Various clinical and radiologic variables impact the neurologic prognosis of patients with ischemic cerebrovascular accidents. About 30% of ischemic cerebrovascular accidents are caused by proximal obstruction of the anterior circulation; in these cases, systemic thrombolysis is of limited usefulness. CT angiography is indicated in candidates for endovascular treatment. Various radiologic factors, including the grade of leptomeningeal collateral circulation, as well as the length, density, and extension of the thrombus, have been identified as predictors of neurologic prognosis after anterior ischemic cerebrovascular accidents due to proximal vascular obstruction. Final infarct volume correlations with mortality and long-term functional outcome in these patients. This study aimed to determine the best predictors of final infarct volume on CT angiography in patients with ischemic cerebral accidents due to proximal occlusion.
Materials and methodsThis retrospective observational study included adults with ischemic cerebrovascular accidents due to obstruction of the anterior circulation diagnosed by CT angiography in the period comprising June 2009 through December 2019. We measured the length and density of the thrombus in unenhanced CT images, and we used the clot burden score to record the grade of leptomeningeal collateral circulation and the extension of the thrombus. Then we measured the final infarct volume on follow-up CT and analyzed the correlations among these radiologic factors in the infarct volume.
ResultsWe included 54 patients [mean age, 82 y; 41 (75%) women] with ischemic cerebrovascular accidents due to proximal occlusion. About 60% of the cerebrovascular accidents affected the right cerebral hemisphere, and the most commonly affected vessel was the M1 segment of the medial cerebral artery (40.7%). Final infarct volume correlated with the grade of leptomeningeal collateral circulation (p=0.03) and with the clot burden score (p=0.01). Neither the length nor the density of the thrombus correlated with final infarct volume.
ConclusionThe final infarct volume can be estimated on the initial CT angiogram. Nevertheless, we found no useful predictive factors in unenhanced CT images. The best independent radiologic predictors of the final infarct volume are the grade of collateral circulation and the clot burden score, especially in patients who did not undergo mechanical thrombectomy, because mechanical thrombectomy improves outcomes. These factors are important for decision making in the management of patients with ischemic cerebrovascular accidents due to proximal occlusion.
Múltiples variables clínicas y radiológicas están involucradas en el pronóstico neurológico de los pacientes con accidente cerebrovascular (ACV) isquémico. Alrededor del 30% de los ACV isquémicos son causados por la obstrucción vascular proximal del circuito anterior; en estos casos, la utilidad de la trombólisis sistémica es limitada. La angiotomografía está indicada en los pacientes que pueden ser candidatos a tratamiento endovascular. Diferentes factores radiológicos como el grado de colaterales leptomeníngeas, o el largo, densidad o extensión del trombo, fueron descritos como predictores del pronóstico neurológico tras un ACV isquémico con compromiso vascular proximal. El volumen final del infarto cerebral se correlaciona con la mortalidad y el grado funcional a largo plazo de estos pacientes. El propósito de este estudio es determinar los mejores predictores radiológicos del volumen final del infarto cerebral en pacientes con ACV isquémico con compromiso proximal, utilizando angiotomografía.
Materiales y métodosRealizamos un estudio observacional retrospectivo. Incluimos pacientes adultos con ACV isquémico causado por la obstrucción de un vaso proximal, diagnosticados mediante angiotomografía en el período de junio de 2009 a diciembre de 2019. Medimos la densidad y el largo del trombo en la adquisición sin contraste, registramos el grado de colaterales leptomeníngeas y la extensión del trombo utilizando el clot burden score. Luego medimos el volumen final del infarto en una tomografía de control y analizamos el grado de correlación entre estos factores radiológicos en el volumen infartado.
ResultadosIncluimos 54 pacientes con ACV isquémico por compromiso vascular proximal; 41 (75%) fueron mujeres. La mediana de edad fue de 82 años. Alrededor del 60% de los ACV comprometieron el hemisferio derecho y el vaso más afectado fue el segmento M1 de la arteria cerebral media (40,7%). Encontramos una asociación entre el grado de colaterales leptomeníngeas (p=0,03) y el clot burden score (p=0,01) con el volumen final del infarto. Tanto el largo como la densidad del trombo no se correlacionaron con el volumen final del infarto.
ConclusiónEs posible estimar el volumen final infartado en la angiotomografía realizada al momento del evento isquémico. Sin embargo, no se hallaron factores predictivos útiles en la tomografía computarizada sin contraste. Los mejores predictores radiológicos del volumen final infartado como factores independientes son el grado de vasos colaterales y la escala de extensión del trombo o clot burden score, en especial en los pacientes que no fueron sometidos a trombectomía mecánica, ya que este tratamiento modifica positivamente la evolución natural de la enfermedad. Estos factores son de importancia para la toma de decisiones en el manejo de los pacientes con ACV isquémico con compromiso vascular proximal.
Cerebrovascular accident (CVA) or stroke continues to be a major health burden worldwide. It is the leading cause of disability and the second most common cause of death,1 as few patients are able to access treatment, largely because they are outside the therapeutic window. The leading cause of stroke is ischaemia, usually due to occlusion of a cerebral artery as a result of progressive atherosclerosis or an embolism from the heart or neck vessels.2
There are multiple clinical and radiological variables involved in the neurological prognosis of patients with ischaemic stroke.3 One of these is the location and extent of the occlusion, both of which are important factors in determining the likelihood of recanalisation with systemic thrombolytics.4 Proximal occlusions cause up to 30% of anterior circulation strokes5; in these cases, the usefulness of systemic thrombolysis is limited. Better functional prognoses have been described with thrombectomy compared to systemic thrombolysis in patients with an acute proximal vessel occlusion.6,7
Non-contrast computed tomography (CT) and CT-angiography are the methods of choice for indicating endovascular treatment in patients with proximal vessel occlusion,8 as they are accessible, fast and highly sensitive for ruling out bleeding.
It is estimated that following vascular occlusion, necrosis occurs at a rate of almost 1.9 million neurons per minute. However, some patients have been found to lose neurons at a faster rate and even early reperfusion may not provide benefits.9 Different radiological factors, such as the grade of collateral circulation,1,10–13 thrombus length4,14 and thrombus extent,11,15,16 have been analysed in an attempt to predict neurological prognosis after ischaemic stroke with proximal vessel involvement. However, other factors such as patient age,17 National Institutes of Health Stroke Scale (NIHSS) on admission18 and time to reperfusion19 may also affect the neurological prognosis of patients with ischaemic stroke.
Most published studies correlate imaging factors with the neurological prognosis of patients based on clinical scales. Final infarct volume (FIV) correlates with mortality and functional status three months after the ischaemic event.20 The aim of this study was to determine by non-contrast CT and CT-angiography the best predictors of cerebral FIV in patients with proximal cerebral vessel occlusion.
Materials and methodsThe study design was of a retrospective cohort of patients diagnosed with ischaemic stroke by diffusion magnetic resonance imaging (MRI) or CT-angiography who came to Hospital Italiano de Buenos Aires from June 2009 to December 2019.
Inclusion criteria were patients over 18 years of age with ischaemic stroke due to acute thrombosis of a proximal vessel in the anterior cerebral circulation, taking as proximal vessel the internal carotid artery, the A1 segment of the anterior cerebral artery and the M1 and M2 segments of the middle cerebral artery. They also needed to have CT-angiography images from the time of the event and follow-up data; either clinical outcome data or diffusion CT or MRI within 15 days post-event.
We excluded patients with images showing motion or metallic artefact or inadequate passage of contrast, patients with a transient ischaemic attack or a stroke involving perforating vessels or from a distal branch of the anterior circulation, or patients with haemorrhagic stroke.
Clinical dataDemographic data such as age and gender, NIHSS on admission and modified Rankin scale 90 days after the event were recorded in all cases. We also collected the information on whether or not the patient had undergone mechanical thrombectomy, which had been at the discretion of the team, consisting of a vascular neurologist and an interventional neuroradiologist.
Computed tomography angiography protocolThe CT-angiograms were performed on a 64-detector-row Aquilion (Toshiba, Japan) and a 320-detector-row Aquilion One (Toshiba, Japan). Non-contrast and contrast-enhanced phases were acquired using the following parameters: 0.5mm slices every 0.3mm, 300mAs, 120kV, 0.5s rotation and a pitch of 0.641. A field of view of approximately 350×220mm was used. The non-contrast phase was acquired in the caudocranial direction, while the CT-angiogram was acquired in the craniocaudal direction. Non-ionic contrast (Iobitridol, Xenetix® 350; Guerbet, France) was used in the contrast phase at a dose of 1ml/kg using an automatic injection pump, with a flow rate of 4ml/s. The images were then subtracted and all the volumes were sent to the Vitrea 2 workstation (Vital Images, Inc, Minnesota, USA) for evaluation. Multiplanar reconstructions with maximum intensity projection (MIP) and 3-D render and MIP reconstructions were performed.
Imaging analysisWe reviewed the intracranial vessel CT-angiograms, which were performed for care purposes in patients with an acute neurological focus, to assess the presence of acute brain lesions. We evaluated the characteristics of the filling defect in the cerebral vessels of the patients included. All images were retrospectively reviewed by a specialist in training and by a neuroradiologist with eight years of experience. We recorded the length of the thrombus measured in millimetres, the density of the thrombus in the non-contrast phase in Hounsfield units, the grade of collateral circulation18,20 and lastly, the degree of occlusion using the clot burden score (CBS).15 To measure the density of the thrombus, the window was first adjusted to W=40L=40 and a region of interest (ROI) was placed in the densest portion of the thrombus according to the perception of the observer.
The grade of collateral circulation was estimated according to the Boulouis et al. classification18 into four groups:
- •
Grade 1: no collaterals or less than 50% of the middle cerebral artery (MCA) territory.
- •
Grade 2: collateral vessels smaller than on the healthy side but more than 50% of the MCA territory.
- •
Grade 3: collateral vessels equal to the healthy side.
- •
Grade 4: collateral vessels larger than on the healthy side or prominent.
The CBS scale has a maximum of 10 points and subtracts points according to the occluded vessel(s) as follows:
- •
1pt cervical internal carotid.
- •
2pt supraclinoid internal carotid.
- •
1pt A1.
- •
2pts proximal M1.
- •
2pts distal M1.
- •
1pt superior branch M2.
- •
1pt inferior branch M2.
The variables were adjusted into two groups according to whether patients had mechanical thrombectomy or not. In the control studies, patients were identified for haemorrhagic transformation and FIV was measured using IntelliSpace Portal PHILIPS® software. Manual segmentation of the FIV was performed using the diffusion hyperintensity on magnetic resonance imaging (MRI) and hypodensity on follow-up CT as the reference limit.
Study procedureWe carried out an exhaustive search of secondary hospital databases to recruit the cases to be included, using various key words in order to find all the cases. The hospital's electronic medical records have multiple structured fields coded with SNOMED CT (Systematized Nomenclature of Medicine - Clinical Terms) terminology, which makes it possible to conduct multiple searches and has an information management area for research.
The scans were evaluated in a standardised manner by two operators, blinded to the previous report on the imaging study.
With regard to sample size calculation, in order to evaluate predictors in CT and CTangiography of final infarct volume as a continuous variable, the sample size was calculated for a mean difference between two independent samples of at least 25ml (considering a difference of 75–100ml, with a power of 80% and a confidence level of 95%, we obtained an estimate of 12 patients). For a model with four or five predictor variables, the estimated n was between 48 and 60 patients.
Statistical analysisQuantitative variables are described as mean with standard deviation (SD) or median with interquartile range (IQR) according to their distribution. Qualitative variables are described as proportions (absolute frequencies) and percentages (relative frequencies) with corresponding 95% confidence intervals. Quantitative variables were compared with Student's t or Mann–Whitney tests according to distribution. Categorical variables were assessed with χ2 or Fisher tests according to assumptions.
The linear correlation (Spearman's coefficient) between the final infarct volume and the different predictor variables was assessed by univariate analysis. Statistically significant univariate variables were included in a multiple linear regression model to assess the association with final infarct volume.
The CBS analysis was dichotomised into two groups: one with scores from 0 to 6, and one with scores from 7 to 10.
A subgroup analysis was performed by dividing patients according to whether or not mechanical thrombectomy was performed.
Statistical significance was set at p<0.05. STATA 13 software was used to perform the analysis.
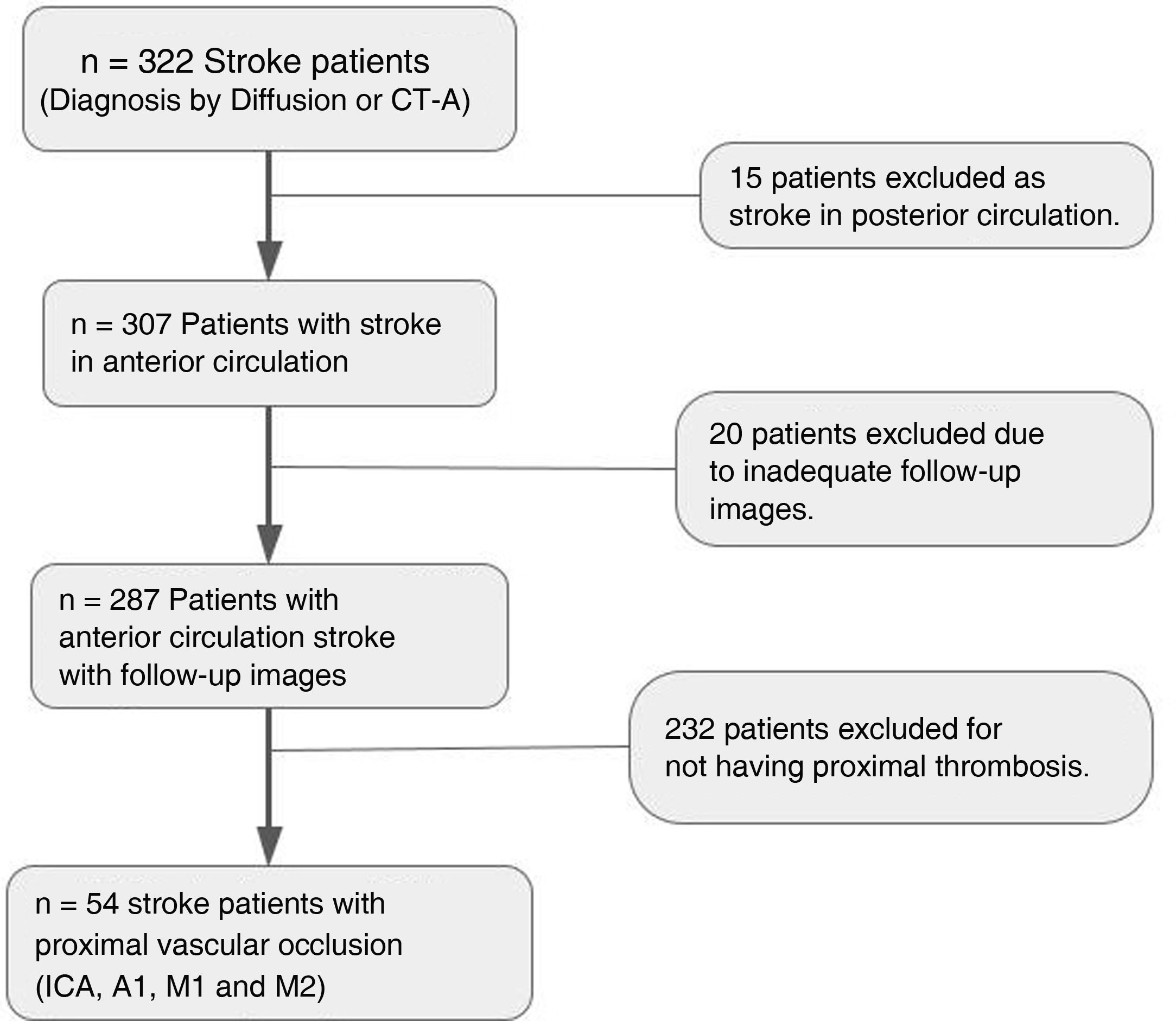
ResultsThe initial sample consisted of 322 patients diagnosed with ischaemic stroke in the defined period and who met all inclusion criteria. We excluded 232 patients in whom no thrombosis was found at the time of the event, 15 patients who had ischaemia in the posterior circulation and 20 patients who were not followed up at our institution and did not have repeat scans after the event. There were eight patients who died within 48h of suffering the stroke, so no imaging follow-up was performed. An overall analysis of the patients between predictors and clinical outcome as measured by the modified Rankin scale and an analysis of correlation with the FIV was therefore performed excluding those patients. This is summarised in the patient inclusion/exclusion flowchart (Fig. 1).
We included 54 patients who had suffered ischaemic stroke due to proximal vessel occlusion and had angiographic studies available for evaluation. Of the total number of patients, 41 (75%) were female. The average age was 82 (74–87). The initial NIHSS was 15.5 (11–20). The time from symptom onset to imaging averaged 160min (90-240min).
Approximately 60% of the events involved the right side, and the vessel most affected was the M1 segment of the MCA (40.7%). One patient had total occlusion of both internal carotid arteries. In terms of collateral circulation, 75.9% of patients had a low grade, 44.4% had no collaterals and 31.5% had less collateral circulation compared to the healthy side. Only three patients had more prominent collateral circulation on the affected side than the healthy side. Mechanical thrombectomy was performed in 30 (55%) of the patients in the series, with a thrombolysis in cerebral infarction (TICI) score of no less than 2b. The imaging findings are summarised in Table 1.
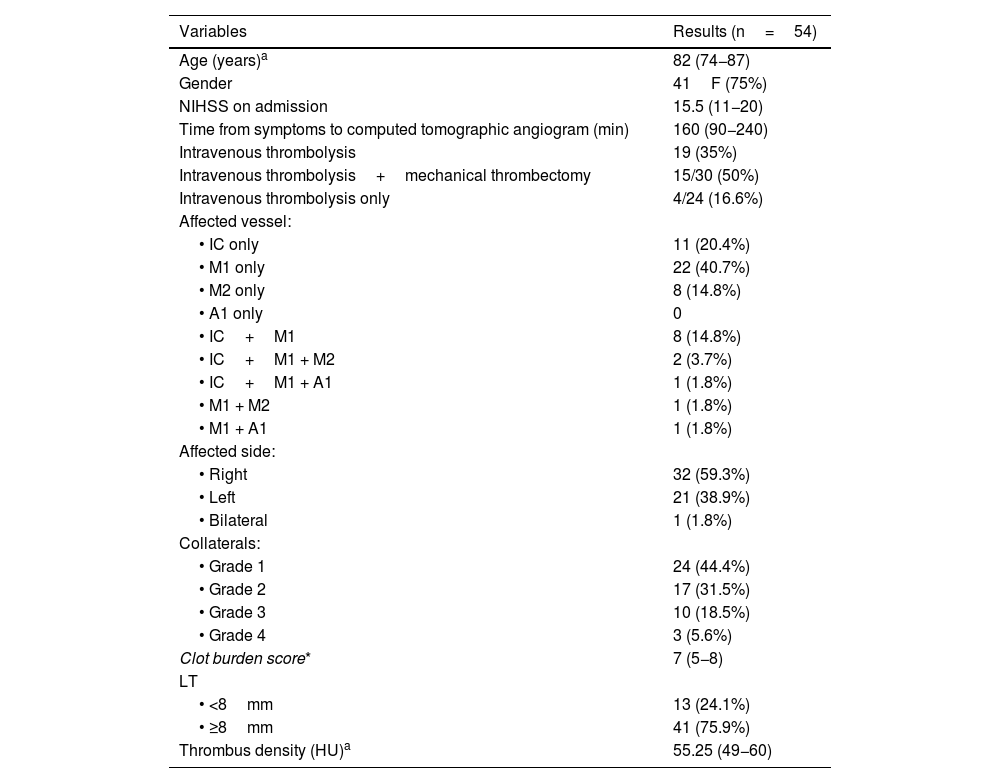
Demographic and imaging variables.
| Variables | Results (n=54) |
|---|---|
| Age (years)a | 82 (74−87) |
| Gender | 41F (75%) |
| NIHSS on admission | 15.5 (11−20) |
| Time from symptoms to computed tomographic angiogram (min) | 160 (90−240) |
| Intravenous thrombolysis | 19 (35%) |
| Intravenous thrombolysis+mechanical thrombectomy | 15/30 (50%) |
| Intravenous thrombolysis only | 4/24 (16.6%) |
| Affected vessel: | |
| • IC only | 11 (20.4%) |
| • M1 only | 22 (40.7%) |
| • M2 only | 8 (14.8%) |
| • A1 only | 0 |
| • IC+M1 | 8 (14.8%) |
| • IC+M1 + M2 | 2 (3.7%) |
| • IC+M1 + A1 | 1 (1.8%) |
| • M1 + M2 | 1 (1.8%) |
| • M1 + A1 | 1 (1.8%) |
| Affected side: | |
| • Right | 32 (59.3%) |
| • Left | 21 (38.9%) |
| • Bilateral | 1 (1.8%) |
| Collaterals: | |
| • Grade 1 | 24 (44.4%) |
| • Grade 2 | 17 (31.5%) |
| • Grade 3 | 10 (18.5%) |
| • Grade 4 | 3 (5.6%) |
| Clot burden score* | 7 (5−8) |
| LT | |
| • <8mm | 13 (24.1%) |
| • ≥8mm | 41 (75.9%) |
| Thrombus density (HU)a | 55.25 (49−60) |
A1, anterior cerebral artery segment; CBS, clot burden score; IC, internal carotid artery; LT, length of thrombus; M1, M2, middle cerebral artery segments; NIHSS, National Institutes of Health Stroke Scale.
bAccording to the Boulouis et al. classification.18.
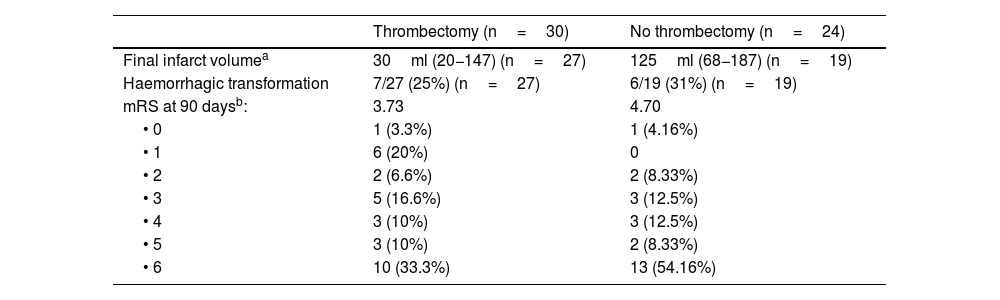
Fifteen percent of the patients did not have a follow-up study because they died before it could be performed; 46 (85%) patients did have follow-up imaging. Ninety-five per cent of the imaging tests were performed by non-contrast CT, the rest by diffusion MRI. The median time to follow-up imaging was three days (IQR=1–5). The FIV was a median of 30ml in the thrombectomy group vs. 125ml in the no-thrombectomy group. The remaining follow-up variables are shown in Table 2.
Follow-up.
| Thrombectomy (n=30) | No thrombectomy (n=24) | |
|---|---|---|
| Final infarct volumea | 30ml (20−147) (n=27) | 125ml (68−187) (n=19) |
| Haemorrhagic transformation | 7/27 (25%) (n=27) | 6/19 (31%) (n=19) |
| mRS at 90 daysb: | 3.73 | 4.70 |
| • 0 | 1 (3.3%) | 1 (4.16%) |
| • 1 | 6 (20%) | 0 |
| • 2 | 2 (6.6%) | 2 (8.33%) |
| • 3 | 5 (16.6%) | 3 (12.5%) |
| • 4 | 3 (10%) | 3 (12.5%) |
| • 5 | 3 (10%) | 2 (8.33%) |
| • 6 | 10 (33.3%) | 13 (54.16%) |
mRS, modified Rankin Scale; FIV, final infarct volume.
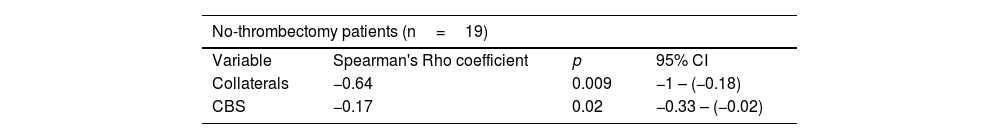
Multiple linear regression was performed and a statistically significant correlation was found between FIV and collateral circulation (p=0.009) (Fig. 2). This association only held in the group that did not have mechanical thrombectomy (p=0.009 vs. p=0.5 in patients with thrombectomy). An inverse linear correlation was found between the CBS scale and FIV in the subgroup of patients who did not have thrombectomy (p=0.02), whereby the larger the thrombus, the lower the score on the scale and the larger the infarct volume in the follow-up scan (Table 3, Fig. 3). After dichotomisation, patients with high CBS scores showed lower FIV; 87ml (63–132) (median and IQR) in contrast to 187ml (121–260) in patients with low CBS scores. However, in our series this difference was not statistically significant (p=0.069). This difference is lost in the group of patients who were treated by mechanical thrombectomy.
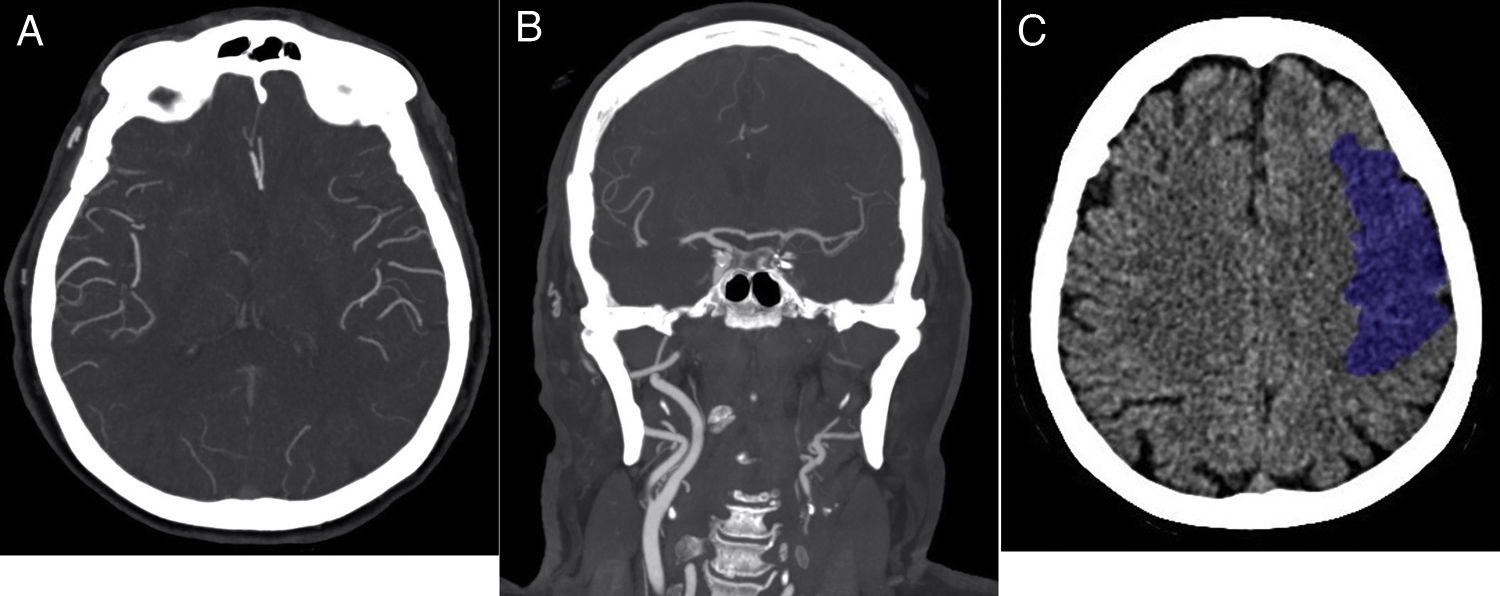
80-year-old female patient with a history of hypertension, type II diabetes, dyslipidaemia and atrial fibrillation, NIHSS on admission 19 points. She consulted with sudden onset right brachial paresis and dysarthria. This patient did not have mechanical thrombectomy. (A) CT-angiogram of the brain, axial slice, maximum intensity projection (MIP), 120min after the onset of symptoms, showing absence of brain parenchyma enhancement in the territory of the left middle cerebral artery. The leptomeningeal collateral branches have similar lumen and post-contrast filling as those on the healthy side (grade 3). (B) MIP coronal slice CT-angiogram of the brain showing absence of opacification of the left internal carotid artery in its cervical tract (−1 point) and in its supraclinoid portion (−2 points). Clot burden score=7 points. (C) Non-contrast CT scan of the brain 48h after symptom onset showed a left frontal hypodense lesion. Volumetric measurement of the infarcted brain parenchyma measuring approximately 75 cc.
Multiple linear regression to evaluate the association between clot burden score and collaterals with respect to final infarct volume, according to whether or not thrombectomy performed.
| No-thrombectomy patients (n=19) | |||
|---|---|---|---|
| Variable | Spearman's Rho coefficient | p | 95% CI |
| Collaterals | −0.64 | 0.009 | −1 – (−0.18) |
| CBS | −0.17 | 0.02 | −0.33 – (−0.02) |
| Thrombectomy patients (n=27) | |||
|---|---|---|---|
| Variable | Spearman's Rho coefficient | p | 95% CI |
| Collaterals | 0.16 | 0.5 | −0.33–0.65 |
| CBS | 0.04 | 0.7 | −0.28–0.19 |
CBS, clot burden score; Collaterals, leptomeningeal collateral circulation.
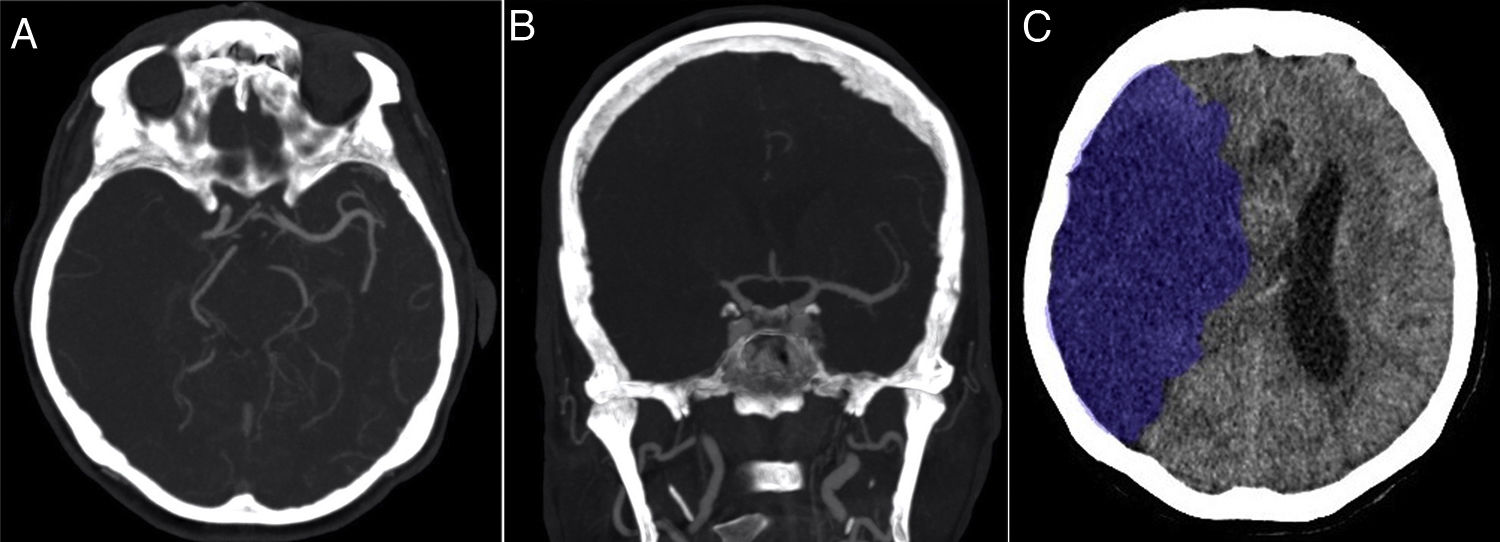
79-year-old female patient with a history of hypertension. She consulted with sudden onset of severe left hemiparesis and dysarthria. National Institutes of Health Stroke Scale (NIHSS) on admission: 16 points. This patient did not have mechanical thrombectomy. (A) Axial MIP CT-angiogram of the brain 60min after the onset of symptoms showing absence of brain parenchyma enhancement in the territory of the right middle cerebral artery. The leptomeningeal collateral branches are markedly diminished compared to the healthy contralateral territory (grade 1). (B) Coronal slice from computed tomographic angiography of the brain, maximum intensity projection (MIP), showing absence of opacification of the right middle cerebral artery in both the proximal (−2 points) and distal (−2 points) portions of the M1 segment, as well as both M2 branches (−2 points). Clot burden score=4 points. (C) Non-contrast computed tomography of the brain 24h after symptom onset showed an extensive right hemisphere hypodense lesion. Volumetric measurement of infarcted brain parenchyma measuring approximately 346 cc.
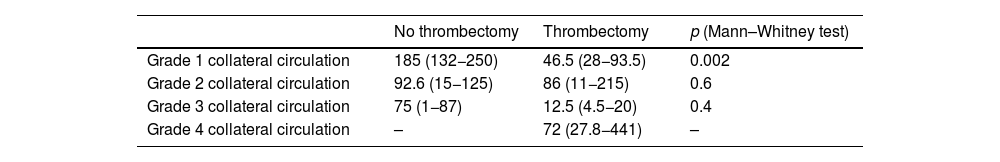
The median FIV was analysed according to the grade of collateral circulation and divided according to whether or not mechanical thrombectomy was performed. The highest FIV were found in patients with the lowest grade of collateral circulation. A lower FIV was found in patients who had mechanical thrombectomy, but this difference was only statistically significant in patients with grade 1 collateral circulation (p=0.002) (Table 4).
Association between median final infarct volume (ml) by grade of collateral circulation, according to whether or not mechanical thrombectomy performed.
| No thrombectomy | Thrombectomy | p (Mann–Whitney test) | |
|---|---|---|---|
| Grade 1 collateral circulation | 185 (132−250) | 46.5 (28−93.5) | 0.002 |
| Grade 2 collateral circulation | 92.6 (15−125) | 86 (11−215) | 0.6 |
| Grade 3 collateral circulation | 75 (1−87) | 12.5 (4.5−20) | 0.4 |
| Grade 4 collateral circulation | – | 72 (27.8−441) | – |
However, there was no correlation between FIV and thrombus length in either the thrombectomy group (p=0.98) or the no-thrombectomy group (p=0.17). There was also no correlation between FIV and thrombus density in either the thrombectomy group (p=0.86) or the no-thrombectomy group (p=0.68).
DiscussionWe assessed radiological factors which might predict FIV after ischaemic stroke with proximal vascular involvement. We found as predictors of FIV the grade of collateral circulation and the length of thrombus assessed by the CBS (Spearman's Rho = −0.17) (p=0.02), the correlation with collateral circulation being stronger (Spearman's Rho = −0.64) (p=0.009). This correlation is lost in the group of patients undergoing mechanical thrombectomy, as this influences FIV. Mechanical thrombectomy indicated in patients with a small infarct core modifies the course of the disease, so the association between the predictors and FIV is not significant in this group.
There is a correlation between the degree of leptomeningeal collateral circulation visualised in the study at the time of the ischaemic event and the FIV, whereby less presence of collateral circulation means greater FIV. The patients who benefited most from mechanical thrombectomy were those with no or poor collateral circulation; in this group there were statistically significant differences in FIV compared to patients who did not have thrombectomy (p=0.002). Patients with a good grade of collateral circulation have lower FIV. This may be explained by the fact that a good grade of collateral circulation is associated with a larger area of ischaemic penumbra limiting the size of the infarct core until reperfusion is possible.12 In addition, these patients have better recanalisation rates after thrombolytics and this could be related to the arrival of the drug at both sides of the thrombus. The grade of collateral circulation is also associated with functional prognosis at 90 days, with the average modified Rankin Scale (mRS) score being lower in patients with better collateral circulation. In line with Menon et al.,10 the percentage of patients with good functional outcome is low in patients with poor collateral circulation, as poor collateral circulation is associated with a larger infarct core and proportionally smaller penumbra area, so mechanical thrombectomy is not recommended in these cases. We found that only 8.3% of patients with poor collateral circulation had a 90-day mRS scale of 0 or 1. The time since onset of the cerebral ischaemia-infarction process in relation to the grade of collateral circulation and other factors such as age, baseline NIHSS and other cardiovascular risk factors was different in each patient. The grade of collateral circulation correlates with the FIV and with long-term neurological prognosis.10,18,21,22
According to Treurniet et al.,16 the thrombus length measured by the CBS is an independent predictor of the patient's neurological prognosis regardless of the treatment given. In line with other authors,11,15 after adjusting the analysis for patients who did and did not have mechanical thrombectomy, we found that a low CBS score was associated with a lower recanalisation rate and higher FIV in the group of patients who did not undergo mechanical thrombectomy. Patients with high CBS scores had lower FIV.
An association between the hyperdense artery sign on CT and thrombus histology has been reported, and spontaneously dense thrombi were found to have a higher red blood cell component (47% vs. 22%). The absence of this sign was correlated with fibrin-rich thrombi.23 According to Moftakhar et al.,24 less dense thrombi are more resistant to both thrombolytic therapies and mechanical thrombectomy due to their high platelet and fibrin content, whereas patients with the hyperdense artery sign have thrombi with a higher proportion of erythrocytes and a higher recanalisation rate. Mokin et al.25 reported thrombus density measured in Hounsfield units as a powerful predictor of recanalisation using the Solitaire stent. However, other authors reported that the absence or presence of the hyperdense artery sign did not affect the recanalisation rate when using stent-retriever or aspiration thrombectomy.17,26 It would be expected that the higher the recanalisation rate, the larger the area of penumbra which can be salvaged and the lower the FIV, such that thrombus density may have an indirect predictive value for FIV. However, there remains a lack of consensus in this area relating to the material used to perform the thrombectomy. In our series, we found no statistically significant association between thrombus density and FIV whether or not patients had mechanical thrombectomy.
There was also no statistically significant association between thrombus length and FIV. In extensive thrombi, measurement was difficult and poorly reproducible at the time of emergency decision making, so we consider this parameter of little use in predicting FIV. Such measurement may be important in small thrombi less than 8mm long, where measurement is quicker, but a very low reperfusion rate has been reported with intravenous thrombolysis for thrombi longer than 8mm.14,19 In these cases, in the absence of contraindications, mechanical thrombectomy is of greater value.
Our study has several limitations. Firstly, being a retrospective study, the information was obtained from the electronic medical records and the images stored in the PACS. In addition, being a single-centre study, we did not have sufficient power for a subgroup analysis.
ConclusionsIt is possible to estimate the final infarct volume on CT-angiograms performed at the time of the ischaemic event. However, no useful predictive factors were found on non-contrast CT. The best radiological predictors of final infarct volume as independent factors are the grade of collateral vessels and the scale of thrombus extension or CBS, particularly in patients who did not have mechanical thrombectomy, as this treatment positively modifies the natural course of the disease. These factors are important for decision making in the management of patients with ischaemic stroke and proximal vascular involvement.
Authorship- 1
Responsible for the integrity of the study:
- 2
Study conception: MJR, AG and JSS.
- 3
Study design: MJR.
- 4
Data collection: MJR, AG and JSS.
- 5
Data analysis and interpretation: MJR, AG and JSS.
- 6
Statistical processing:
- 7
Literature search:
- 8
Drafting of the article:
- 9
Critical review of the manuscript with intellectually relevant contributions: LDN and MPA.
- 10
Approval of the final version: CB.
The authors declare that they have no conflicts of interest.