The endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) is the technique of choice for the evaluation of hilar-mediastinal lymphadenopathy and staging of non-small cell lung cancer. The endobronchial ultrasound-guided transbronchial mediastinal cryobiopsy (EBUS-TBMC) has emerged as an alternative to optimize diagnostic yield for benign mediastinal pathology, rare conditions, and lymphoproliferative processes that require larger (histological) samples.1–4
In a recent meta-analysis,1 only two case series from those included used three types of needles (19G/21G/22G) to perform EBUS TBNA prior to mediastinal cryobiopsy. In one of these series of 4 cases, the 21G needle was used in half of the cases, without describing the number of punctures for tunneling, with a freezing time of 3s using a 1.1mm cryoprobe and no complications. In the other series of 27 cases, the 22G needle was used in 19 cases, performing 3 punctures for tunneling, with a freezing time of 3–4s using a 1.1mm cryoprobe, and no complications as well.
We present a series of 15 cases in which we performed EBUS-TBMC immediately after performing EBUS-TBNA,5–8 using different needle gauges to tunnel the pathway, allowing the passage of the cryoprobe. The characteristics of these cases, the needle used in each case, the studied lymph node stations, the number of punctures for tunneling and cryobiopsies, and results are summarized in Table 1.
Case series, needle used, number of punctures for tunneling and cryobiopsies, explored lymph node stations and results of EBUS-TBNA and EBUS-TBMC.
| C | Age | Reason for the endoscopic study | N | EBUS-TBNA (LNS) | PFT | EBUS-TBMC (LNS) | NC | Outcome TBNA/Cell block | Outcome cryobiopsy | Complications |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 68 | Staging of lung cancer | 21G | 11R, 4R, 7 | 2 | 7 | 2 | Anthracosis | Anthracosis | No |
| 2 | 77 | Diagnosis and staging | 21G | 11L,7 | 2 | 7 | 2 | SCLC | SCLC | No |
| 3 | 70 | Diagnosis and staging | 19G | 11L, 7, 11R | 2 | 7 | 3 | Adenocarcinoma | Adenocarcinoma | No |
| 4 | 60 | Diagnosis and staging | 21G | 4L, 7 | 3 | 7 | 2 | Adenocarcinoma | Adenocarcinoma | No |
| 5 | 41 | Lymphadenopathies | 22G | 7, 11L | 2 | 11L | 3 | N-NGL | N-NGL | No |
| 6 | 41 | Lymphadenopathies | 19G | 7 | 2 | 7 | 3 | Necrosis. TB-PCR (+) | NGL | No |
| 7 | 52 | Lymphadenopathies | 19G | 7, 11L | 2 | 11L | 3 | N-NGL | N-NGL | No |
| 8 | 52 | Lymphadenopathies | 21-19G | 7, 11R | 3 y 1 | 7 y 11R | 1 y 2 | Anthracosis | N-NGL | No |
| 9 | 54 | Lymphadenopathies | 22G | 7, 11L | 2 | 11L | 3 | N-NGL | N-NGL | No |
| 10 | 71 | Lymphadenopathies | 22G | 11L, 4R, 7 | 2 | 7 | 2 | N-NGL | N-NGL | No |
| 11 | 72 | Lymphadenopathies | 22G | 11L, 7 | 2 | 7 | 3 | Anthracosis | Anthracosis | No |
| 12 | 61 | Lymphadenopathies | 19G | 7, 11L | 2 y 1 | 7 y 11L | 2 y 2 | Anthracosis | Sinus Histiocytosis | No |
| 13 | 61 | Lymphadenopathies | 19G | 7, 11L | 1 y 1 | 7 y 11L | 1 y 2 | Anthracosis | N-NGL | Pneumomediastinum |
| 14 | 55 | Lymphadenopathies | 22G | 11L, 7,11R | 1 | 11L | 2 | Anthracosis | Anthracosis | No |
| 15 | 71 | Lymphadenopathies | 22G | 11L,7 | 2 | 11L | 1 | Anthracosis | Anthracosis | No |
C: case. N: needle (Gauges). EBUS-TBNA: Endobronchial Ultrasound-guided Transbronchial Needle Aspiration. LNS: lymph node station. PFT: punctures for tunneling. EBUS-TBMC: Endobronchial Ultrasound-guided Transbronchial Mediastinal Cryobiopsy. NC: number of cryobiopsies. SCLC: small cell lung cancer. N-NGL: non-necrotizing granulomatous lymphadenitis (Sarcoidosis). NGL: necrotizing granulomatous lymphadenitis. TB-PCR: Tuberculosis Polymerase Chain Reaction.
All endobronchial ultrasound procedures were performed with day-hospital admissions, scheduled stays of 5–6h (unless incidents occurred), under intravenous general anesthesia with an airway secured via a laryngeal mask or endotracheal tube, using an EBUS bronchoscope (BF-UC180F, Olympus, Tokyo, Japan). EBUS-TBNA was performed with cytological sampling and tunneling using a 21G (ViziShot 2, Olympus) or 19G needle (ViziShot 2 Flex, Olympus) or 22G needle (SonoTip TopGain; Medi-Globe, Rohrdorf, Germany), and EBUS-TBMC with the ERBECRYO 2 system and a 1.1-mm cryoprobe (Erbecryo 2; Erbe, Tubingen, Germany) for histological sampling.
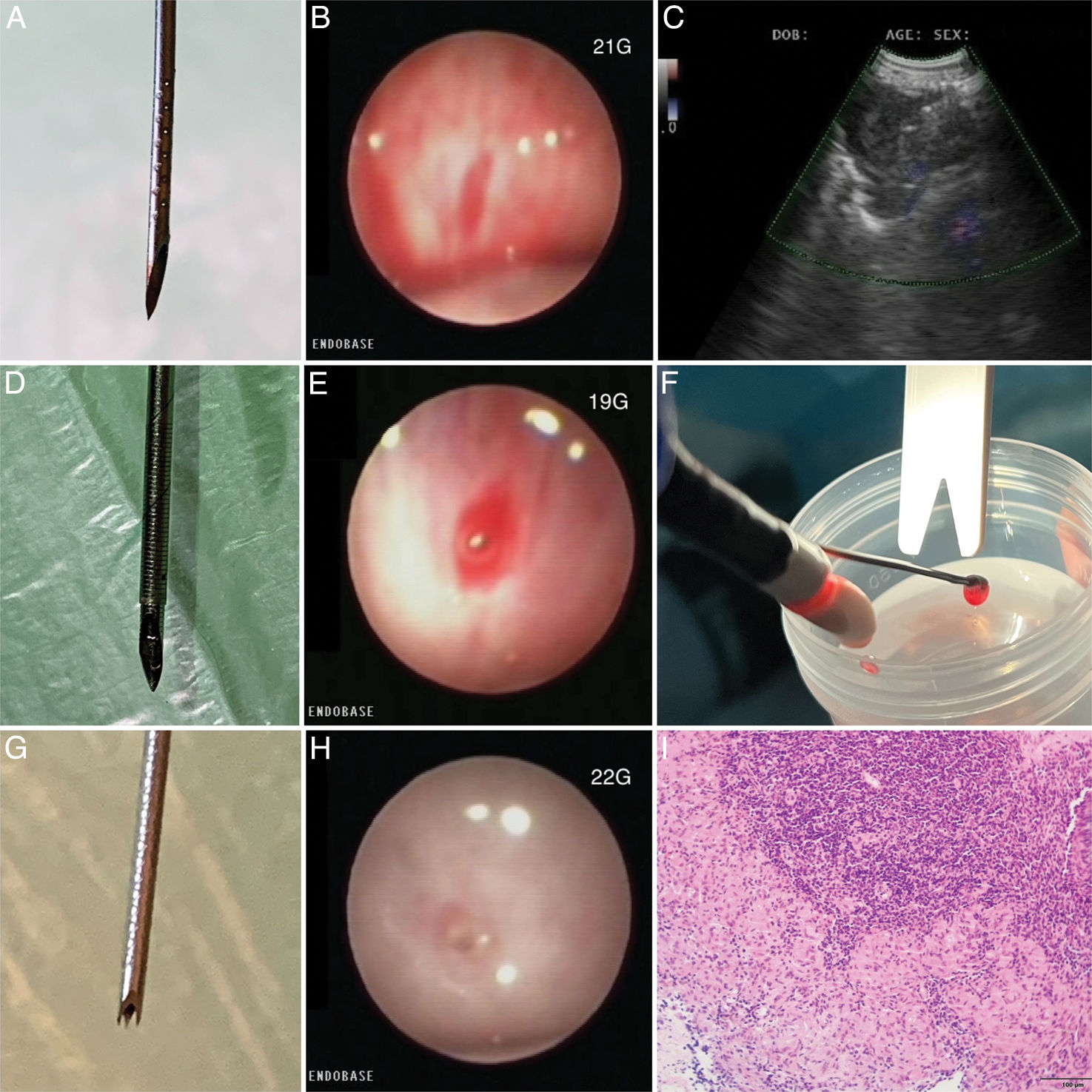
EBUS-TBNA with a 21G needle1,6 (Fig. 1A) was performed in 4 cases, requiring 2–3 punctures in each nodal station with approximately 40 passes (needle agitations) into the lesion per puncture for sampling and tunneling, being the last 5 passes at the proximal end of the lymph node capsule to achieve tunnelization (Fig. 1B), except in one case where we had to switch to a larger gauge needle (19G) to achieve tunneling. The 19G needle1,5,6 (Fig. 1D) was used in 6 cases, requiring 1–2 punctures per nodal station with approximately 40 passes into the lesion for sampling and tunneling, being the last 5 passes at the proximal end of the lymph node capsule to achieve tunnelization (Fig. 1E). Finally, the 22G needle1–3 (Fig. 1G) was used in 6 cases, requiring 1–2 punctures per nodal station with approximately 40 passes into the lesion for sampling and tunneling, being the last 5 passes at the proximal end of the lymph node capsule to achieve tunnelization (Fig. 1H). There was no ROSE (rapid on-site evaluation) in any case.
(A) 21G needle. (B) Puncture site with 21G needle. (C) 1.1mm cryoprobe inside lymphadenopathy with Doppler mode activated. (D) 19G needle. (E) Puncture site with 19G needle. (F) EBUS bronchoscope with cryobiopsy sample. (G) 22G needle. (H) Puncture site with 22G needle. (I) Polymorphic lymphoid population with the presence of granulomatous structures without necrosis, N-NGL.
EBUS-TBMC (Cryo-EBUS) was performed by inserting the 1.1mm cryoprobe through the working channel of the EBUS bronchoscope by tunneled opening via, and after confirming the absence of vascularization using doppler mode (Fig. 1C), a freezing cycle of 4–5s was applied, followed by the removal of the EBUS bronchoscope in one block, and the samples were placed in a formalin container (Fig. 1F). On average, 2 cryobiopsies were performed per selected nodal station. The nodal station where the most cryobiopsies were performed was station 7 (ten cases), followed by station 11L (seven cases), and finally station 11R (one case).
The results of this series of 15 cases were as follows: 6 cases of non-necrotizing granulomatous lymphadenitis (sarcoidosis, Fig. 1I), 4 cases of nodal anthracosis, 2 cases of pulmonary adenocarcinoma, 1 case of small cell neoplasia, 1 case of nodal tuberculosis with necrotizing granulomatous lymphadenitis, and 1 case of sinus histiocytosis. The diagnostic concordance between TBNA/cell block and cryobiopsy was 80% (in twelve cases). There was only one complication, a pneumomediastinum, which required hospitalization with conservative management and discharge after 72h of observation.
In conclusion, in this series of cases, we observed the potential of EBUS-TBMC in the diagnosis of benign pathologies requiring larger samples, with sarcoidosis being the most frequent diagnosis, with 6 cases, 3 of which were diagnosed through histology (cryobiopsy) and not through cytology (TBNA). We were also able to observe the technical potential of three different needles for tunneling, finding greater difficulty with the 21G needle, requiring two or three punctures to tunnel the pathway compared to the 19G needle (larger gauge) and the 22G needle (smaller gauge but with 3 cutting tips, crown-shaped), where only one or two punctures were required to achieve the same. The only complication was a pneumomediastinum following tunneling with the larger gauge needle (19G) and cryobiopsy, which may influence the initial choice and favor a smaller gauge needle, as well as consider performing fewer passes (needle agitations), prioritizing direct puncture-tunnelization with cryobiopsy and applying a shorter freezing time. Our experience in this series would require a larger, prospective patient cohort to assess the reliability, safety, and reproducibility of these findings.
Informed consentWritten informed consent was obtained in all cases for the publication of the article.
FundingThere was no funding source in this study.
Authors’ contributionsMTR: Performance of endobronchial ultrasound and cryobiopsy. Data and image collection. Drafting of the manuscript.
AAB: Performance of endobronchial ultrasound and cryobiopsy. Review and approval of the manuscript.
TRA: Case selection. Review and approval of the manuscript.
FDM: Review and analysis of data. Review and approval of the manuscript.
KCC: Case selection. Participation in the performance of endobronchial ultrasound. Review and approval of the manuscript.
CBD: Case selection. Review and approval of the manuscript.
PGM: Anatomopathological study and contribution of histological image. Review and approval of the manuscript.
Conflicts of interestThe authors declare that they have no conflict of interest to the publication of this article.
The authors thank the support of our own department of pulmonology, the departments of anesthesiology and pathology, and our nursing and auxiliary staff: Pilar Revilla, Gloria Martínez, Josefa Villar, Carmen San José y Mónica García.