Thorium dioxide in suspension (ThO2), known commercially as “Thorotrast®”, was, until the 1950s, considered an apparently harmless contrast medium without adverse effects. It was initially used in angiography and, later, due to its high X-ray absorption capacity, in practically all radiological studies. However, it is a radioactive element, which is deposited in the reticuloendothelial system, including the liver, spleen and lymph nodes, so these organs are exposed to ionising radiation throughout life.1,2
We present the complex case of Thorotrast® deposition in a 54-year-old female patient under follow-up by Internal Medicine and Gastroenterology. The patient's history included type 2 diabetes mellitus, high blood pressure, Sjögren’s syndrome and chronic autoimmune gastritis with vitamin B12 deficiency. No Thorotrast® deposition was found in the biopsies.
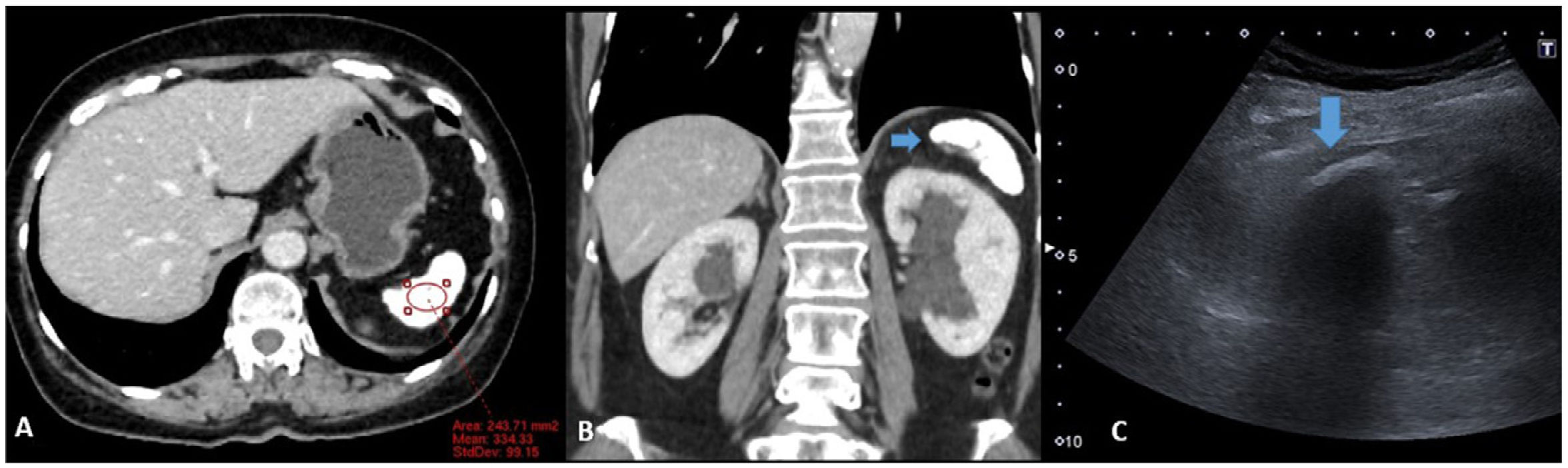
An intestinal MRI was performed due to diarrhoea and diffuse abdominal pain, which showed findings suggestive of Thorotrast® deposition in the spleen (Fig. 1), even though the patient denied having had contact with this contrast. CT of chest and abdomen was requested to look for other possible causes of the findings, but the additional studies ruled out other differential diagnoses. The patient is currently being followed up with ultrasounds due to the risk of developing radiation-induced cancer (Fig. 2).
A) T2-weighted axial sequence, showing the small, hypointense spleen (arrow). B) Axial TRUFI sequence (True Fisp) showing a concentric magnetic susceptibility artefact in the spleen (arrowhead). C) Diffusion study. ADC where the artefact produced by the spleen can be seen. D) Coronal T1 VIBE (Volume Interpolated Breath-hold Examination) sequence after contrast administration, again showing a magnetic susceptibility artefact in the spleen (arrowhead and arrow).
A) Axial contrast-enhanced CT with spleen ROI. Small, hyperdense spleen (334 HU), with punctate images of diffuse distribution. B) Coronal reconstruction where the small spleen is seen. C) Ultrasound focused on the left hypochondrium. Subcostal longitudinal section of the spleen. Hyperechoic linear image with posterior acoustic shadowing (arrow) corresponding to the spleen.
The thorium in Thorotrast® is an alpha and beta radiation emitter, with a biological half-life of 400 years, retained in the body if administered intravascularly.1,2 All this exposure to ionising radiation has been associated with a 100-fold increase in the risk of abdominal cancer and even vascular neoplasms such as angiosarcomas.1,3
Diagnosis of Thorotrast® deposition can be challenging, especially when the patient denies exposure. The differential diagnosis includes previous granulomatous infections, mineral deposition diseases such as iron overload, amiodarone deposition in cardiac patients, or gold deposition in those treated for rheumatoid arthritis. Glycogen storage diseases and exposure to cisplatin may also present similar findings.3 The possibility of other conditions, such as sickle cell disease, lupus erythematosus and autosplenectomy, was also considered in our patient.
A thorium density with Hounsfield units greater than 500 on CT can be key in the diagnosis.3 However, in our case, these attenuation values were not observed. In a study evaluating the performance of Thorotrast® on MRI, a marked decrease in signal intensity was observed on T1- and T2-weighted images. However, this study concluded that Thorotrast® deposition does not cause artifacts on MRI,4 which differs from our case. These discrepancies may be due to differences in magnetic field intensity (0.5 T vs 1.5 T), as there are currently sequences which are very sensitive to magnetic field heterogeneities.
Lastly, because radium-228 (the first product of thorium decay) is chemically similar to calcium,1 we believe that the ultrasound artefact of the spleen, with acoustic shadowing, could be due to this similarity between the elements. Although there are few scientific references on autoimmune disorders and Thorotrast®,5 given its mutagenic nature, it is conceivable that it played a role in the development of the patient’s autoimmune syndromes, despite the absence of Thorotrast® deposition in the biopsies.
Thorotrast® is a clear example of how an initially promising radiological contrast agent became a health risk and continues to affect patients who received it many years ago. The current generation of radiologists must therefore be able to recognise its imaging presentation for cases in which the exposure is not described in the patient's medical records.
Informed consentInformed consent was obtained from the patient.
Authorship/collaborators- 1
Responsible for the integrity of the study: AAM.
- 2
Study conception: AAM.
- 3
Study design: AAM and EPP.
- 4
Data collection: AAM.
- 5
Data analysis and interpretation: AAM.
- 6
Literature search: AAM and EPP.
- 7
Drafting of the article: AAM and EPP.
- 8
Critical review of the manuscript with intellectually relevant contributions: AAM, EPP, RLL and LSO.
- 9
Approval of the final version: AAM, EPP, RLL and LSO.
The authors declare that they have no conflicts of interest.