The present case study analyzes semen quality, nutritional patterns, and hormonal and oxidative status of an international high-level triathlete with a low-volume, high-intensity training load.
MethodThe athlete was 26 years old, having participated in competitions since he was 13 years old, and practiced professional triathlon for the last five years. The qualitative sperm parameters analyzed were volume, sperm count, motility, morphology, and DNA fragmentation (additional testing performed as needed). Salivary hormones measured were T, C, and T/C. Seminal plasma total antioxidant capacity was measured. Maximum oxygen consumption and training characteristics were assessed. To determine habitual food intake and its possible repercussion on oxidative status, a quali-quantitative frequency questionnaire of 136 different foods was used and subsequently analyzed using specific software.
ResultsHormonal and physical semen parameters were within normal ranges. Sperm morphology and DNA fragmentation showed abnormal values (3.9% and 38.33%). Round cells in semen were higher than normal (2.3million/ml), with presence of macrophages. Apoptotic and necrotic events were observed. Total antioxidant capacity, although not compromised, was low. Dietetic intake was characterized by excess protein and appropriate overall antioxidant intake (with slight deficit and excess of some specific nutrients) according to recommended values.
ConclusionsIn this high-intensity endurance athlete, sperm parameters, mainly sperm morphology and DNA fragmentation, are altered. Further knowledge is needed with regards nutritional antioxidant intake and other dietetic strategies oriented toward avoiding oxidative damage in semen of high-performance triathletes. Moreover, adequate nutritional strategies must be found and nutritional advice given to athletes so as to palliate or dampen the effects of exercise on semen quality.
El presente estudio de caso analiza la calidad de semen, patrones nutricionales y status hormonal y oxidativo de un triatleta internacional de alto nivel con carga de entrenamiento de bajo volumen y alta intensidad.
MétodoAtleta de 26 años de edad, con una práctica deportiva competitiva desde los 13 años, y cinco años de triatlón profesional. Los parámetros cualitativos analizados en semen fueron: volumen, contaje espermático, motilidad, morfología, y fragmentación de ADN, con pruebas adicionales según necesidad. Las hormonas salivares cuantificadas fueron: T, C, y T/C. La capacidad antioxidante total fue medida en plasma seminal. También se analizó el consumo máximo de oxígeno y características de entrenamiento. Para determinar la ingesta alimentaria habitual y su posible repercusión sobre el estado oxidativo, se utilizó un cuestionario de frecuencia de consumo cuali-cuantitativa, de 136 alimentos, analizado mediante software informático.
ResultadosLos valores hormonales y parámetros físicos seminales estaban dentro de la normalidad. La morfología y fragmentación de ADN espermático mostraron valores anormales (3.9% y 38.33%). Aparecía un elevado número de células redondas (2.3 millones/mL), con presencia de macrófagos. Se observaron eventos apoptóticos y necróticos. La capacidad antioxidante total, aunque no alterada, estaba baja. La ingesta dietética se caracterizó por un exceso proteico y consumo adecuado de antioxidantes (con ligero déficit y exceso de algunos nutrientes específicos), según valores recomendados.
ConclusionesEl ejercicio de resistencia de alta intensidad altera los parámetros espermáticos, principalmente morfología y fragmentación de ADN. Es necesario obtener mayor información sobre el efecto de antioxidantes y otras estrategias dietéticas con relación al daño oxidativo en el semen de triatletas de alto rendimiento, al igual que hallar estrategias nutricionales adecuadas para paliar o amortiguar los efectos del ejercicio sobre la calidad del semen.
O presente estudo de caso analisa a qualidade de sêmen, padrões nutricionais, estado hormonal e oxidativo de um triatleta internacional de alto nível com carga de treinamento de baixo volume e alta intensidade.
MétodoAtleta de 26 anos de idade, com uma pratica esportiva desde os 13 anos e cinco anos de triátlon profissional. Os parâmetros qualitativos analisados no sêmen foram: volume, contagem espermática, motilidade, morfologia, e fragmentação de DNA com testes adicionais dada necessidade. Os testes hormonais salivares foram: Testosterona, Cortisol e a razão testosterona/cortisol. A capacidade antioxidante total foi medida no plasma seminal. Também foi analisado o consumo máximo de oxigênio e características do treinamento. Para determinar a ingesta alimentar habitual e sua possível repercussão sobre o estado oxidativo, foi utilizado um questionário de frequência de consumo quali-quantitativo de 136 alimentos, a partir do uso de um software especifico.
Resultadosos valores e parâmetros hormonais e físicos seminais estavam dentro da normalidade. A morfologia e fragmentação do DNA espermático mostraram valores anormais (3,9% e 38,33%). Apresentando um elevado número de células redondas (2,3 milhoes/mL), com presença de macrófagos. Se observaram eventos apoptóticos e necróticos. A capacidade antioxidante total, ainda que não alterada, estava baixa. A ingesta dietética foi caracterizada por um excesso proteico e consumo adequado de antioxidantes (com ligeiro déficit excesso de alguns nutrientes específicos) segundo valores recomendados.
Conclusõeso exercício de resistência de alta intensidade altera os parâmetros espermáticos, principalmente morfologia e fragmentação do DNA. Faz-se necessário obter maiores informações sobre o efeito de antioxidantes e outras estratégias dietéticas com relação ao dano oxidativo no sêmen de triatletas de alto rendimento, e traçar estratégias nutricionais adequadas para atenuar os efeitos do exercício sobre a qualidade do sêmen.
It has been recently postulated that exhaustive endurance exercise may be a factor altering the seminological and hormonal profiles in elite athletes. Triathlon competition imposes great physical and mental demands on the athletes, forcing them to undergo high training loads and long-duration competitions.1
The Ironman is certainly one of the most extenuating competitions, with athletes spending between 9 and 10h in order to complete a 3.86km-swim, 180km-cycling, and 42.2km-run. Athletes aim in this competition to achieve the best possible position and scores. As a result of the high training loads used by the triathletes, the organism undergoes great physical and mental stress. Moreover, physical training may produce negative physiological changes that may lead to overreaching/overtraining status.2
There are many studies that report a relationship between intense endurance exercise and hormonal (suprarenal and gonadal) and semen alterations.3 From the studies, it has been observed that training volume is one of the characteristics most exerting an influence on hormone and semen parameters. Some authors have even established a theory of a volume-threshold.4
Previous studies have related triathlon practice to semen alterations.5,6 This relationship has been observed to be related to training volume. However, studies have suggested that training intensity may also be deleterious for hormone and semen profiles and may induce an oxidative status.7,8 Moreover, inappropriate nutrition may also influence these parameters.9 In line with this, we have assessed semen, hormone and total antioxidant capacity values in a high-intensity elite triathlete as well as careful nutritional surveillance in order to determine any possible alterations in the reproductive profile of this particular athlete.
MethodSubjectThe athlete, who was 26 years old, had a VO2max of 72.11ml/kg/min, as measured by a gas analyzer (Erich Jaeger, Viasys Healthcare, Germany).
His mean annual weekly training regime is as follows: 15km of swimming, 155.4km of cycling, and 30km of running. With regards to cycling alone, his mean annual weekly training characteristics were as follow: 18.5h and a total covered distance of 155.4km. His mean training values for the last week of training were, therefore, diminished in comparison to the mean annual values (4.1km of swimming, 112.8km of cycling, and 14km of running). Due to the athlete's own training regime, he had not performed any cycling in the week of the testing (performed on a Wednesday). The intensity at which the athlete trained in the three different disciplines is as follows: 75% of maximum HR for swimming, 75% for cycling, and 68% for running.
It has to be mentioned that the athlete completed a detailed fertility questionnaire which did not reveal any possible previous fertility-related issues.
ProceduresThe athlete underwent complete physical exam as well as completed a fertility, a training and a nutritional questionnaire. Routine blood analysis was performed with normal results for all common biochemical and physiological parameters (no iron or zinc deficiency, cholesterol, 25-hydroxyvitamin D or glycemic alterations). Semen, hormone, total antioxidant capacity (TAC), nutritional habits and training parameters were carefully analyzed.
With regards to training, the athlete was in a tapering period preparing for a non-official competition. As previously described, the week before testing was characterized by a low volume training. All testing and questionnaires were done on the same day. The athlete collected semen and afterwards he gave the saliva sample and answered the nutritional questionnaire. For better semen characterization, he gave another semen sample 3 days later. He was asked to drastically lower the load 2 days before sample collection, and to avoid any cycling exercise. Moreover, the day before the testing, he decreased training volume to 50% of the total of that week's volume.
Semen analysisThe athlete kept the required abstinence for adequate sample collection (4 days). World Health Organization (WHO) values for volume, number and velocity were chosen for sample normality assessment, while Kruger's strict criteria were chosen for morphology normality assessment.10 Total motility (PR+NP) was assessed and motility was graded as follows: progressive motility (PR), non-progressive motility (NP) and immotile sperm (IM). For determination of leucocytes in the sample the leucoscreen test was used (Fertipro, Belgium). For morphology, two sperm smears were stained (Diff Quick, Panreac, Barcelona, Spain) and observed at 100× under oil. DNA fragmentation evaluation was performed using a sperm chromatin dispersion test (Halosperm®, Halotech DNA S.L., Madrid, Spain). Apoptosis and necrosis determination was performed using the Annexin V testing (Miltenyi Biotec, Germany) and observing sperm with a fluorescence microscope. TAC determination was performed in seminal plasma after centrifugation by ELISA with commercially available kits (Cayman Chemical, Michigan, USA).
Hormone analysisFor saliva collection, the athlete avoided any food or caffeine products for a period of at least 2h before sampling. Saliva collection was performed by inserting a collection pad inside the athlete's mouth and keeping it inside the mouth to absorb saliva during 2min. Afterwards, the pad was removed and stored at −80°C until assayed. Commercial kits for nonradioactive ELISA were used for hormonal assessment (Salivary Testosterone EIA Kit and Salivary Cortisol EIA Kit, Salimetrics LLC, Suffolk, UK). Sensitivity was <0.003μg/dL for Cs and 1pg/ml for Ts. With regards to reproducibility of measures, the ICC was 0.8 for Ts and 0.78 for Cs.
Nutritional analysisThe material used for data collection and analysis with regards to the nutritional intake was based on the completion of a food frequency questionnaire, using a computerized support, and an individual personal interview, the purpose of which is to gather knowledge on the dietary habits before, during and after training for the studied athlete.
In order to assess the athlete's nutrition, he was asked to complete, during a personalized interview, a food frequency survey obtained from the “Study Predimed. Nodo Pamplona AP-UNAV. Epidemiology and Public Health, University of Navarra”. By means of this survey, knowledge is obtained about which foods are eaten, how frequently and in what quantity. The survey consists of 136 foods divided into seven groups (dairy, vegetables, eggs–meats–fish, fruits, cereals–vegetables, oils and fats, and miscellaneous), asking the athlete to refer his daily, weekly and monthly intake of each one of them and even three seasonal categories can be differentiated (Spring, Fall–Winter and Summer) for better assess met due to seasonal differences in obtaining certain foods. In addition, a manual with pictures of different foods and dishes,11 and food replicas were used. With all these data, the portion size of each food consumed by the athlete was established so as to later determine the amount ingested.
These surveys have been analyzed with the software V.2.0 Dietsource TM obtaining final data on micronutrients, macronutrients and energy for the triathlete.
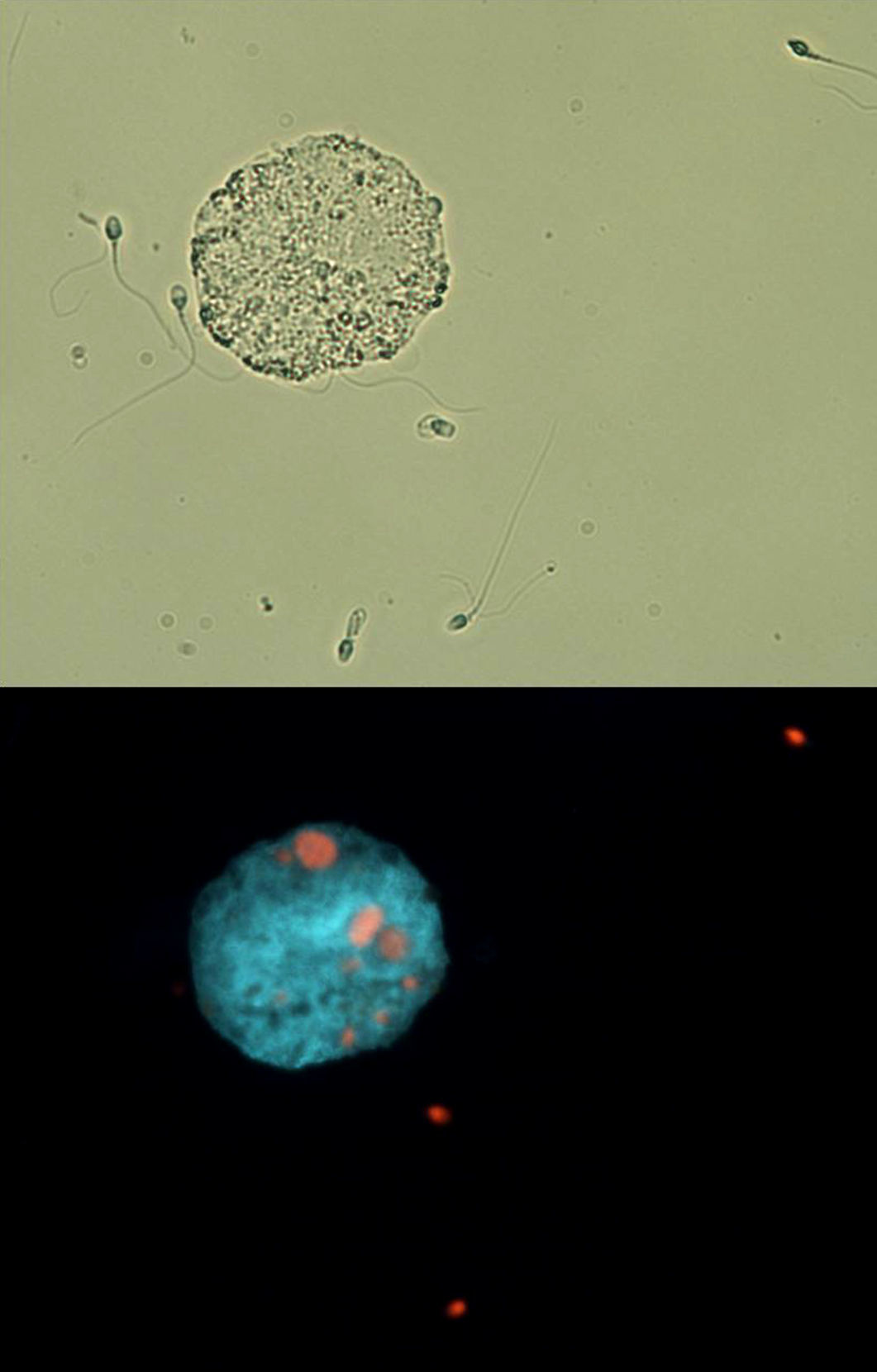
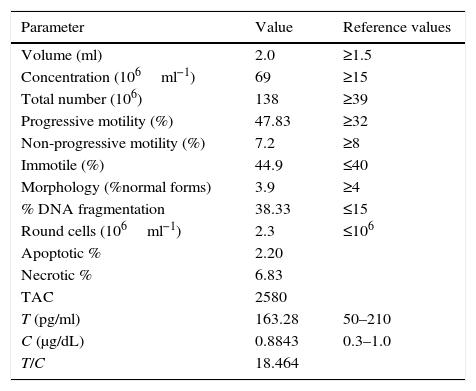
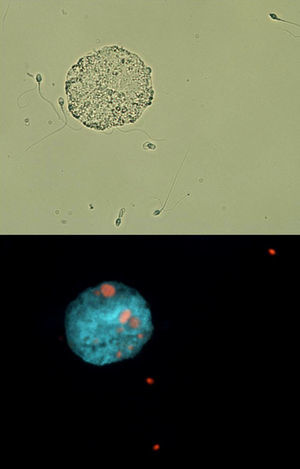
ResultsSemen and hormone valuesValues for semen physical parameters were within normal ranges, as well as values for sperm concentration and velocity. However, both morphology and DNA fragmentation showed abnormal values (3.9% and 38.33% respectively). Moreover, it was observed that the presence of round cells in semen was higher than normal (Table 1). Active macrophages were found to be present in the semen sample (Fig. 1). Hormonal values were within normal. Table 1 also shows the values for TAC from seminal plasmas as well as the values obtained for salivary testosterone, salivary cortisol, and the T/C ratio resulting from both hormones.
Seminological and hormonal values of the studied athlete.
| Parameter | Value | Reference values |
|---|---|---|
| Volume (ml) | 2.0 | ≥1.5 |
| Concentration (106ml−1) | 69 | ≥15 |
| Total number (106) | 138 | ≥39 |
| Progressive motility (%) | 47.83 | ≥32 |
| Non-progressive motility (%) | 7.2 | ≥8 |
| Immotile (%) | 44.9 | ≤40 |
| Morphology (%normal forms) | 3.9 | ≥4 |
| % DNA fragmentation | 38.33 | ≤15 |
| Round cells (106ml−1) | 2.3 | ≤106 |
| Apoptotic % | 2.20 | |
| Necrotic % | 6.83 | |
| TAC | 2580 | |
| T (pg/ml) | 163.28 | 50–210 |
| C (μg/dL) | 0.8843 | 0.3–1.0 |
| T/C | 18.464 |
Fluorescence and bright field microscopy images. A macrophage can be seen along with several abnormal sperm in bright field (A). Fluorescence microscopy shows, in the same field, how several of these sperm cells are undergoing necrosis; moreover, several sperm cells (red color) have been phagocitized by the macrophage (B). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
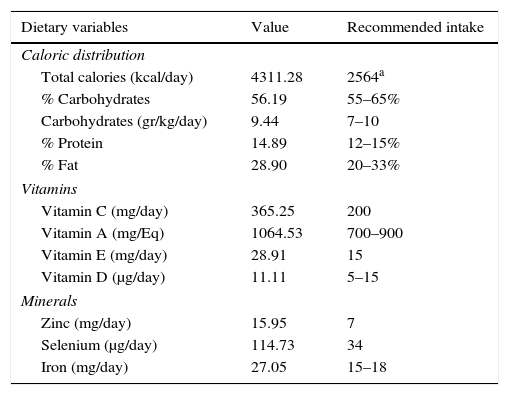
After analyzing the different dietary data obtained from the interview with the athlete, the following observations were found (Table 2), indicating an overall suitable nutritional intake for the requirements for the kind of the athlete studied.
Habitual dietary composition on the studied athlete.
| Dietary variables | Value | Recommended intake |
|---|---|---|
| Caloric distribution | ||
| Total calories (kcal/day) | 4311.28 | 2564a |
| % Carbohydrates | 56.19 | 55–65% |
| Carbohydrates (gr/kg/day) | 9.44 | 7–10 |
| % Protein | 14.89 | 12–15% |
| % Fat | 28.90 | 20–33% |
| Vitamins | ||
| Vitamin C (mg/day) | 365.25 | 200 |
| Vitamin A (mg/Eq) | 1064.53 | 700–900 |
| Vitamin E (mg/day) | 28.91 | 15 |
| Vitamin D (μg/day) | 11.11 | 5–15 |
| Minerals | ||
| Zinc (mg/day) | 15.95 | 7 |
| Selenium (μg/day) | 114.73 | 34 |
| Iron (mg/day) | 27.05 | 15–18 |
The main finding of the present study is an altered value for sperm morphology and sperm DNA fragmentation despite adequate testosterone value and nutrition and TAC values.
Several studies have shown a potential relationship of exercise to hormonal and seminological alterations.4–7 The characteristics observed in the present case seem to be in accordance with previously published observations,4–7,12 the triathlete did not show any alterations for sperm number or velocity but showed significant alterations in sperm morphology and a great degree of sperm DNA fragmentation. Significant alterations in morphology and sperm DNA fragmentation have been correlated with longer times to conception and less chances of live birth.13,14
In the present case, the athlete is characterized by performing a high-intensity, low-volume training; when his training is compared to other triathletes is notorious his priority is intensity rather than volume.
Testosterone is essential for proper spermatogenesis to occur. Our triathlete showed testosterone levels within the normal range for healthy male adults, although somewhat lower than the values reported for other sports modalities, especially with regards to strength sports.15 Yet it has also been documented that Ironman competitors, and even other endurance athletes (running, cycling, rowing, swimming), may present lowered testosterone values,16 especially immediately after competition.17
Although endurance athletes may have T values below normal range,18 the triathlete in this study showed normal values, even greater than those observed in other triathletes; this may be because he is characterized by a high-intensity training.
Intensity defined as “high-intensity training” has been shown to be the key factor in modulating endocrine response as increase in T and decrease in C.19 Moreover, an increase in salivary T could be associated with a greater metabolic efficiency toward the pre-competition and competition phases.20 Zahavi and Perel20 reported an improvement in the efficiency of the mitochondrial proton pump when producing ATP, despite suggesting that this metabolic advantage could be associated to an increase in oxidative damage. The fact that TAC values are somewhat low, can refer to the subject not having an appropriate antioxidant defense and, therefore, he may not be able to mitigate the oxidative damage.
In highly trained male athletes, as in female athletes21 exercise is usually associated with a decrease in the hypothalamic–pituitary–suprarrenal axis, although basal cortisol levels are usually greater than normal, reflecting a chronic response to training.22
Macrophage presence was observed in the athlete's semen. Although macrophages should not be present in semen, phosphatidylserine exposure, like in the case of apoptotic cells, serves as a triggering signal for macrophages23; this may also be true for removing defective sperm; in fact, some authors have reported presence of macrophages along impaired sperm parameters in men.24 Macrophages may, thus, have a function in the differentiated tissues of the reproductive system. In this regard, we have observed high numbers of sperm with morphological anomalies as well as with fragmented DNA and undergoing apoptosis and necrosis. Other authors have observed a direct relationship between the presence of macrophages and the number of sperm exhibiting denatured DNA.25 High-load endurance training promotes the appearance of muscle injury markers along with an increased white cell count in blood.26 During inflammation, additional circulating macrophages help testes-residing macrophages by arriving and invading the site of inflammation.27
Nonetheless, the number of round cells is elevated, with the presence not only of macrophages and other leucoytic cells but also a greater than normal presence of germinal cells. This fact may indicate a moderate alteration of spermatogenesis.
When analyzing the micronutrient intake with regards established recommendations,9,28,29 it can be observed that the athlete has a deficit in the intake of calcium, iodine and vitamin B12, as shown in Table 2. In addition, it can be observed there is a high consumption of Niacin, Riboflavin (Vitamin B2), Vitamin B6, Vitamin C, Folate and Vitamin E, Iron and Zinc.
The observed excess can lead to oxidative DNA damage in testicular and epididymal sperm, as well as a significant increase in 8-oxodG.30 Especially, an accumulation of certain minerals, such as iron and zinc, can produce damage in sperm and other reproduction-related cells, as well as degeneration of the germinal epithelium of the testes, multinucleated giant cell formation, and lack of mature sperm, along with a decrease in spermatogenesis and sperm motility with subsequent reduction of fertility.31,32
The intake and use of vitamin C and vitamin E (with different modes of action) as antioxidants should be carefully considered because, even though they have a protective effect on the polyunsaturated acids (PUFAs) present in the sperm membrane33 with a subsequent improvement in sperm function, in some cases (with the concurrent presence of metals such as Fe and Cu, etc.) these vitamins can act as potent prooxidants. In fact, it has been reported that continued Vitamin C supplementation in athletes may alter performance and elevate oxidative markers.34 In the present case, the athlete showed an elevated intake of both vitamin C and Iron and this imbalance may lead to a pro-oxidative state.
The macronutrients intake analysis reveals high protein intake and, to a lesser extent, of carbohydrates. High consumption of meat and dairy products has been associated with the incorporation of xenoestrogens, powerful endocrine disruptors, and thus to a deterioration in semen quality.35 Overall daily energy intake for this athlete is estimated at 4311.28kcal/day. Although this energy value is much higher than for normal individuals, it is adequate for a high-level athlete.
In the present case, it can be observed how training load, even if training volume is not high, promotes alterations in semen parameters and leucocytic profiles. Sperm DNA fragmentation, as well as morphology, are affected by high-intensity endurance exercise. Further studies are needed to clarify this fact and to seek for preventive measures in this specific population. Moreover, even though nutrition seems to be appropriate, despite slight excess and deficiency of some specific nutrients and a slight excess protein intake, this parameter needs to be further assessed with regards to the relation of exercise and fertility. Moreover, appropriate nutritional strategies should be assessed for avoiding the deleterious effects on the reproductive system that are associated to sports practice.
Financial supportGrant (IMD2010SC0001) from the Consejeria de Turismo, Comercio y Deporte de la Junta de Andalucia.
Conflict of interestThe authors declare that they have no conflict of interest.
Grant from the Consejeria de Turismo, Comercio y Deporte de la Junta de Andalucia.