VPI 10463 is able to release a higher concentration of toxins than other nosocomial strains but it is not considered a hypervirulent strain29.
Although the role of the binary toxin during infection is not well understood, the presence of the cdt gene could contribute to the virulence of C. difficile ALCD3. Indeed, a mouse model of infection with strain R20291 CDT+ produced higher mortality than the isogenic CDT− strain7,27.
Isolate ALCD3 showed high ratios of sporulated forms (Fig. 2b). The sporulation ratios and free spore ratios (Figs. 2b and c) evidence the efficiency in sporogenesis of the isolate.
Spore germination is a paramount step in the infective cycle. Dormant spores must sense environmental signals to germinate thus allowing bacterial growth. Germination of C. difficile spores is triggered by natural germinants and co-germinants such as taurocholate and glycine, respectively19,36. Moreover, divalent ions such as calcium play a role as factors enhancing germination23. As a particular mechanism to detect germinants, C. difficile uses the CspC pseudoprotease to sense cholate-derived bile acids such as taurocholate36.
Isolate ALCD3 demonstrated high response to taurocholate (Table 1 and Fig. 4). Interestingly, the release of DPA was evident in the first 10min of incubation with taurocholate. This indicates that the germinant rapidly diffuses in the spore coat thus reaching CspC. However, some spores required further activation since heat treatment and the presence of glycine as co-germinant in addition to taurocholate, significantly enhanced DPA release from the ALCD3 isolate.
In the present report, we demonstrate that isolate ALCD3 is efficient in germination (Figs. 4 and 5), which contrasts with the reference strain VPI10463 that was able to produce high amounts of spores but showed lower rate of cortex hydrolysis in the presence of 100mM taurocholate. It must be pointed out that during germination of C. difficile spores, cortex hydrolysis precedes DPA release. This is opposite to B. subtilis germination13. Therefore, results shown in Figures 4 and 5 correspond to consecutive events. As expected, isolate ALCD3 responds to the co-germinant glycine but, noteworthy, it is also able to germinate when only taurocholate was used. These findings agree with the demonstrated effect of CspC conformation on germination. Indeed, a single point mutation in cspC dramatically modifies the mobility of relevant domains thus allowing germination with otherwise competitive inhibitors such as chenodeoxycholate12. In addition, it has been proposed that there are mutants (yabG mutants) that fail to process CspBA to give rise to CspB, the cortex hydrolase11,30. This condition leads to spores that are insensitive to the presence of co-germinants40. Differences in response to germinants can be related to differences in the diffusion of germinants due to variations in the exosporium composition31.
It can be noted that isolate ALCD3 responds to increasing taurocholate concentrations by increasing the rate of cortex hydrolysis (Table 1). However, the results shown in Figure 5B demonstrated that DPA release peaked at 12.5mM.
It is worth noting that isolate ALCD3 was obtained from a recurrent event, 10 days after the patient completed the standard treatment with oral vancomycin for 14 days.
Our study reports for the first-time the circulation of C. difficile ST293 outside China. We demonstrated that C. difficile ALCD3 ST293 isolate presented high sporulation/germination efficiencies that could contribute to its high pathogenic and spread potential. The present report encourages further research to understand the real impact of this lineage in Argentina.
Conflict of interestNone declared.
Authors acknowledge financial support from Facultad de Ciencias Exactas (Universidad Nacional de La Plata, X816), CONICET (PIP 2018-511) and ANPCyT (PICT 2018-3512). Isolate ALCD3 was from Hospital Alemán, Ciudad Autónoma de Buenos Aires, Argentina. PCSP is fellow of the CONICET, Argentina. FMT, DC and PFP are members of the Carrera del Investigador Científico y Tecnológico, CONICET, Argentina.