Claudin-4, a component of the tight junction, plays an important role in tumorigenesis and metastasis of ovarian cancer, but its role in platinum resistance has not been elucidated.
Aim of the workTo determine the presence of claudin-4 in ovarian cancer tissues in relation to platinum compounds resistance.
Patients and methodsPatients with advanced ovarian malignancy (FIGO stages III and IV) that have undergone primary surgery for maximal cytoreduction, followed by first line chemotherapy with platinum compounds and paclitaxel, were followed up for 6 months to determine chemotherapeutic response. Claudin-4 expression in ovarian cancer tissue resected from the patients surgically was evaluated immunohistochemically.
ResultsClaudin-4 is associated with more aggressive behavior of ovarian tumors and the advanced stage of the tumors. High expression of claudin-4 was found in high grade tumors, of the papillary serous subtype. High expression is linked to chemotherapeutic resistance, whereas low expression is associated with good response to first line chemotherapy.
ConclusionHigh claudin-4 expression can predict poor chemotherapeutic response in advanced ovarian cancer.
La claudina-4, componente de la zona de oclusión, desempeña un papel importante en la oncogenia y metástasis del cáncer ovárico, pero su papel en la resistencia al platino no se ha aclarado todavía.
ObjetivoEstablecer la presencia de claudina-4 en los tejidos de cáncer ovárico con relación a la resistencia a compuestos de platino.
Pacientes y métodosPacientes con neoplasia maligna de ovario (clasificación FIGO fase III y IV) sometidos a una intervención citorreductora primaria seguida de quimioterapia de primera línea con compuestos de platino y paclitaxel; se realizó un seguimiento durante 6 meses para valorar la reacción a la quimioterapia. Se llevó a cabo un análisis inmunohistoquímico de la expresión de la claudina-4 en los tejidos de cáncer ovárico.
ResultadosLa claudina-4 se asocia con un comportamiento más agresivo de los tumores ováricos y con una fase más avanzada de los mismos. Se encontró una alta manifestación de claudina-4 en tumores de grado alto, de subtipo papiloma seroso. Se relaciona la expresión alta con la resistencia quimioterápica, mientras que una expresión baja se asocia a una buena reacción a la quimioterapia de primera línea.
ConclusiónUna expresión alta de claudina-4 puede predecir una mala respuesta quimioterápica en el cáncer de ovario avanzado.
Ovarian cancer is the most common fatal cancer of the female reproductive tract and is responsible for 5% of all cancer deaths in women. About 70% of ovarian cancers are diagnosed in Stages III/IV due to lack of specific signs and symptoms.1 Patients undergo surgery aiming for maximal cytoreduction and this is followed by first line chemotherapy with platinum compounds and paclitaxel.2 Approximately 80% of the patients show an initial response but unfortunately, the majority of the patients relapse.3 The specific mechanisms for drug resistance have not been yet identified. Therefore, there is a pressing need for the identification of prognostic markers for first line chemotherapy.
Apical junctional complexes are responsible for holding epithelial cells together and they are formed of tight junctions (TJs), adherens junctions and desmosomes. Claudins are the main constituents of TJs and comprise 24 closely related transmembrane proteins.4 Tight junctions are essential for the tight sealing of the cellular sheets, controlling para-cellular ion flux and therefore maintaining tissue homeostasis and cell polarity.5 Also, because of the ability of tight junction proteins to recruit signaling proteins, tight junctions have been hypothesized to be involved in the regulation of proliferation, differentiation, and other cellular functions.6 The expression of claudins is variable in different cancers.7 Regarding cancer ovary, it was found that claudin-3 and claudin-4 are up-regulated,8 and claudin-4 is over-expressed in ovarian cancer in comparison with ovarian cystadenomas.9 This high expression suggests that claudins are responsible for increased invasion, motility and cell survival, the characteristics required for metastasis.10 This can also suggest that high expression of claudins could be associated with chemoresistance. Therefore, this study was conducted to investigate the relationship between the expression of claudin-4 and the response to first line chemotherapy, aiming to identify a prognostic marker to therapy.
Patients and methodsThis study was conducted on 25 patients with advanced ovarian malignancy (FIGO stages III and IV). Informed consent was taken from the patients and they were all subjected to history taking, clinical examination, radiological investigation with trans-vaginal ultrasound (TVUS) and CT, and laboratory detection of pre-operative serum CA 125 levels. All patients underwent maximal cytoreductive surgery in El Shatby University Hospital, aiming at leaving a residual tumor <1cm. The operation comprised of ascetic fluid aspiration, total abdominal hysterectomy and bilateral salpingo-opherectomy, omentectomy and lymph node sampling (pelvic and para-aortic). The patients then received adjuvant chemotherapy of platinum compounds and paclitaxel. The patients were followed up for a period of six months to assess the response to chemotherapy. Resistance was identified by the detection of pelvic masses either clinically or radiologically (TVUS or CT), and by the level of CA 125, either as a failure to decrease, or as an increase after an initial decrease. The patients were divided into two groups according to chemotherapeutic response: the resistant group and the responder group.
ImmunohistochemistryOvarian cancer tissues were fixed with 10% buffered formalin and embedded in paraffin. Only epithelial ovarian tumors were included in this study. Three-micrometer sections were deparaffinized and hydrated. They were incubated in the microwave for 10min. Claudin-4 protein expression was detected by primary rabbit monoclonal claudin-4 antibody (Cat. No. RB-9266-R7, Thermo Fisher Scientific, Lab Vision Corporation, Fremont, CA, USA). The antibody was diluted at 1:300 in antibody diluents and incubated with the tissue specimens for two hours. Ten randomly selected areas of each slide were analyzed using high power fields objective (×40) with 100 cells counted per field, and the positively stained cells were determined in respect to the total number of cells. For semiquantitative evaluation, tumorous and non-tumorous epithelia were considered negative if less than 5% of the cells reacted. The following further values were given: 1 (6–20% positivity), 2 (21–40% positivity), 3 (41–60% positivity), 4 (61–80% positivity), and 5 (81–100% positivity). Only membranous staining was classified as positive. Intensity scores were from 0 to 3; where 0 is negative, 1 is weakly positive, 2 is moderately positive and 3 is strongly positive. Both scores were multiplied together (Intensity X Percentage) to obtain the combined claudin-4 score (CLDN4 score).
ResultsThe patients were divided into two groups according to chemotherapeutic response: the resistant group (52%) and the responder group (48%). The two groups were then compared regarding the demographic characteristics, tumor histopathology, grade, immunohistochemical staining, stage and chemotherapeutic response, all in relation to claudin-4 expression.
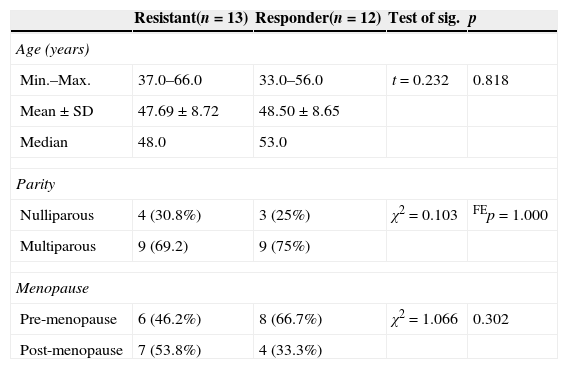
There was no statistical difference between the two studied groups in relation to the demographic data (Table 1).
Comparison between the two studied groups according to demographic data.
| Resistant(n=13) | Responder(n=12) | Test of sig. | p | |
|---|---|---|---|---|
| Age (years) | ||||
| Min.–Max. | 37.0–66.0 | 33.0–56.0 | t=0.232 | 0.818 |
| Mean±SD | 47.69±8.72 | 48.50±8.65 | ||
| Median | 48.0 | 53.0 | ||
| Parity | ||||
| Nulliparous | 4 (30.8%) | 3 (25%) | χ2=0.103 | FEp=1.000 |
| Multiparous | 9 (69.2) | 9 (75%) | ||
| Menopause | ||||
| Pre-menopause | 6 (46.2%) | 8 (66.7%) | χ2=1.066 | 0.302 |
| Post-menopause | 7 (53.8%) | 4 (33.3%) | ||
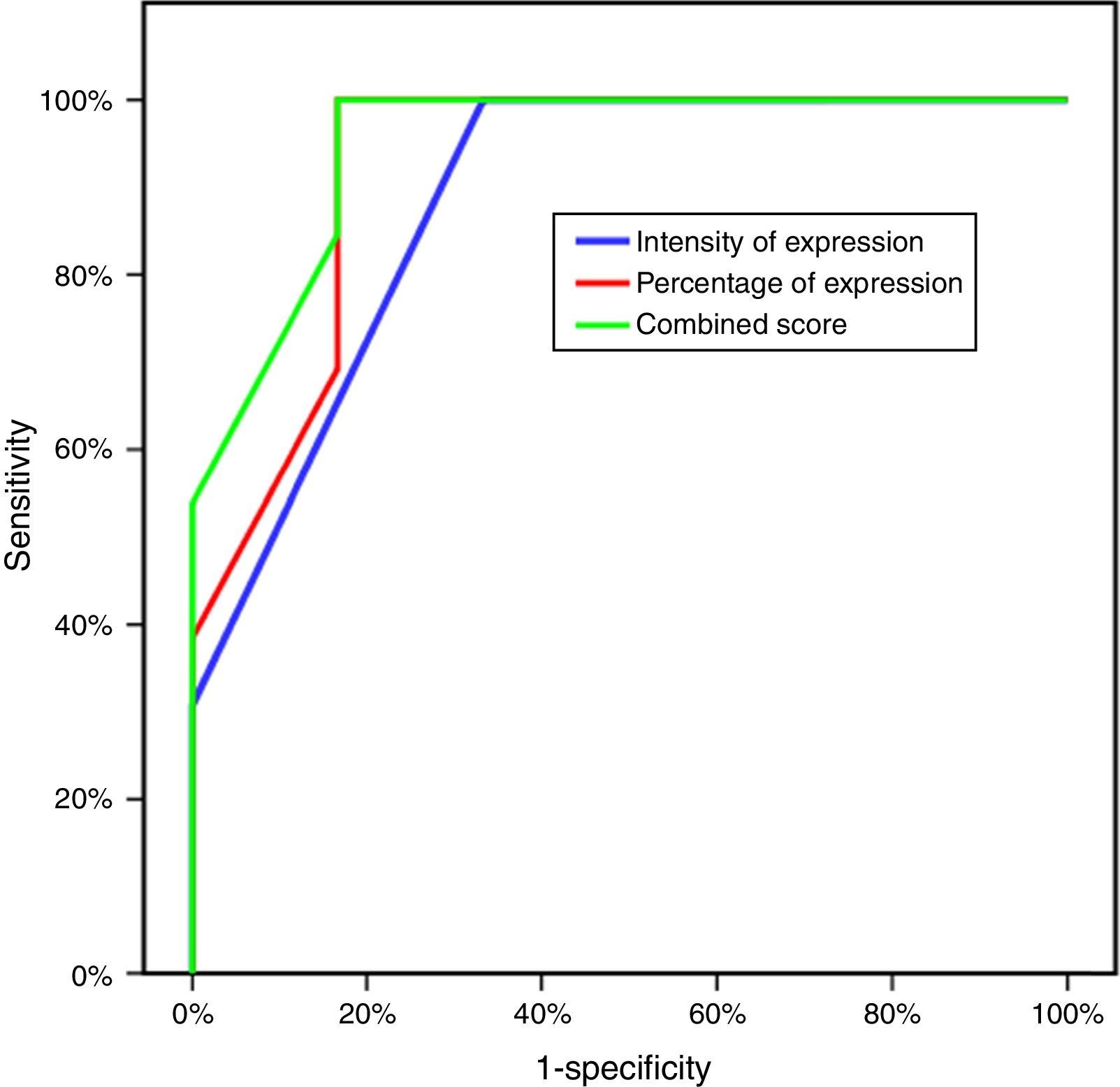
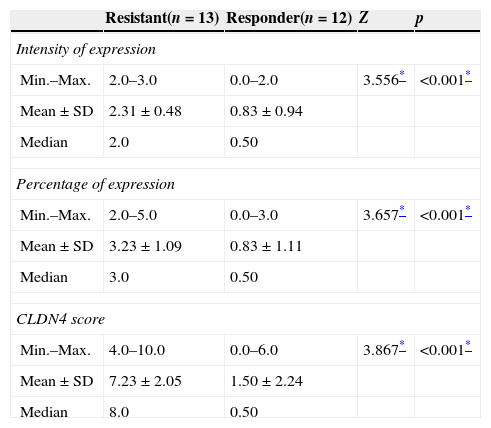
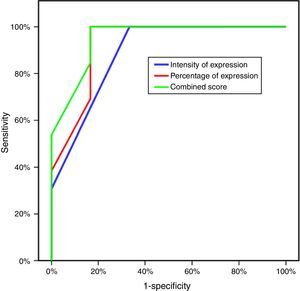
Three parameters were utilized to evaluate the difference of expression of CLDN4 in the two study groups: the intensity of expression, the percentage of expression and the combined CLDN4 score (percentage×intensity). All showed statistically significant difference, with greater intensity of expression, percentage of expression and CLDN4 score in the resistant group (Table 2). Plotting this data on a ROC curve enabled us to identify CLDN4 score as the single most descriptive parameter of expression to be used in further comparative tests in this study, with the greatest area under the curve of 94.9%, which is of statistical significance. A score of four was taken as the cut-off value for expression, above which is considered high expression of CLDN4, and a score of four or less means low expression. This value has a sensitivity of 84.62% and a specificity of 83.33% (Fig. 1)
Comparison between the two studied groups according to the intensity of expression, percentage of expression and CLDN4 score.
| Resistant(n=13) | Responder(n=12) | Z | p | |
|---|---|---|---|---|
| Intensity of expression | ||||
| Min.–Max. | 2.0–3.0 | 0.0–2.0 | 3.556* | <0.001* |
| Mean±SD | 2.31±0.48 | 0.83±0.94 | ||
| Median | 2.0 | 0.50 | ||
| Percentage of expression | ||||
| Min.–Max. | 2.0–5.0 | 0.0–3.0 | 3.657* | <0.001* |
| Mean±SD | 3.23±1.09 | 0.83±1.11 | ||
| Median | 3.0 | 0.50 | ||
| CLDN4 score | ||||
| Min.–Max. | 4.0–10.0 | 0.0–6.0 | 3.867* | <0.001* |
| Mean±SD | 7.23±2.05 | 1.50±2.24 | ||
| Median | 8.0 | 0.50 | ||
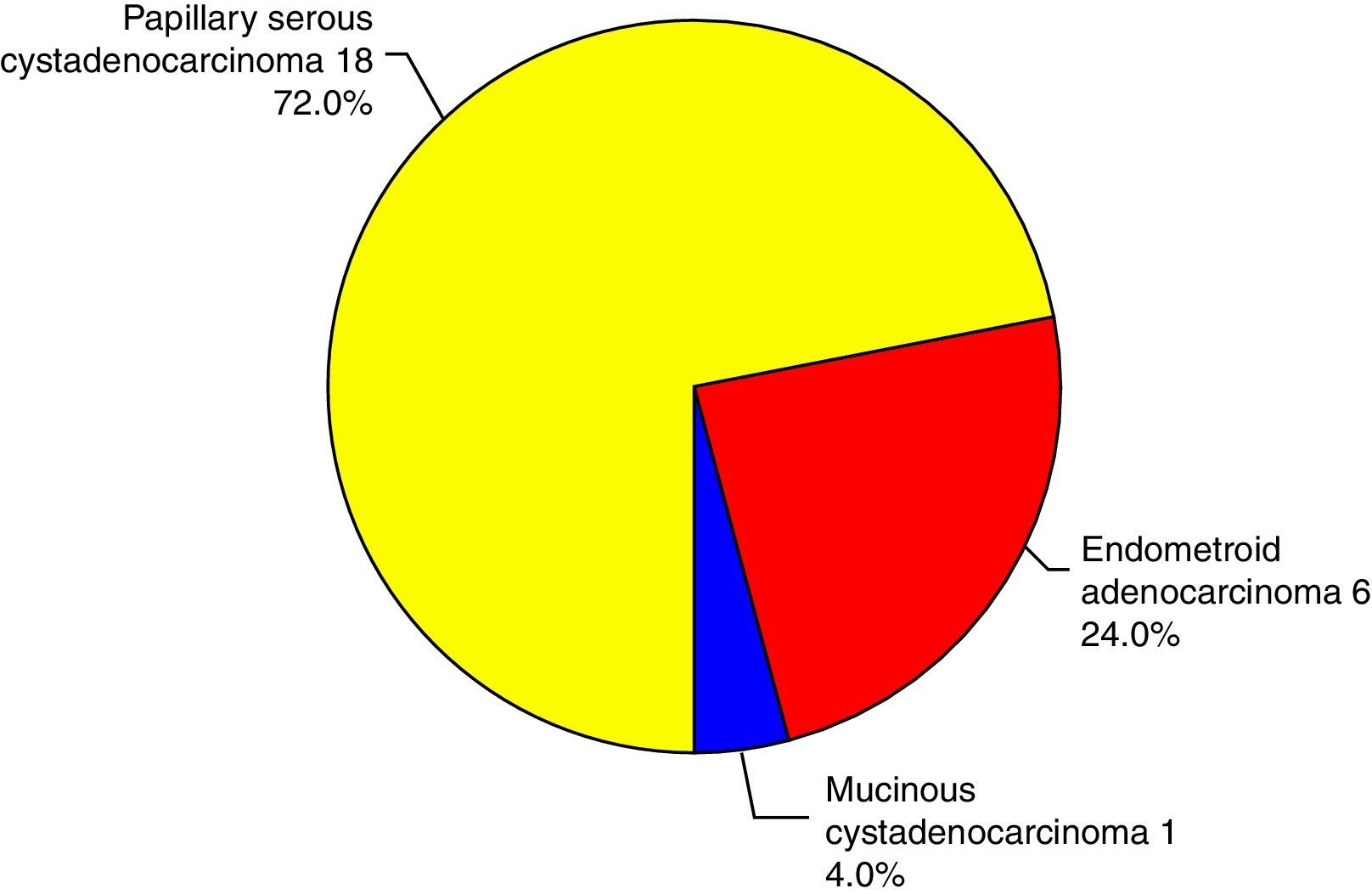
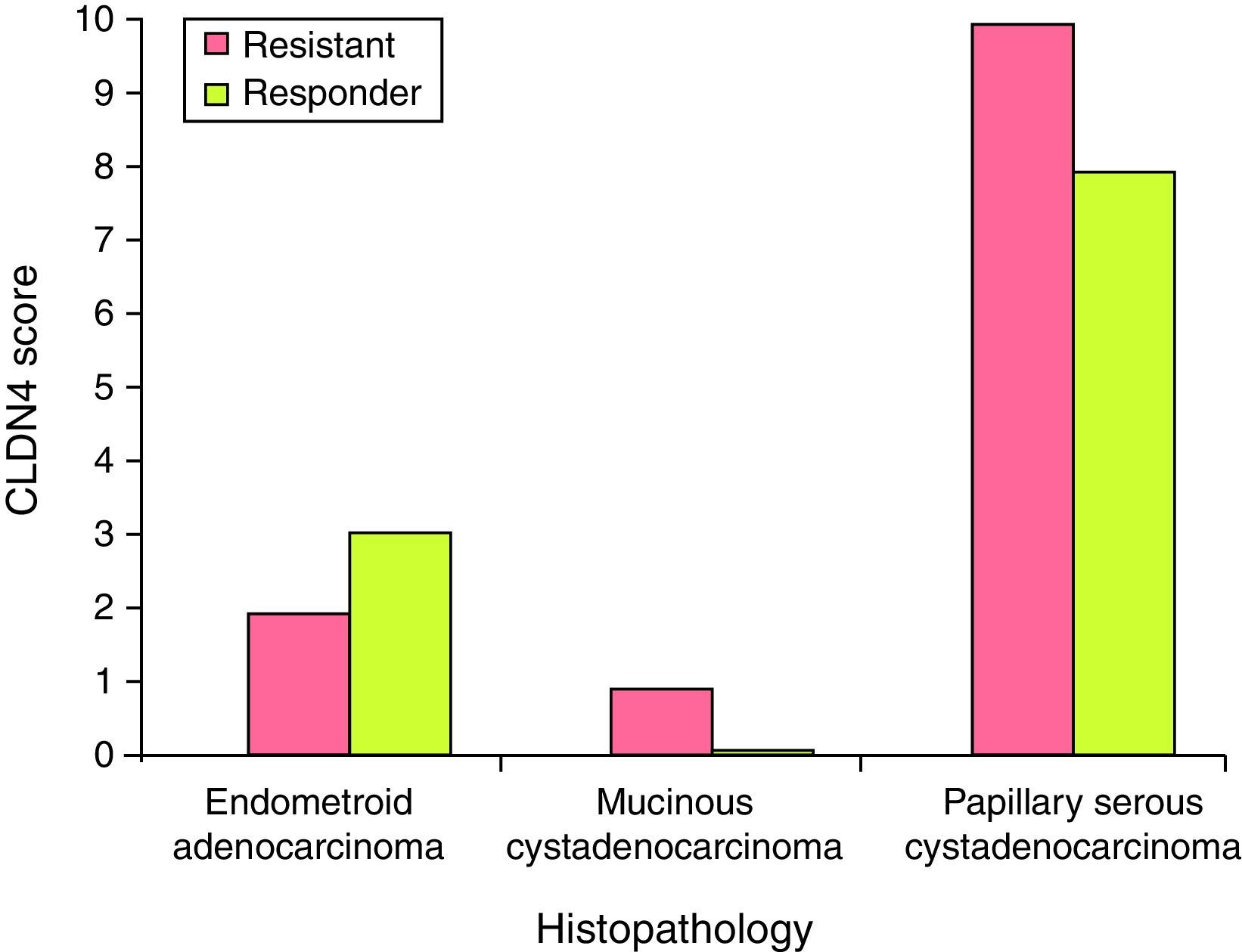
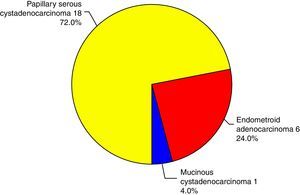
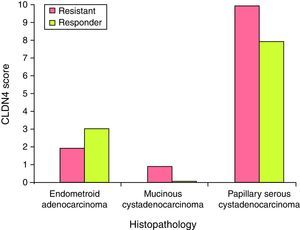
There was no statistically significant difference in the expression of CLDN4 between the different histopathological types encountered in this study. Claudin-4 was expressed in 76% of the specimens. Only epithelial ovarian tumors were included in this study, with three subtypes: papillary serous, endometrioid and mucinous (Fig. 2). Being only a single case, the mucinous type was excluded from the comparison and there was no statistically significant difference in the expression of CLDN4 between the papillary serous and the endometrioid type of ovarian cancer (Fig. 3).
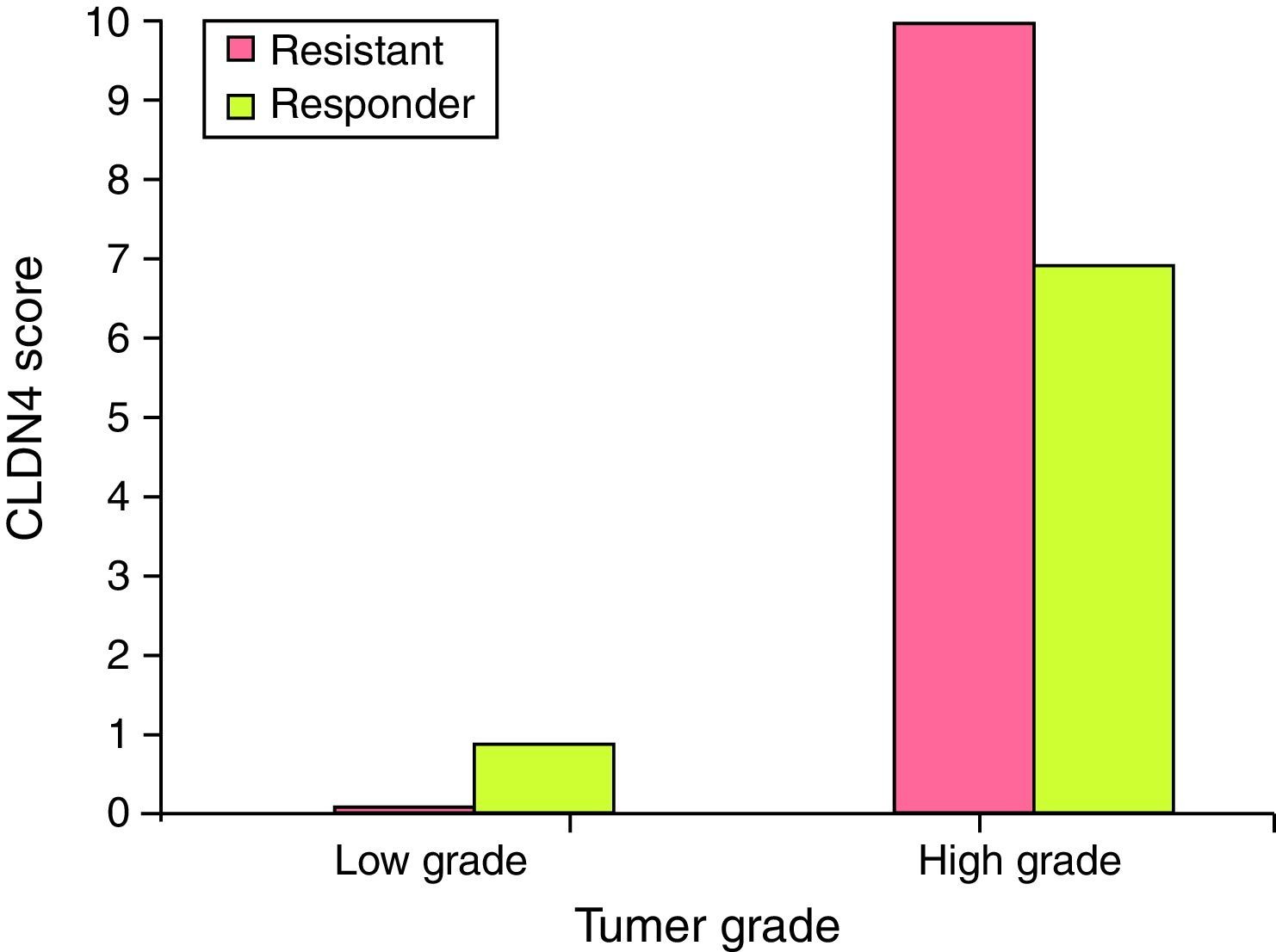
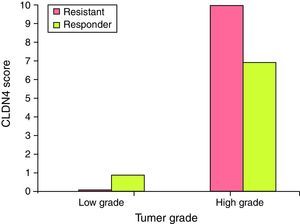
Claudin-4 is over expressed in high grade tumors of the resistant group. It was found that CLDN4 score >4 was found in high grade tumors of the resistant group (80%), while CLDN4 score ≤4 represented 100% of the low grade tumors in the responder group. This difference is statistically significant (Fig. 4).
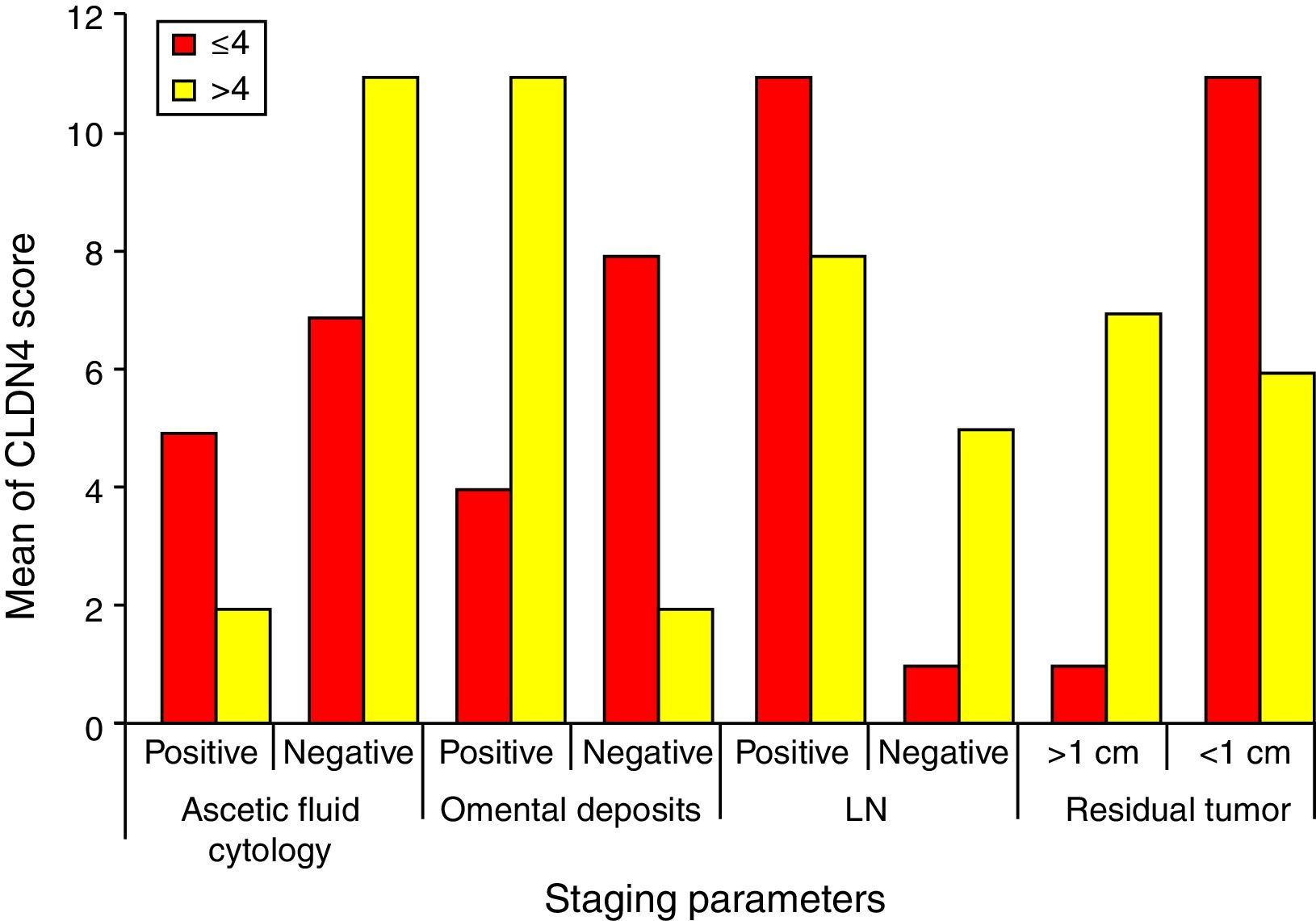
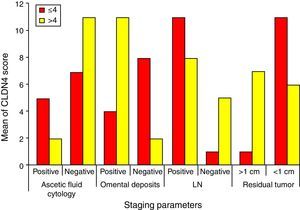
Higher CLDN4 scores are associated with higher stages of the tumor. It is statistically proven that higher CLDN4 scores are associated with more aggressive tumors, those with positive ascetic fluid cytology, omental deposits, nodal affection and completely unresectable tumors. Regarding the score cut-off level, positive omental deposits (84.6%) showed significant association with scores of >4, and tumor residues of <1cm (91.7%) showed significant association with scores of <4 (Fig. 5).
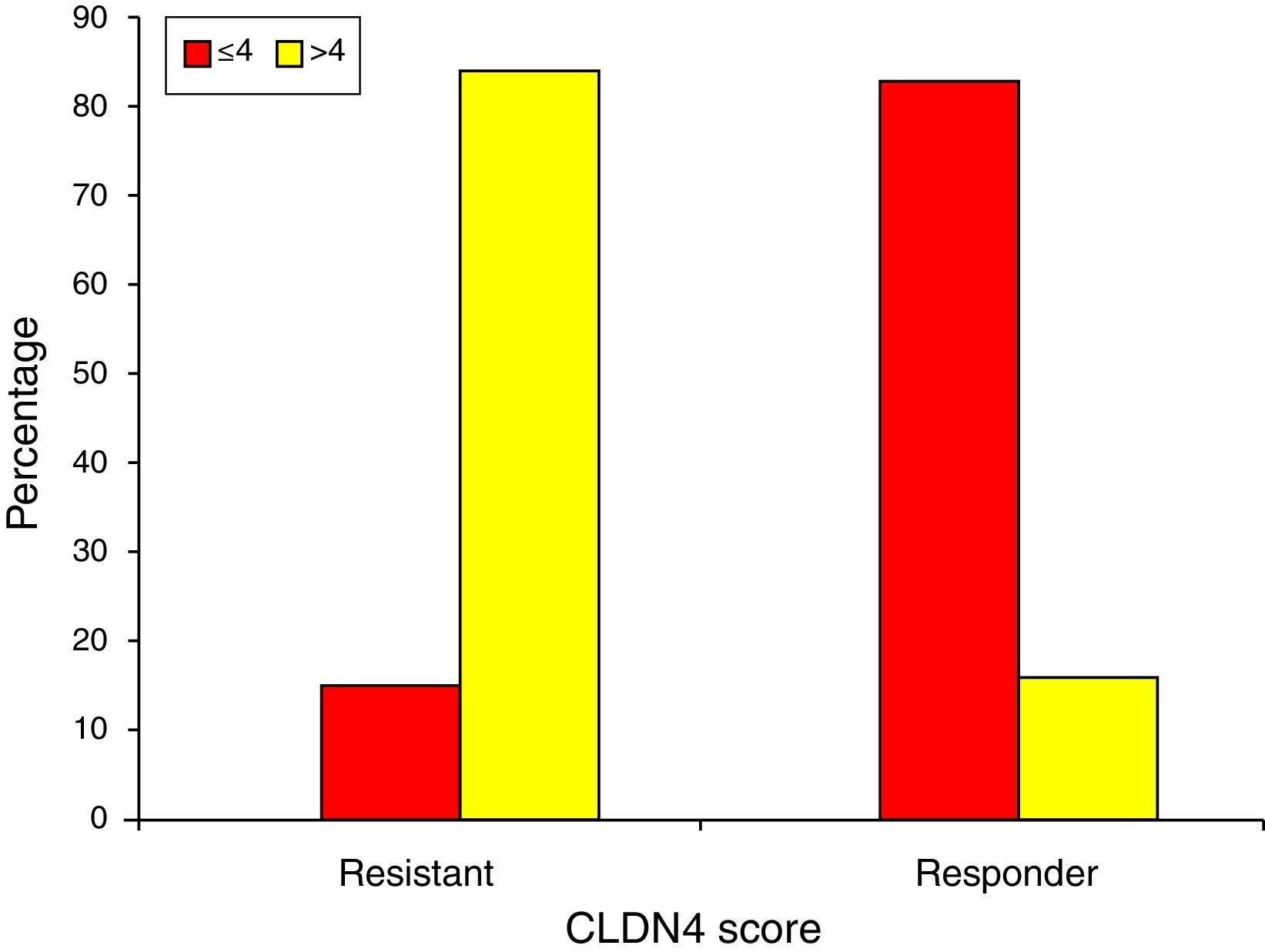
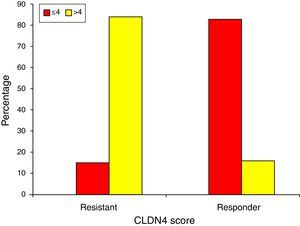
Finally, chemotherapeutic resistance is significantly associated with CLDN4 scores of >4 (84.6%), while chemotherapeutic response is seen with scores ≤4 (83.3%) (Fig. 6).
DiscussionOvarian cancer is the leading cause of death among gynecological cancers, the commonest type being epithelial ovarian cancer. Most of the cases are advanced at the time of diagnosis, and the majority of these patients develop recurrent disease despite an initial response to primary treatment consisting of surgical debulking and chemotherapy with platinum and paclitaxel. Therefore, novel strategies that may be effective in enhancing sensitivity or overcoming resistance are needed.
This study was conducted to correlate the expression of CLDN4 in advanced epithelial ovarian cancer and the chemotherapeutic response. Twenty-five patients with advanced epithelial ovarian cancer were included in this study and were divided into resistant and responder groups.
In this study, CLDN4 was expressed in 76% of the specimens. Fourteen were in the papillary serous subtype (77.8%), four in the endometrioid subtype (66.7%), and the single mucinous case was positive for CLDN4. This was in accordance with Boylan et al.,11 as their study demonstrated the expression of CLDN4 in nearly 70% of their cases. The highest percentage of expression was detected in the endometrioid and mucinous subtypes (both 77.4% positive) followed by the serous subtype (72.17%) and the clear cell (57.58%) subtype. Taking into account the high prevalence of papillary serous subtype in our study, it is likely that we found the highest expression in this subtype. Litkouhi et al.12 found the highest percentage of claudin-4 expression in clear cell and endometrioid subtypes of ovarian cancer.
Considering the papillary serous subtype as the commonest in this study, they were divided into high and low grade tumors. Statistically significant correlation was found between high CLDN4 expression and high grade tumors. In contrast, Zhu et al. found in a study to investigate the expression of claudins 1, 3, 4 and 5 that claudin-3, but not claudin-4, was significantly increased in moderately, poorly and undifferentiated adenocarcinomas compared to well-differentiated and borderline-type tumors.13 They also reported that claudins were not expressed in sex cord stromal tumors and dysgerminomas.
This study demonstrated the increased aggression and invasiveness of ovarian tumors highly expressing claudin-4, through the presence of ascites, omental deposits, lymph node metastasis, and inability to perform maximal cytoreductive surgery (residual tumor>1cm) due to multiple colonic or splenic invasion or the invasion of the porta hepatis. Morin et al.7 has agreed with our study, and so did Agarwal et al.10 As the identification of metastatic cells in serous effusions has prognostic and therapeutic implications, Lonardi et al.14 have diagnosed the presence of claudin-4 in serous effusion of 67 tumors of the female genital tract, suggesting that claudin-4 might be used as an ideal “single-shot” marker for the identification of metastatic epithelial cells in serous effusions.
Conversely to our results, Choi et al.15 found that the intensity of CLDN3 and CLDN4 staining did not correlate statistically significantly with patient age, tumor grade, advanced stage or the presence of ascites in ovarian cancers. However, intense CLDN4 staining did tend to correlate with higher grade cancers, although this trend did not reach statistical significance (p=0.068).
In determining the relationship between claudin-4 expression and chemotherapeutic response, we have found that resistant tumors demonstrated high expression of claudin-4 (84.6% of the resistant group had CLDN4 scores >4). On the other hand, 83.3% of the sensitive group recorded scores ≤4. This suggests that high claudin-4 expression is associated with chemoresistance and can be used as a prognostic indicator.
Similarly, in an attempt to identify prognostic markers for therapy, Stewart et al.16have studied the expression of 1117 proteins in sensitive and resistant ovarian tumors. Utilizing quantitative proteomic technology integrated with mRNA-expression levels, they have identified that claudin-4 was one of the top proteins associated with cisplatin resistance in ovarian cells with a 7.2 fold over-expression level.
In agreement with our study, Yoshida et al.,17 in a study conducted on 43 cases of ovarian cancer, demonstrated that claudin-4 expression was significantly greater in ovarian cancer tissue from chemoresistant patients compared to chemosensitive patients, and the overall survival was significantly shorter for claudin-4-positive than claudin-4-negative cases. They also demonstrated that suppression of claudin-4, via transfection of ovarian cancer cells with siRNA, resulted in a significant increase of cisplatin sensitivity and cellular accumulation of fluorescence-labeled cisplatin.
In contrast, Litkouhi et al.12 stated that there is no relation between the effectiveness of chemotherapy in ovarian cancer and claudin-4 expression.
Overcoming chemotherapy resistance is of crucial important for improving the prognosis of patients with ovarian cancer. However, understanding the mechanism of the chemotherapy resistance and overcoming it is still not fully unraveled, as it is not yet ascertained whether claudin-4 plays a role as an influx or efflux transporter of cisplatin. In this context, Shang et al.18 have identified that claudin-3 and claudin-4 affect sensitivity of the ovarian cancer cells to the cytotoxic effect of cisplatin by regulating the expression of the copper transporter CTR1, hence affecting its influx to the cells.
It can be inferred that high claudin-4 expression can predict poor chemotherapeutic response in advanced ovarian cancer. Still the possibility of its use as a chemotherapeutic target seems promising, but remains to be elucidated.
ConclusionsClaudin-4 is associated with more aggressive behavior of ovarian tumors and the advanced stage of the tumors. High expression of claudin-4 was found in high grade tumors, of the papillary serous subtypes. High expression is also linked to chemotherapeutic resistance, whereas low expression is associated with good response to first line chemotherapy. Claudin-4 can be used as a prognostic marker for chemotherapy.
RecommendationsUsing claudin-4 as a prognostic tool to predict response to chemotherapy in advanced ovarian cancers. Studying the role of other members of the claudin family in relation to ovarian cancer, especially claudins 3, 6 and 7. Exploring the possibility of using claudin-4 as a targeted therapeutic modality.
Ethical responsibilitiesProtection of people and animalsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Data confidentialityThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestThe authors have nothing to disclose and declare no conflict of interest, whether personal or financial.