Cryoablation is a percutaneous thermal ablation technique developed to destroy focal groups of cells using extreme cold. It is an outpatient and image-guided procedure, performed under local anaesthesia, with typically short recovery times and good outcomes. Percutaneous cryoablation has good success rates for treating breast fibroadenomas. It is used in cases of malignant tumours mainly when a patient refuses surgery or is not considered to be a suitable candidate. Some studies indicate that cryoablation could be as effective and safe as lumpectomy in cases of low-risk early-stage breast cancer. Moreover, some studies have reported a regression of metastatic foci after ablation of a primary tumour thanks to an anti-tumour immune response stimulated by cryoablation. This article describes the technique and its indications with regards to breast tumours according to the existing literature.
La crioablación es una técnica de ablación térmica, que provoca destrucción celular gracias a los efectos del frío. Es un procedimiento que se realiza guiado por imagen, de forma ambulatoria y con anestesia local. Es sencillo, seguro y bien tolerado. Ha demostrado su eficacia en el manejo de los fibroadenomas. Se emplea en el tratamiento del cáncer de mama en mujeres que rechazan o no son candidatas a cirugía. Según el resultado de los estudios publicados, en un futuro próximo, podría ser una alternativa a la cirugía en el cáncer de mama precoz y de bajo riesgo. Asimismo, se ha demostrado que la crioablación estimula la respuesta inmune antitumoral, por lo que podría tener también efecto sistémico. El objetivo de este artículo es describir la técnica de la crioablación en las lesiones de la mama y revisar sus indicaciones de acuerdo con la evidencia científica disponible.
Breast cancer is the most common cancer diagnosed in women worldwide. Over the years, surgical management has evolved and less invasive strategies have been sought. Following this trend, thermal ablation techniques emerged as an alternative to surgery. These procedures destroy the viable cells of a previously selected tissue using cold or heat. One of the most studied and used in the breast is cryoablation, also called cryosurgery.
History of cryoablationThe use of cold for therapeutic purposes, known as cryotherapy, began in ancient times. Cryoablation is a form of cryotherapy, which uses sub-zero temperatures to achieve tissue necrosis.
James Arnott (1797–1883) is recognised as the first physician to use extreme cold to cause tissue destruction. He used a mixture of saline solutions and crushed ice to obtain temperatures as low as −24°C to treat advanced cancers in accessible sites, including breast and cervical tumours, with the aim of reducing pain and bleeding.1 He also hypothesised that cold was probably capable of curing neoplasms at early stages. However, to make this possible, advances in technology were necessary, in particular the development of better cryogenic agents. At the end of the 19th century, Campbell White in the United States was the first to perform cryotherapy with liquefied gases, which allowed very low temperatures to be reached. Until the mid-20th century, there was debate as to whether air, oxygen or CO2 was the most effective, and its use was limited to skin lesions. Liquid nitrogen began to be used in the middle of the last century and the first cryoprobe was developed in the United States in 1961, which made it possible to treat deeper lesions. Over the following years, cryosurgery experienced rapid growth both in its use and indications, including prostate, brain, liver, head and neck, bone and heart. Other cryogenic agents, such as argon, were also tested. In the late 1980s, cryoablation was an accepted treatment in specialities such as dermatology and gynaecology, but was replaced by surgery in many others. At the end of the 20th century, interest in this procedure experienced something of a revival with the development of ultrasound to monitor the freezing process and the improvement in cryosurgery equipment.
Cryoablation was the first ablation technique used for breast cancer. The first experimental procedure on a resectable breast tumour was performed by Rand et al. in 1985.
Mechanism of actionCryoablation causes tissue necrosis by the direct mechanism of cold, and by indirect mechanisms, causing changes in the cellular microenvironment that affect tissue viability.
The procedure consists of freeze-thaw cycles. During freezing, intracellular crystals form and destroy membranes and organelles, the growth of which may continue during thawing, exacerbating cell damage. This phenomenon occurs adjacent to the cryoprobe and when cooling is very rapid or at very low temperatures. The formation of extracellular ice, which occurs when the temperature decreases more slowly, results in the sequestration of free water, increasing the osmolarity of the extracellular space, with dehydration of the cells. During thawing, extracellular ice melts before intracellular ice, creating an osmotic imbalance that causes the cells to burst.2
Cells that are not destroyed by the direct effect of the cold die by apoptosis, a phenomenon usually seen in the periphery of the frozen area, which is exposed to temperatures that are not immediately lethal but do cause non-recoverable cell damage.2
Frostbite causes tissue ischaemia due to thrombosis of the blood vessels, complicating repair. Inflammatory cells, including macrophages and neutrophils, clear cell debris.
This process can continue for weeks or months after ablation, ending in an area of coagulation necrosis surrounded by neutrophils.2
Cells in most tissues will necrotise if rapidly cooled below −40 to −60°C. The effectiveness of cryoablation depends on the speed, duration and number of freeze-thaw cycles. Necrosis will be more effective the lower the temperature and the faster it is reached and if thawing is passive and slow. According to current knowledge, the use of at least two cycles of fast freezing and slow thawing is effective.2
Cryoablation systemsArgon and nitrogen are the most commonly-used cryogenic gases. In equipment using argon, freezing at the tip of the probe occurs by decompression of the gas, according to the Joule-Thomson effect. It has 12–17G cryoprobes and several can be used at the same time.
Liquid nitrogen is cheaper and easier to store than argon. In equipment using nitrogen, the cryoablation probe freezes the tumours simply because the gas circulates in a closed circuit. As the nitrogen cools the tissue at the tip of the cryoprobe, heat absorption by the lesion causes the cryogenic agent to change from liquid to gas. This system uses 13G and 10G needles. They are insulated to protect healthy tissue and to keep temperatures below freezing, except at their tip, to allow rapid freezing of the selected tissue. Freezing volumes of up to 6.5cm can be achieved with a single needle.
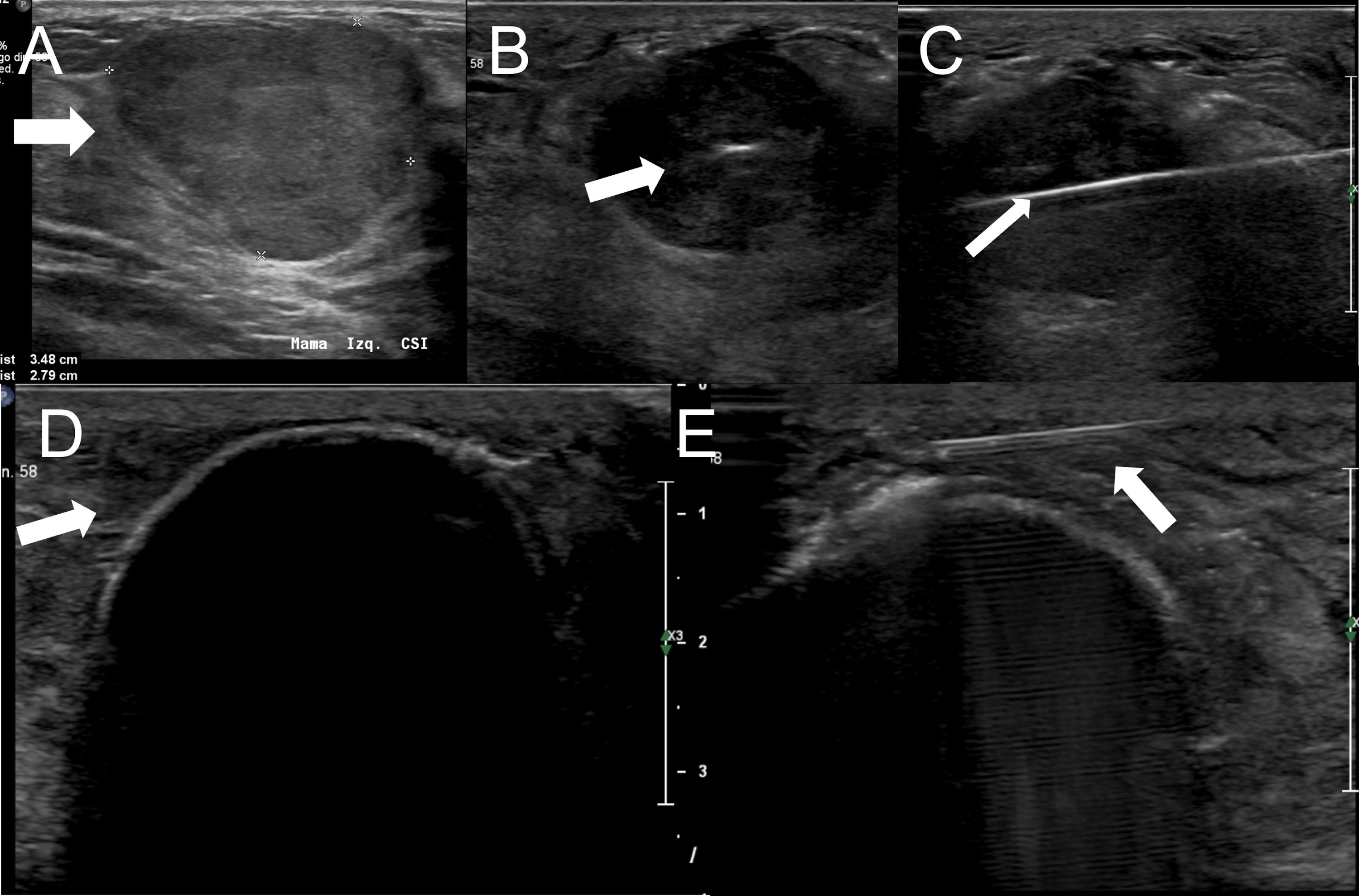
TechniqueCryoablation is an image-guided outpatient procedure; in the breast, ultrasound is typically used. It requires only local anaesthesia on the skin and around the lesion to be treated, just as when a nodule is removed percutaneously under vacuum. An incision of about 4mm is made in the skin and the cryoprobe is inserted through the long axis of the tumour; it is important for it to be positioned in the centre of the tumour on both the longitudinal and transverse axes. As mentioned above, there are two freezing cycles, separated by a thawing cycle, which must be of equal duration. This will depend on the size of the target lesion. In benign lesions the aim is to achieve a lethal zone of the same size as the nodule. However, in malignant tumours, a safety margin, typically measuring 1cm, is necessary. It is important to note that the lethal zone will be smaller than the freezing volume reached. With ultrasound it is possible to precisely monitor the entire process. The ice is seen as an echogenic line between treated and untreated tissue (Fig. 1). Cycle duration will vary from two to 15min and can be decided at the beginning of the procedure, depending on the size of the tumour and the manufacturer's recommendations, which provide information about the dimensions of the freezing volume achieved according to time. Another option is to work manually, which involves measuring the longitudinal and transverse diameters of the ice ball several times during the first cycle and stopping when the desired size is reached. At the end of the procedure, the system automatically heats the tip of the cryoprobe so that it can be removed from the breast.
Fibroadenoma measuring 3.5cm (arrow in A). The cryoprobe is placed in the centre of the mass, along its longer axis (arrow in B and C). The boundary between treated and untreated tissue is seen as an echogenic border with posterior shadowing (arrow in D). If the ice gets close to the skin surface, hydrodissection can be performed by injecting warm saline between the freezing volume and the skin to avoid burns (arrow in E).
Undesirable effects are mild and uncommon. The cold has an analgesic and vasoconstrictor effect, so pain and bleeding are rare. As with any interventional procedure, there is a small chance of infection. A discolouration of the skin superficial to the treated area (tattoo) may appear, which disappears spontaneously. If the ice reaches the fascia of the pectoral muscles, there may be pain when moving the arm for a few days. If the freezing volume reaches the skin, a burn will appear, usually mild, which can be avoided by increasing the distance between the ice and the skin surface by injecting warm saline during the procedure and placing warm towels on the skin after the procedure is completed.3
IndicationsThe American Society of Breast Surgeons (ASBrS) considers cryoablation indicated for the treatment of fibroadenomas up to 4cm in largest diameter.4 Several articles have been published demonstrating that cold ablation achieves volume reduction with a very low rate of mild complications and no major adverse effects.5 At present, there are no studies in the literature evaluating its use in lesions of uncertain malignant potential (B3).
For malignant breast tumours, we now use percutaneous treatment in patients who do not want or are not candidates for surgery, mainly older patients. Cazzato et al.6 examined the use of cold ablation to treat breast cancer in this group of women. Twenty-three tumours measuring 3cm or less in size were included. Median follow-up was 14.6 months and local disease control was achieved in 78% of patients (18/23). In patients who refuse surgery or are considered to be at high surgical risk, percutaneous treatment would be curative or palliative, depending on the size of the lesion. According to the results of a systematic review published in 2022,7 currently available percutaneous techniques could be an alternative to surgery for the treatment of tumours up to 2cm in size and their efficacy is questionable for tumours of 3cm or less in size. There is insufficient evidence for larger lesions, but the authors suggest that percutaneous management could be useful for local control of unresectable cancers.
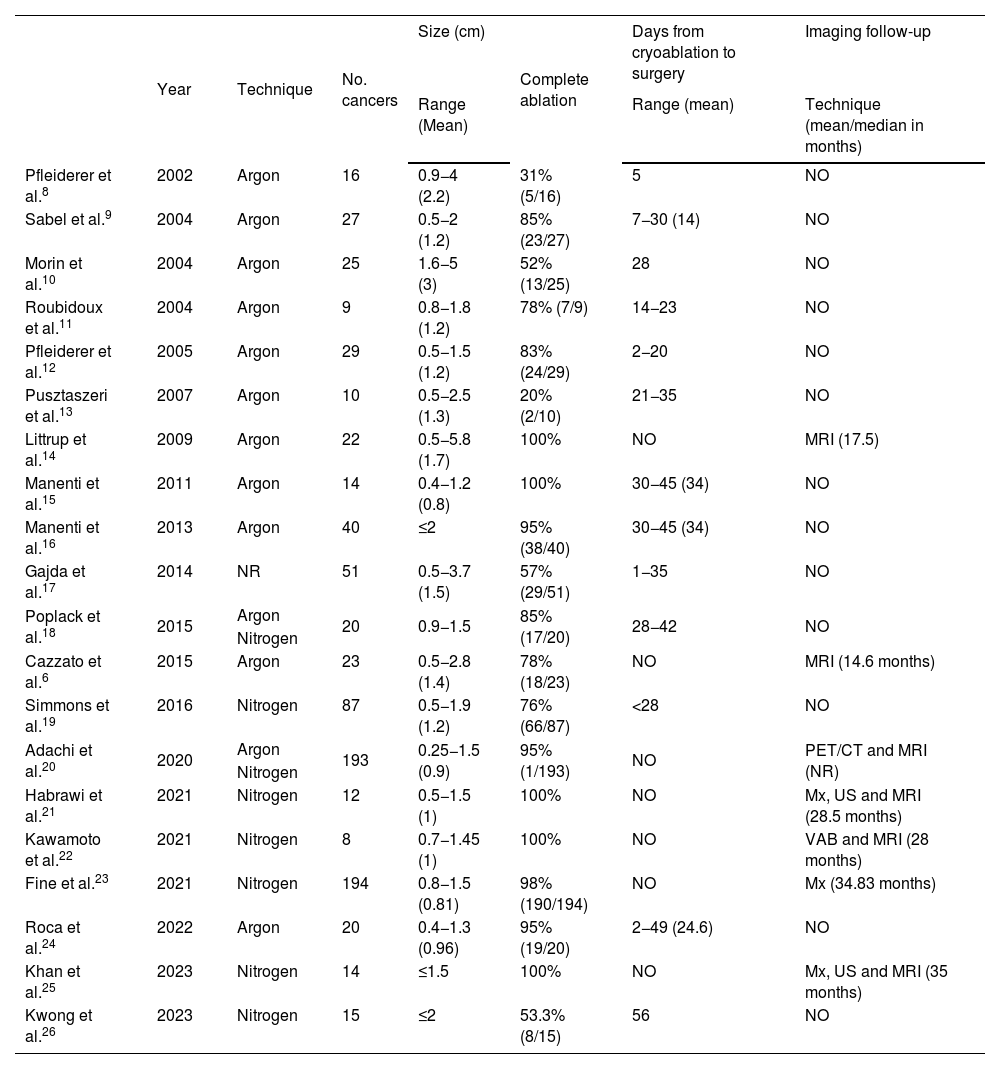
There are a number of articles in the literature evaluating the efficacy of cryoablation as a substitute for surgery in breast cancer6–26 (Table 1). These studies are heterogeneous in terms of tumour size, time, follow-up and adjuvant therapy (radiotherapy, hormone therapy). Moreover, the sample size in the majority is small.
Characteristics of the main studies published on the efficacy of cryoablation in the treatment of breast cancer.
| Year | Technique | No. cancers | Size (cm) | Complete ablation | Days from cryoablation to surgery | Imaging follow-up | |
|---|---|---|---|---|---|---|---|
| Range (Mean) | Range (mean) | Technique (mean/median in months) | |||||
| Pfleiderer et al.8 | 2002 | Argon | 16 | 0.9−4 (2.2) | 31% (5/16) | 5 | NO |
| Sabel et al.9 | 2004 | Argon | 27 | 0.5−2 (1.2) | 85% (23/27) | 7−30 (14) | NO |
| Morin et al.10 | 2004 | Argon | 25 | 1.6−5 (3) | 52% (13/25) | 28 | NO |
| Roubidoux et al.11 | 2004 | Argon | 9 | 0.8−1.8 (1.2) | 78% (7/9) | 14−23 | NO |
| Pfleiderer et al.12 | 2005 | Argon | 29 | 0.5−1.5 (1.2) | 83% (24/29) | 2−20 | NO |
| Pusztaszeri et al.13 | 2007 | Argon | 10 | 0.5−2.5 (1.3) | 20% (2/10) | 21−35 | NO |
| Littrup et al.14 | 2009 | Argon | 22 | 0.5−5.8 (1.7) | 100% | NO | MRI (17.5) |
| Manenti et al.15 | 2011 | Argon | 14 | 0.4−1.2 (0.8) | 100% | 30−45 (34) | NO |
| Manenti et al.16 | 2013 | Argon | 40 | ≤2 | 95% (38/40) | 30−45 (34) | NO |
| Gajda et al.17 | 2014 | NR | 51 | 0.5−3.7 (1.5) | 57% (29/51) | 1−35 | NO |
| Poplack et al.18 | 2015 | Argon | 20 | 0.9−1.5 | 85% (17/20) | 28−42 | NO |
| Nitrogen | |||||||
| Cazzato et al.6 | 2015 | Argon | 23 | 0.5−2.8 (1.4) | 78% (18/23) | NO | MRI (14.6 months) |
| Simmons et al.19 | 2016 | Nitrogen | 87 | 0.5−1.9 (1.2) | 76% (66/87) | <28 | NO |
| Adachi et al.20 | 2020 | Argon | 193 | 0.25−1.5 (0.9) | 95% (1/193) | NO | PET/CT and MRI (NR) |
| Nitrogen | |||||||
| Habrawi et al.21 | 2021 | Nitrogen | 12 | 0.5−1.5 (1) | 100% | NO | Mx, US and MRI (28.5 months) |
| Kawamoto et al.22 | 2021 | Nitrogen | 8 | 0.7−1.45 (1) | 100% | NO | VAB and MRI (28 months) |
| Fine et al.23 | 2021 | Nitrogen | 194 | 0.8−1.5 (0.81) | 98% (190/194) | NO | Mx (34.83 months) |
| Roca et al.24 | 2022 | Argon | 20 | 0.4−1.3 (0.96) | 95% (19/20) | 2−49 (24.6) | NO |
| Khan et al.25 | 2023 | Nitrogen | 14 | ≤1.5 | 100% | NO | Mx, US and MRI (35 months) |
| Kwong et al.26 | 2023 | Nitrogen | 15 | ≤2 | 53.3% (8/15) | 56 | NO |
Mx: mammography; US: ultrasound; MRI: magnetic resonance imaging; VAB: vacuum-assisted biopsy; NR: not reported in the article; NO: not performed; PET/CT: positron emission tomography/computerised tomography.
A meta-analysis published in 20213 included 397 patients with breast cancer up to 2cm in size treated with cryoablation, with a complete ablation rate of 80.3% (95% CI, 66%–89%). Habrawi et al.21 designed a study to assess the feasibility of cryoablation for early and low-risk breast cancer in 12 women. The complete ablation rate was 100% after a median follow-up of 28.5 months. Simmons et al.19 published the results after cryoablation of 86 female patients diagnosed with invasive breast cancer up to 2cm in size. After cryoablation, no residual tumour was demonstrated in 76% of cases (66/87). Roca et al.24 achieved complete ablation of 95% (19/20) of low-risk malignant breast tumours≤1.5cm in size treated with argon cryoablation. In 2023, Khan et al.25 compared the cost-effectiveness and quality of life of cryoablation vs surgery in women with early, low-risk breast cancer up to 1.5cm in size. The authors concluded that cryoablation provides benefits compared to surgical resection in terms of cost and physical and cosmetic results.
According to the published results, cryoablation could, in the future, be an alternative to surgery in low-risk breast cancer: invasive ductal hormone-receptor positive, HER2-negative carcinoma up to 1.5−2cm in size, with less than 25% intraductal component; and postmenopausal women. However, studies with larger sample sizes and longer follow-up times are needed to assess the recurrence rate before cryoablation can be considered as an alternative to surgery in patients who are able and willing to undergo the procedure.
Two prospective clinical trials are currently underway to evaluate the use of cryoablation as an alternative to surgery in the treatment of early, low-risk breast cancer (≤1.5cm); Cryoablation of Low Risk Small Breast Cancer-Ice3 Trial (ICE3)27 and Freezing instead of Resection Of Small breast Tumors (FROST).28 In both cases, patients are followed up clinically and with imaging. In ICE3 mammography is used, while in FROST patients are followed up with ultrasound and magnetic resonance imaging (MRI), and biopsy is performed six months after the procedure. Fine et al.23 published the results three years after the start of the ICE3 trial. After a mean follow-up of 34.83 months, the recurrence rate was 2.06% (4/194 patients). The FROST study started in 2016 and is estimated to end in 2023, but has not yet published its results.
In the future, cryoablation may also be indicated in stage IV or high-risk tumours. It has been reported that cold ablation of the primary cancer causes inflammation and releases antigens that trigger a tumour-specific immune response,29 which would provide a systemic benefit in addition to the local treatment. This is known as the abscopal effect. A study is currently ongoing to determine the impact of preoperative cryoablation combined with immunotherapy (ipilimumab and nivolumab) on three-year disease-free survival in women with triple negative breast cancer treated with neoadjuvant chemotherapy using taxanes.30
ContraindicationsSome articles mention that lesions close to the skin and pectoral muscles are not candidates for cryoablation.3 However, it has been reported that it is possible to freeze tumours in close proximity to or even in contact with both without complications. No other contraindications have been reported.
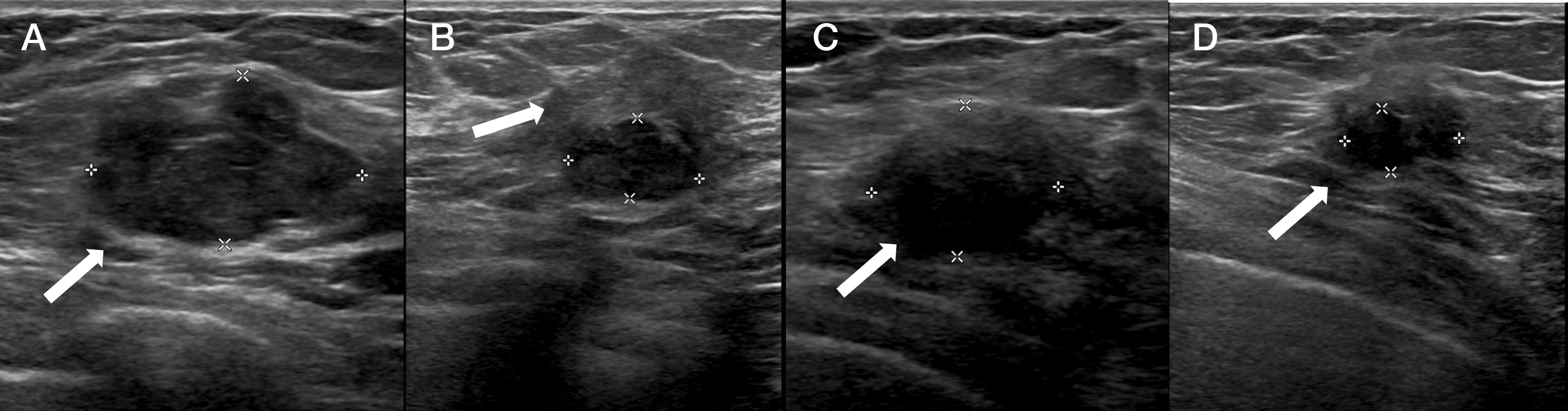
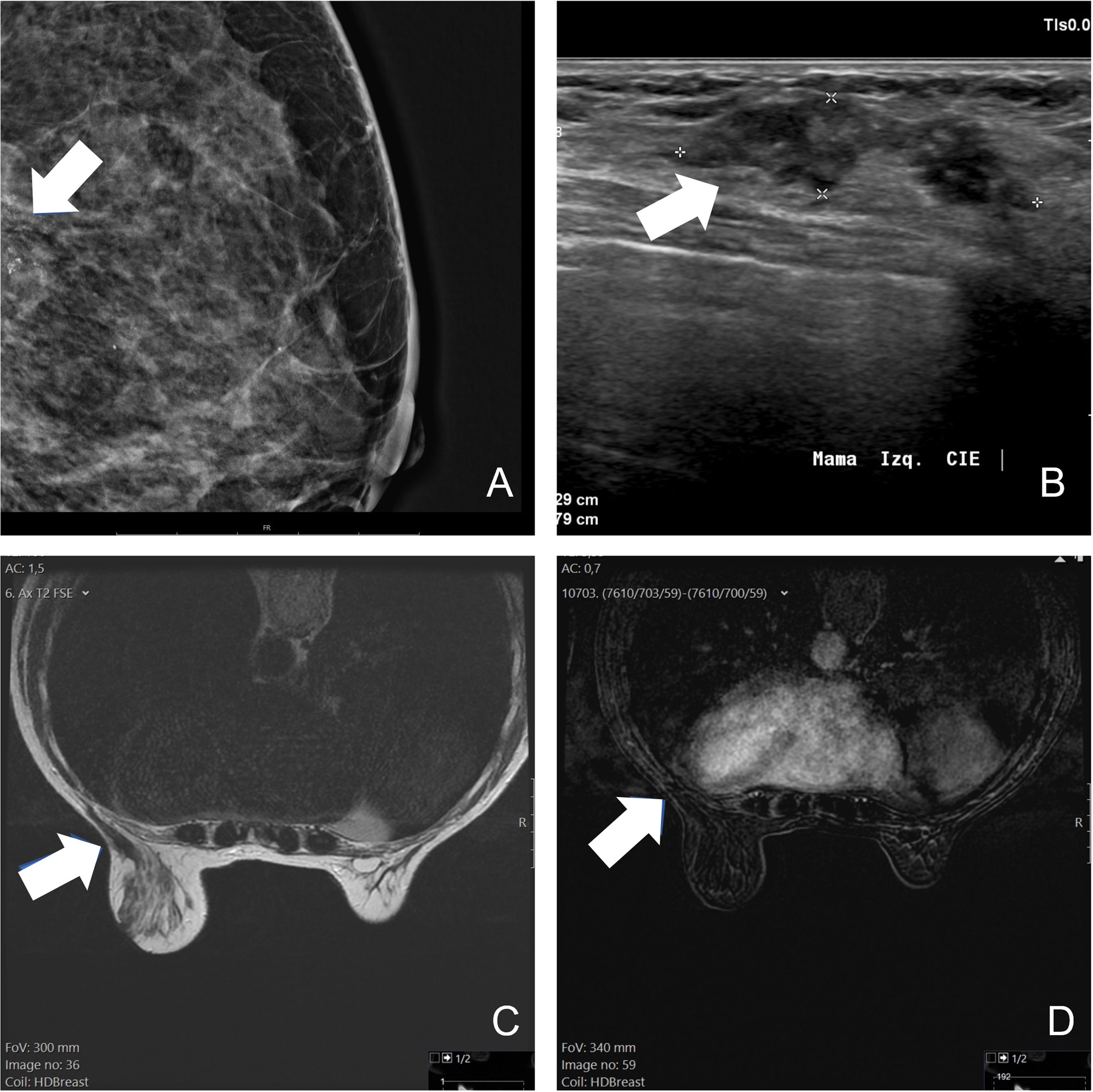
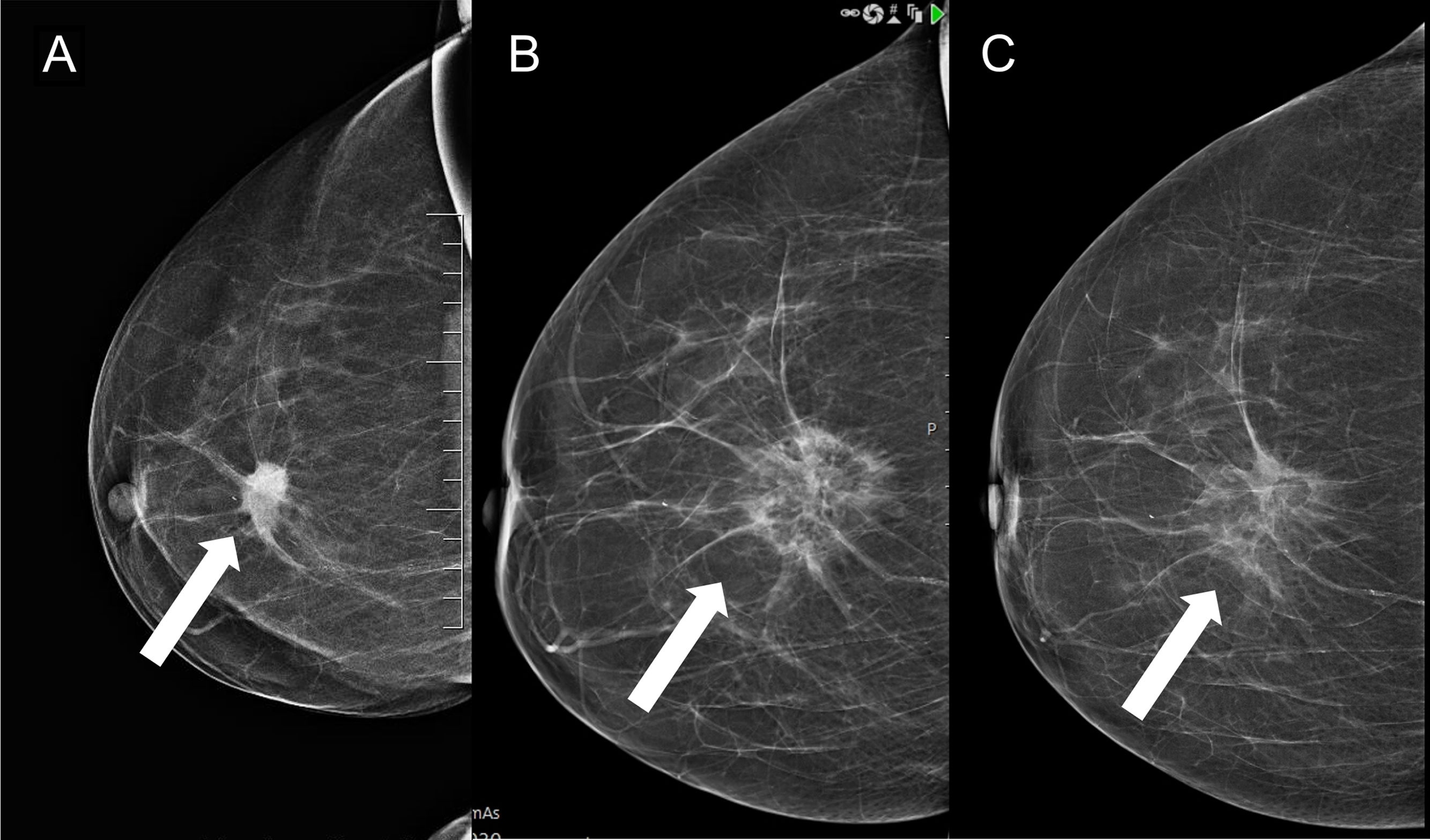
Follow-upThere is insufficient evidence in the literature to recommend a specific imaging technique or specific time intervals. For the follow-up of patients under 35 years of age with fibroadenomas treated with cryoablation, ultrasound monitoring is probably sufficient (Fig. 2). In older patients, screening could be done with mammography and/or ultrasound. For malignant neoplasms, functional techniques (MRI or contrast-enhanced mammography) should be used, possibly every six months for at least the first few years (Fig. 3). However, digital mammography is also useful (Fig. 4). If there is evidence of recurrence or residual tumour, a biopsy should be performed and cryoablation may be performed again to treat it (Fig. 5).
Fibroadenoma measuring 2.7cm treated with cryoablation (arrow in A). The six-month follow-up ultrasound (B) shows the smaller, hypoechogenic nodule (measured in the image) surrounded by an echogenic area resulting from the ablation (arrow in B). In follow-up at 13 and 24 months (C and D, respectively), the interface between the two zones is less evident and the size decreases over time (arrows in C and D).
Invasive ductal breast carcinoma, luminal B HER2 negative, 2.3cm in size, treated with cryoablation. Magnified mammography at diagnosis shows pleomorphic microcalcifications located in the posterior third of the breast (arrow in A). The ultrasound shows an oval nodule with parallel undefined borders (arrow in B). On MRI performed one year after the procedure, the lesion is evident on the T2-weighted sequence (arrow in C), but shows no enhancement after contrast administration (arrow in D).
Luminal A invasive ductal carcinoma, 2.3cm in size, treated with cryoablation (arrow in A). Follow-up mammograms at six and 12 months (arrows in B and C, respectively) show a lesion with a dense border and a low-density centre, consistent with fat necrosis, which decreases in size over time.
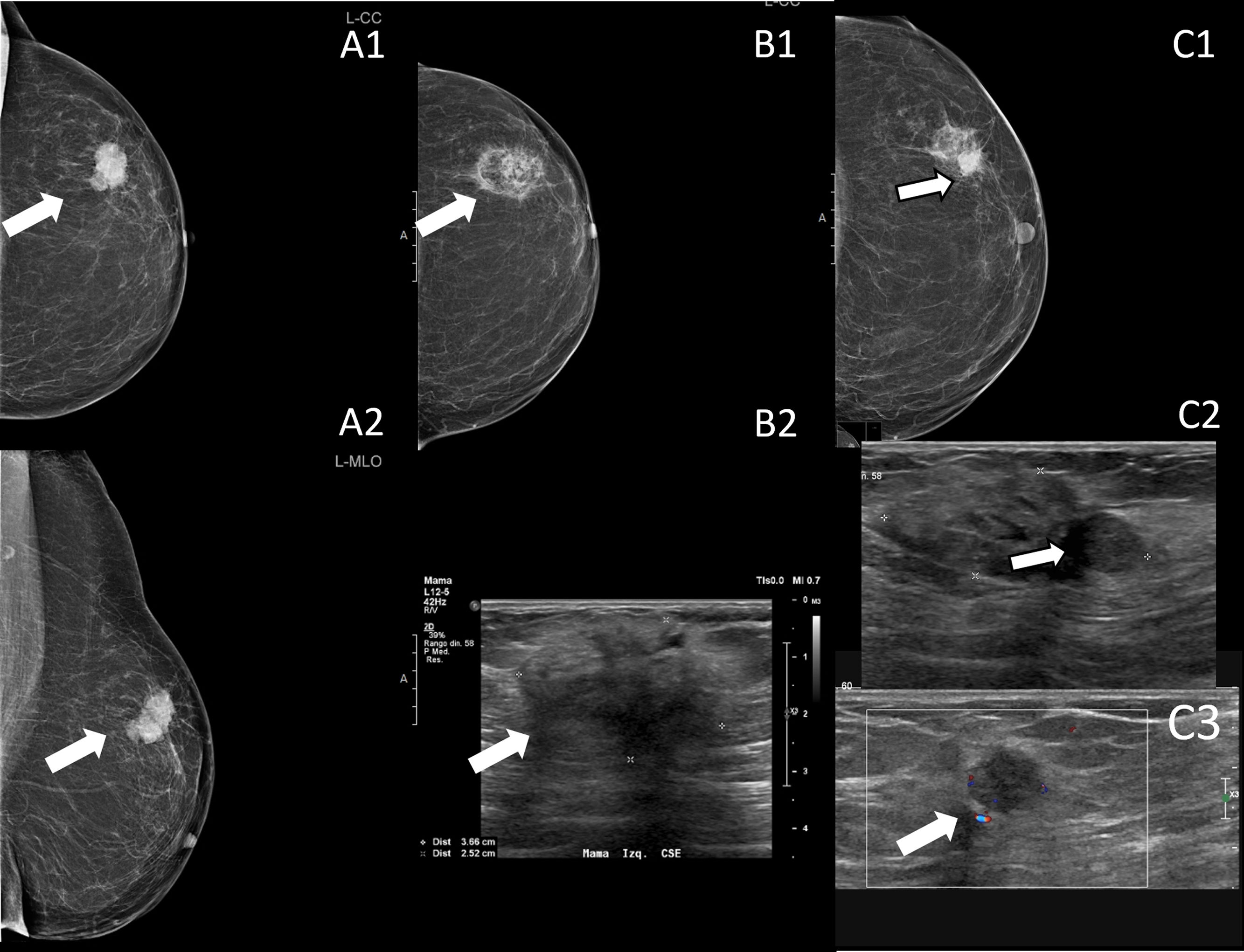
Triple negative breast cancer 3cm in size diagnosed in an 85-year-old woman who does not want surgery (arrows in A). Cryoablation was performed, with no evidence of recurrence on follow-up mammography and ultrasound 12 months after the procedure (arrow in B). At the 18-month follow-up, a dense, rounded image is seen on the mammogram, located at the periphery of the area of fat necrosis (arrow in C1), and a hypoechogenic nodule (arrow in C2) with flow within it (arrow in C3). The findings are consistent with recurrence, which was confirmed by percutaneous biopsy. Cryoablation treatment was performed again.
Cryoablation is a simple technique, performed on an outpatient basis, that is very well tolerated and with a low complication rate. Its efficacy is proven in the treatment of fibroadenomas and presently allows local control of hormone-sensitive breast cancer in frail patients who refuse or are not candidates for surgery. Its role in breast cancer with a more aggressive tumour profile and in a metastatic context is currently being studied. In the near future it is expected to become an alternative to surgery in postmenopausal women with early, hormone receptor-positive, HER2-negative breast cancer.