Multiple sclerosis (MS) is a clinical entity characterised by both inflammatory and neurodegenerative components. Ocrelizumab is an anti-CD20 monoclonal antibody that has demonstrated high clinical efficacy in controlling neuroinflammation, and evidence suggests it may also exert a beneficial effect on neurodegeneration by reducing brain volume loss and progression independent of relapse activity. Our objective was to evaluate the effect of ocrelizumab on brain atrophy in MS patients using bidimensional atrophy measurements obtained from conventional MRI.
Patients and methodsThis was a retrospective study involving 38 MS patients who had been on ocrelizumab treatment for at least 24months. Both relapsing forms (RMS) and active progressive forms (PMS) were included. The brain atrophy indices assessed were bicaudate distance (Bicau, mm), corpus callosum index (CCI), and third ventricle width (3VW, mm).
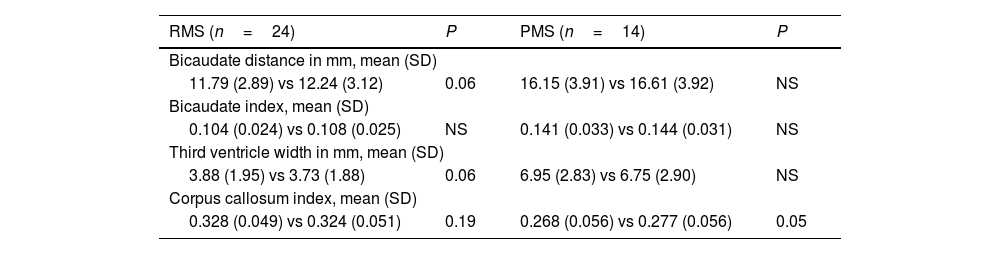
ResultsThe mean age was 45.6±11.6years. The clinical form distribution was RMS (n=24) and PMS (n=14). The median treatment duration was 44.5months (interquartile range: 35–58). In RMS patients, the values of the atrophy indices were 3VW (3.73±1.88 vs. 3.88±1.95, p=.06), CCI (0.33±0.05 vs. 0.32±0.05, p=N.S.), and Bicau (11.79±2.89 vs. 12.24±3.12, p=.06). In PMS patients, no statistically significant differences were observed.
ConclusionOur findings suggest that ocrelizumab is an effective therapy in reducing brain atrophy progression in both relapsing and progressive forms of MS.
La esclerosis múltiple (EM) es una entidad clínica caracterizada por un componente inflamatorio y otro neurodegenerativo. El ocrelizumab es un anticuerpo monoclonal anti-CD20 que muestra una alta eficacia clínica sobre la neuroinflamación y presenta datos que sugieren un efecto positivo sobre la neurodegeneración a través de la reducción de la pérdida del volumen cerebral y de la progresión independiente de los brotes. Nuestro objetivo fue valorar el efecto del ocrelizumab sobre la atrofia cerebral de pacientes con EM utilizando las medidas bidimensionales de atrofia de la RM convencional.
Pacientes y métodosEstudio retrospectivo donde se estudiaron 38 pacientes con EM que llevaban al menos 24 meses en tratamiento con ocrelizumab. Se incluyeron tanto formas recurrentes (EMR) como progresivas con actividad (EMP). Los índices de atrofia cerebral valorados fueron la distancia bicaudado (Bicau, mm), el índice del cuerpo calloso (ICC) y la anchura del III ventrículo (A3V, mm).
ResultadosLa edad media fue de 45,6±11,6 años. La distribución por formas clínicas fue EMR (n=24) y EMP (n=14). La mediana de duración del tratamiento fue de 44,5 meses (rango intercuartílico: 35–58). En los pacientes con EMR el valour de los índices de atrofia fue: A3V (3,73±1,88 vs 3,88±1,95, p=0,06), ICC (0,33±0,05 vs 0,32±0,05, p=N.S.), y Bicau (11,79±2,89 vs 12,24±3,12, p=0,06). En los pacientes con EMP no se encontraron diferencias estadísticamente significativas.
ConclusiónLos datos de nuestra serie muestran que el ocrelizumab es un fármaco eficaz para reducir la progresión de la atrofia cerebral en la EMR y en las formas progresivas.
Multiple sclerosis (MS) is an immune-mediated disease with a neuroinflammatory component that causes clinical relapses and the associated disability, and a neurodegenerative component involved in the independent progression of relapses, known as progression independent of relapse activity (PIRA). Ocrelizumab is an anti-CD20 monoclonal antibody that has been shown to be highly efficient in reducing the annualised relapse rate and the appearance of new MRI lesions.1 Furthermore, it has been suggested that the drug has a positive effect on neurodegeneration, slowing the rate of brain volume loss and reducing the increase in PIRA.2–4
In clinical trials and the research setting, brain atrophy is measured with cerebral volume quantification techniques, whose use is limited to certain tertiary-level hospitals. In everyday clinical practice, cerebral atrophy should be assessed in conventional brain MRI studies using such bidimensional measurements as bicaudate distance (BCD, mm), third ventricle width (TVW, mm), and corpus callosum index (CCI).5–8 These measurements enable indirect assessment of thalamic atrophy (TVW) and subcortical atrophy (BCD); the corpus callosum is the largest interhemispheric commissure and is affected in the majority of patients with MS. Furthermore, these measurements show significant correlations with cognitive impairment as assessed with the Symbol Digit Modalities Test (SDMT),9 as well as cerebral volume measurements.10
The aim of our study was to measure the progression of brain atrophy in patients with MS treated with ocrelizumab using bidimensional measurements in MRI studies.
Patients and methodsWe conducted an observational, descriptive, open study. Patients were recruited from an outpatient neurology consultation. We included patients with MS, aged older than 18years, who were receiving treatment with ocrelizumab according to clinical guidelines. Therefore, we included patients presenting highly active relapsing MS (RMS), secondary progressive MS (SPMS), and primary progressive MS (PPMS) with clinical (relapses) or radiological activity (appearance of new lesions/contrast uptake). Patients had to have been receiving treatment with ocrelizumab for at least 24months.
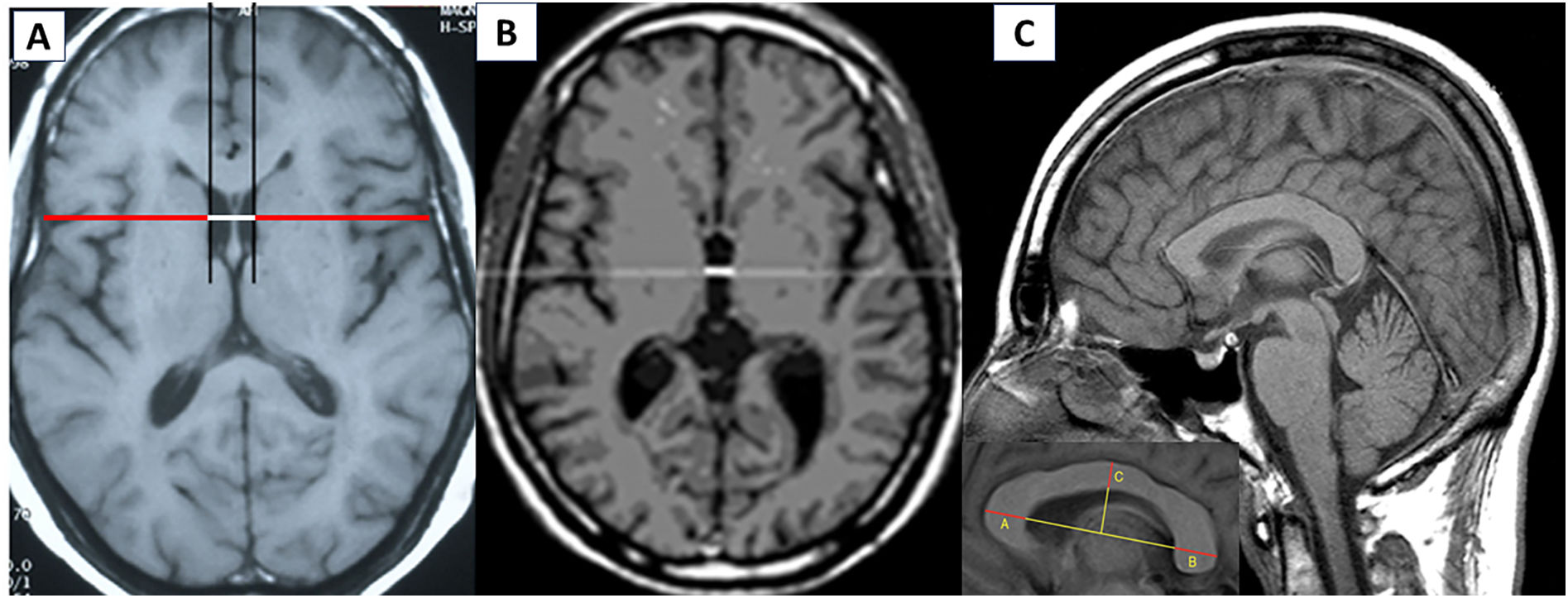
All patients had undergone a baseline MRI study in the 6months prior to the onset of treatment with ocrelizumab. Brain atrophy was estimated using bidimensional measurements, specifically BCD, bicaudate index (BCI), CCI, and TVW (Fig. 1). Measurements were obtained by a neurologist after a training period at our hospital's radiology department. The BCI was measured using the axial FLAIR slice in which the head of the caudate nuclei was most visible and closest together, and was defined as the minimum intercaudate distance divided by brain width along the same line.10 The CCI is measured on the sagittal T1-weighted image with the best view of the corpus callosum, centred on the cerebellar vermis; a straight line is drawn between the most prominent part of the splenium and the genu of the corpus callosum (a–b); the index is obtained by adding the thickness of the genu (a_a′), the splenium (b_b′), and corpus callosum (c_c’), and dividing the sum by the a-b distance ((a_a′+b_b′+c_c′)/a_b).8
The study variables were age, time since diagnosis, Expanded Disability Status Scale (EDSS) score, BCD, BCI, CCI, and TVW.
Data were analysed using the SPSS Statistics software. Firstly, we used the Kolmogorov–Smirnov test to check the normality of variables (goodness of fit). For normally distributed variables, we used parametric statistics (mean, standard deviation, and t test for paired samples) and, for non-normally distributed variables, we used non-parametric statistics (median, range, and Wilcoxon test). P-values <.05 were considered statistically significant.
The study was approved by the drug research ethics committee of the region of Galicia, with the record code 2025/219. Before inclusion in the study, patients gave their written informed consent.
ResultsWe analysed 38 patients with MS (17 women and 21 men) with a mean (SD) age of 45.6 (11.6) years. Twenty-four patients (63.2%) presented RMS, and the remaining patients had active progressive MS (SPMS: 9; PPMS: 5). The mean time since diagnosis was 13.7 (9.1) years. Eleven patients (28.9%) had not previously received treatment with disease-modifying therapies for MS; of the remaining patients, 7 had switched from natalizumab due to a high risk of progressive multifocal leukoencephalopathy, and the other 20 from various other treatments due to treatment failure (relapses) in all cases. The mean EDSS score of the group amounted to 3.8 (2.5) (RRMS: 2.2 [1.4]; progressive MS: 6.7 [0.7]). Patients had been receiving ocrelizumab for a median of 44.5months (range, 25–72months). None presented MS relapses during the follow-up period.
All measurements were significantly correlated (Pearson coefficient) with EDSS score (CCI: r=−0.35, P=.032; TVW: r=0.49, P=.002; BCD: r=0.55, P<.001).
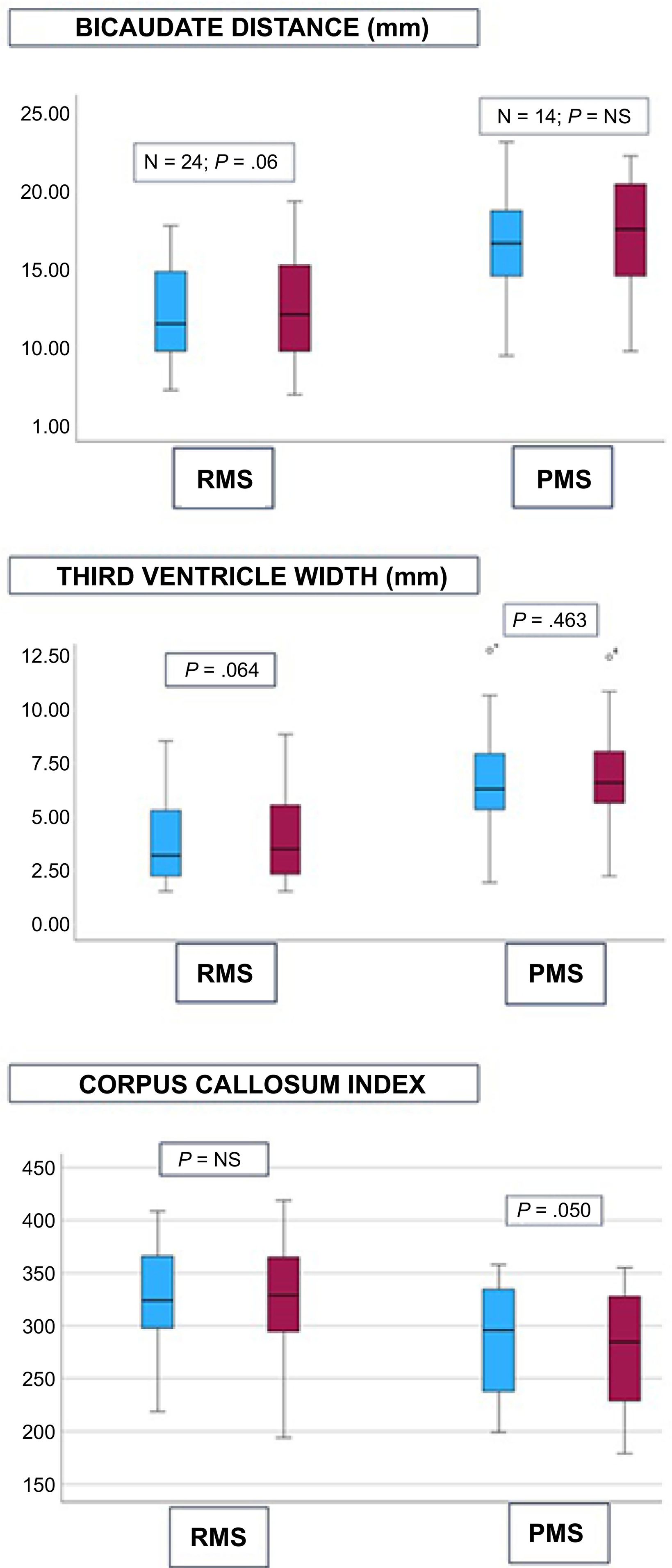
BCI significantly increased by 3% during follow-up (13.40 [3.89] vs 13.86 [4.00] mm, P=.01), which represented an increase of 0.12mm/year. TVW did not significantly change (4.84 [2.70] vs 5.01 [2.73] mm, P=.10). CCI showed a slight improvement of 2% (0.313 [0.055] vs 0.307 [0.057], P=.02). Table 1 and Fig. 2 show the values of the different measurements in patients with RMS and progressive MS. The neuroprotective effect of treatment with ocrelizumab is more evident in progressive forms.
Bidimensional measurements of brain atrophy in relapsing and progressive forms of multiple sclerosis.
| RMS (n=24) | P | PMS (n=14) | P |
|---|---|---|---|
| Bicaudate distance in mm, mean (SD) | |||
| 11.79 (2.89) vs 12.24 (3.12) | 0.06 | 16.15 (3.91) vs 16.61 (3.92) | NS |
| Bicaudate index, mean (SD) | |||
| 0.104 (0.024) vs 0.108 (0.025) | NS | 0.141 (0.033) vs 0.144 (0.031) | NS |
| Third ventricle width in mm, mean (SD) | |||
| 3.88 (1.95) vs 3.73 (1.88) | 0.06 | 6.95 (2.83) vs 6.75 (2.90) | NS |
| Corpus callosum index, mean (SD) | |||
| 0.328 (0.049) vs 0.324 (0.051) | 0.19 | 0.268 (0.056) vs 0.277 (0.056) | 0.05 |
NS: not significant; PMS: progressive multiple sclerosis; RMS: relapsing multiple sclerosis.
Mean values of the different measurements of brain atrophy in brain MRI studies, grouped by relapsing and progressive forms with clinical and/or radiological activity. Blue: baseline values prior to ocrelizumab treatment. Red: values after a median follow-up period of 44.5months.
NS: not significant; PMS: progressive multiple sclerosis; RMS: relapsing multiple sclerosis. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Multiple sclerosis is a disease with a pronounced neurodegenerative component that translates into brain and spinal cord atrophy and is associated with clinical progression and cognitive impairment.11 Scientific evidence suggests that moderately and highly efficacious disease-modifying therapies present a benefit by reducing the progression of cerebral atrophy, mainly measured with cerebral volume quantification techniques.4,12–15
Ocrelizumab has been reported to slow the rate of brain volume loss, approaching the healthy ageing rate,4 and to reduce thalamic atrophy.16 Our clinical series demonstrates the potential effect of ocrelizumab on brain atrophy in patients with MS through the use of bidimensional measurements to quantify atrophy in brain MRI studies. Thus, in our series, for a median follow-up period of 44months, we observed a benefit, with a reduction in the progression of brain atrophy, which seems to be more significant in progressive forms of MS. Studies of patients with MS have reported a mean increase in brain atrophy of 0.46mm/year as measured with BCD and 0.20mm/year with TVW.5 In our series, BCD increased by 0.12mm/year, which represents a 75% reduction in the progression of atrophy with regard to the data available in the literature; this positive effect is reinforced by the absence of significant differences in BCI during follow-up (Fig. 2). In this context of slowing the rate of brain atrophy, the absence of statistically significant differences in TVW and CCI should be noted. When RMS and progressive forms of MS are analysed independently, the positive effect of ocrelizumab on brain atrophy becomes even clearer.
Serum neurofilament light chain levels are elevated in patients with MS and increase with inflammatory activity (relapses and/or gadolinium-enhancing lesions). Treatment with ocrelizumab in RMS and progressive MS has shown a greater effect on thalamic atrophy in patients with high neurofilament light chain levels at treatment onset.6 In our study, we observed no statistically significant differences in TVW for a median follow-up time of almost 4years, supporting the effect of ocrelizumab in reducing thalamic atrophy in patients with RMS and with active progressive forms.
Some limitations of our study are the small number of patients included and its open, retrospective design. However, our findings are in line with the data reported in the literature on the effect of ocrelizumab in reducing the progression of brain atrophy as measured with MRI volumetric quantification techniques.4,16 Furthermore, we highlight the moderate correlation between bidimensional measurements of atrophy in brain MRI studies and MS-related disability as measured with the EDSS.
In conclusion, our data not only corroborate the effectiveness of ocrelizumab treatment in reducing the progression of brain atrophy, but are also consistent with the cerebral volume measurements reported in clinical trials. Furthermore, our findings also support the role of conventional MRI as a useful tool both for assessing brain atrophy in everyday clinical practice9,17 and for rapidly assessing the effect of treatment on disease progression.18
Ethical approval and patient consentThe study was approved by the drug research ethics committee of the region of Galicia (record code: 2025/219).
Declaration of generative AI and AI-assisted technologies in the writing processNot applicable.
FundingThis study has received no external funding of any kind.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.