The anti-CD20 monoclonal antibodies, rituximab (RTX) and ocrelizumab (OCRE), are used routinely for the treatment of neuroimmunological diseases. However, infusion-related reactions (IRRs) are common, and their underlying mechanisms are not well defined.
ObjectivesOur aim was to determine the incidence of IRRs during OCRE and RTX treatment for neuroimmunological diseases and to identify predisposing factors.
MethodsWe conducted a retrospective observational study including patients who received at least one dose of OCRE or RTX between October 2018 and March 2022. Demographic data, comorbidities, immunological variables, and information about treatments and IRRs were collected. Statistical analysis was performed to assess IRR incidence, differences between groups, and predictors of IRR.
ResultsOf 48 patients receiving 239 infusions, 17.6% presented IRRs. The incidence of IRRs was 40% among all patients: 41% in the OCRE group and 38% in the RTX group. Most IRRs were mild (90%). We found statistically significant differences in infusion time between both groups, with longer times associated with higher IRR incidence. Interestingly, a higher percentage of B lymphocytes prior to infusion was associated with increased risk of IRRs.
ConclusionIRRs are common with both treatments, especially in the early stages. The percentage of B lymphocytes before infusion and longer infusion times are associated with a higher risk of developing IRRs. These findings may help to mitigate IRRs during treatment.
El rituximab (RTX) y el ocrelizumab (OCRE), anticuerpos monoclonales anti-CD20, se utilizan en el tratamiento de enfermedades neuroimmunológicas. Las reacciones relacionadas con su infusión (RAI) son frecuentes y sus mecanismos subyacentes no están bien definidos.
ObjetivosDeterminar la incidencia de RAI con OCRE y RTX en pacientes con enfermedades neuroinmunológicas, e identificar factores predisponentes.
MetodologíaEstudio observacional retrospectivo que incluyó pacientes que recibieron al menos una dosis de OCRE o RTX entre octubre de 2018 y marzo de 2022. Se recogieron datos demográficos, comorbilidades, variables inmunológicas e información sobre los tratamientos y las RAI. Se realizó un análisis estadístico para evaluar la incidencia de RAI, las diferencias entre grupos y los factores predictivos de RAI.
ResultadosDe un total de 48 pacientes que recibieron 239 infusiones, el 17,6% presentaron RAI. La incidencia de RAI fue del 40% entre los pacientes, siendo del 41% en el grupo OCRE y del 38% en el grupo RTX y la mayoría, leves (90%). Se observaron diferencias estadísticamente significativas en el tiempo de infusión entre ambos grupos, asociándose tiempos más prolongados con una mayor incidencia de RAI. Se objectiva un mayor porcentaje de células B antes de la infusión se asoció con un mayor riesgo de RAI.
ConclusiónLas RAI son frecuentes con ambos tratamientos, especialmente en las fases iniciales. La presencia de células B antes de la infusión se asocian con un mayor riesgo de desarrollar RAI. Las presencia de RAI aumenta el tiempo de infusión. Estos hallazgos podrían contribuir a mitigar las RAI.
Multiple sclerosis (MS) is an autoimmune disease characterised by inflammation, demyelination, and neurodegeneration of the central nervous system (CNS).1–4 It typically manifests between the ages of 20 and 40 years, and is the leading non-traumatic cause of disability in young people.2 The number of people affected is around 2.9 million worldwide.5 In Spain, around 50 000 people are estimated to be affected.
Neuromyelitis optica spectrum disorder (NMOSD) is an autoimmune disease that mainly affects the optic nerve.6,7 To date, anti-AQP4 antibodies (Ab) have been identified in these patients, suggesting the involvement of humoural immunity in its pathophysiology.8,9 On the other hand, autoimmune encephalopathies are the result of a humoural and cellular response against antigens in the brain parenchyma.10
B lymphocytes, in addition to T lymphocytes, play a crucial role in the pathophysiology of MS.11–15 CD20 is an antigen present on the surface of mature B lymphocytes, which is not expressed in lymphoid stem cells or plasma cells.16,17 The first drug targeting the CD20 receptor of B lymphocytes for the treatment of MS was rituximab (RTX), with intravenous administration. Subsequently, the humanised monoclonal antibody ocrelizumab (OCRE) was introduced; this was the first drug approved for the treatment of both relapsing-remitting (RRMS) and primary progressive MS (PPMS).18–20 RTX is used to treat NMOSD and autoimmune encephalopathies based on evidence from case series, although there are no formal clinical trials to support this indication. B cell depletion may help to curb the autoimmune mechanism of these diseases.
Anti-CD20 Ab differ in terms of molecular structure, the epitopes to which they bind, and the mechanisms by which they destroy B lymphocytes. These differences may affect immunogenicity and possible adverse reactions. Infusion-related reactions (IRRs) are defined as reactions that occur during the infusion of the medication, and include flushing, pruritus, erythema, urticaria, chest pain, dyspnoea, changes in blood pressure and heart rate, abdominal pain, nausea, vomiting, or fever, and can result from various immunological mechanisms.21,22
The most common adverse reaction with OCRE is IRR, whose prevalence ranges from 21% to 40% of treated patients. These reactions are most frequent during the first infusion (22%-27%).23–25 Most are mild or moderate and respond well to treatment.23 Although RTX is generally well tolerated, a study by Yamout et al26 reported that 26% of patients experienced IRRs in 40 administered infusions; these were mostly mild and self-limiting.
The mechanisms causing IRRs are not well defined, and multiple factors may be involved. They may be due to type 1 hypersensitivity (HS), mediated by immunoglobulin E, characterised by activation and degranulation of mast cells with the production and release of such anaphylotoxins as tryptase,27,28 or type 2 HS, mediated by immune complexes and the release of such cytokines as interleukin 6 (IL-6).29,30 IRRs caused by monoclonal Ab are mainly due to type 2 HS reactions.31
These reactions are prevented and managed through premedication, patient monitoring, and symptomatic treatment.21,23,25,31 Since clinical manifestations are similar regardless of the immune process activated, serum tryptase and IL-6 should be determined in patients with IRRs to identify the type of secondary reaction.
The incidence and risk factors of IRRs secondary to the use of anti-CD20 monoclonal Ab are not well established. For this reason, the objective of this study was to determine the incidence of IRR with the use of OCRE and RTX in autoimmune diseases of the CNS, as well as to identify factors predisposing to their appearance, in order to improve their prevention.
MethodologyWe conducted a retrospective observational study at the Multidisciplinary Day Hospital of the Hospital Universitari Germans Trias i Pujol (HUGTiP), a public reference centre for autoimmune diseases of the CNS in the North Metropolitan area of Barcelona, between October 2021 and April 2022. The Research Ethics Committee of HUGTiP approved the study. Patients were informed about the study objectives and procedures, and those who agreed to participate signed informed consent forms.
The inclusion criteria for the study were adult patients diagnosed with MS, NMOSD or autoimmune encephalopathy, based on clinical, radiological or laboratory criteria, followed up at HUGTiP’s MS and Neuroimmunology Unit, who had received at least one dose of treatment with OCRE or RTX according to the usual guidelines, preceded by premedication (dexchlorpheniramine 5 mg, hydrocortisone 100 mg, paracetamol 1000 mg, metoclopramide 10 mg and, in RTX infusion, famotidine 20 mg), between October 2018 and March 2022. OCRE: 2 initial doses of 300 mg separated by an interval of 2 weeks, with maintenance doses of 600 mg every 6 months; RTX: 2 initial doses of 500 mg separated by an interval of 2 weeks and maintenance doses of 500 or 1000 mg every 6 months. Patients treated at other centres and those who did not sign the informed consent form were excluded.
Study variablesThe study variables were gathered from patient medical records. Demographic variables were age, sex, body mass index (BMI), and smoking status (smokers, former smokers, or never smokers). Comorbidity variables were the presence of diabetes, dyslipidaemia, hypertension, chronic kidney disease, and cardiovascular disease. Variables related to atopy were personal and/or family history of allergy or atopy or diseases progressing with elevated tryptase (eg, mastocytosis or mast cell activation syndrome). Variables related to the neuroimmunological disease were the type of disease, the year of diagnosis, Expanded Disability Status Scale score at the start of anti-CD20 treatment and at recruitment, the presence of disease relapses during treatment, and the year and reason for discontinuation of anti-CD20 treatment, if applicable. Variables related to anti-CD20 treatment were the drug administered, dose, and treatment start date. Infusion-related variables were the number of doses received, the infusion time of each dose, the premedication received, and the percentage of B lymphocytes (defined as CD19+ cells) prior to each infusion. Variables related to IRR were the number of infusions in which IRR occurred, the severity of the IRR (IRR was defined as mild when no medication was required, moderate if medication was required to proceed with administration, and severe if medication was required and the infusion had to be suspended), clinical manifestations (pruritic rash, non-pruritic rash, pruritus, ear pain, and odynophagia or oropharyngeal discomfort), and the outcome of the IRR. Immunological variables were serum tryptase and IL-6 levels prior to treatment onset. Subjective patient-related variables on the perception of IRR in some of the infusions received (perception of IRR: yes/no) were collected at the time of inclusion in the study, with specific questions about symptoms, including dyspnoea, pruritus, odynophagia, palpitations, vomiting, rash, or discomfort in any of the infusions received previously.
Statistical analysisFor the calculation of IRR incidence, the numerator was the number of patients who presented at least one IRR, and the denominator was the number of patients treated with each drug.
A descriptive analysis was conducted with frequencies for categorical variables, and with means, standard deviation (SD), medians, and interquartile range (IQR) for continuous variables. For comparisons between groups (RTX and OCRE), we performed the chi-square test of independence for categorical variables and the t test for continuous variables to assess whether patients treated with RTX and OCRE were homogeneous. A bivariate analysis was also conducted to establish the mean number of IRRs per individual and to analyse the proportion of IRRs by treatment number.
The chi-square test and t test were also used to examine differences between categorical and continuous explanatory variables and IRR incidence. A logistic model was created for all treatments to analyse whether having experienced IRR in any of the previous infusions or in the immediately preceding one had a significant effect on the odds ratio (OR) for IRR incidence, excluding the first treatment.
A generalised estimating equation (GEE) logistic model (an extension of the generalised linear model) with first-order autocorrelation was also developed to relate the number of previous infusions and IRRs to the probability of presenting IRR, based on the drug, age, and B lymphocyte population. Statistical analysis was performed using the R Studio software, and all statistical analyses were conducted with a 95% confidence level.
ResultsBetween October 2021 and April 2022, 50 patients meeting the inclusion criteria were selected; 2 of these did not agree to participate in the study. Therefore, a total of 48 patients were recruited.
Demographic and clinical characteristicsTwenty-eight patients (58.3%) were women, with a median age of 47 years (IQR: 17; range, 21-70). Some 18.7% (9/48) had dyslipidaemia, 8.3% (4/48) had diabetes mellitus, 8.3% (4/48) had hypertension, and 2% (1/48) had kidney failure. No patient had cardiovascular disease or conditions associated with elevated tryptase levels. A total of 23% (11/48) had a family history of atopy, and 18.8% (9/48) had a personal history of atopy. Twenty-nine (60.4%) had a history of smoking (active smokers: 13/48; former smokers: 16/48). The mean BMI was 26 kg/m2 (SD: 5.6). Some 83.3% (40/48) had MS (21 RRMS, 12 PPMS, and 7 secondary progressive MS), 14.5% had NMOSD, and 2% presented Morvan syndrome. At baseline, tryptase levels were undetectable (< 3.13 pg/mL) in 60%. In patients with detectable levels, the values ranged between 3.13 and 74 pg/mL, with a median of 6 pg/mL (IQR: 3.5). The median time from diagnosis to onset of anti-CD20 treatment was 2 years (IQR: 6.5), and the median Expanded Disability Status Scale score at treatment onset was 4.25 (IQR: 3). Three patients (6.25%) had at least one relapse during the study period. Some 64% (28/48) of patients reported having experienced infusion-related symptoms during at least one previous infusion.
Comparison of patient characteristics between treatment groupsOf the total sample, 56.2% of patients (27/48) received treatment with OCRE, and 43.7% (21/48) received RTX. All patients in the OCRE group had MS, with a median age of 41.7 years (IQR: 19), and 63% (17/27) were women. In the RTX group, 62% (13/21) had MS, the median age was 52 years (IQR: 16), and 52.3% (11/21) were women (Table 1).
Clinico-demographic characteristics in each treatment group.
| OCRE | RTX | TOTAL | P | |
|---|---|---|---|---|
| n (%) | 27 (56.2%) | 21 (43.7%) | 48 | |
| CNS disease | 100% (27/27) MS | 62% (13/21) MS33% (7/21) NMOSD4.7% (1/21) Morvan syndrome | .002 | |
| Women | 63% (17/27) | 52.3% (11/21) | 58.3% (28/48) | .658 |
| Age in years, median (IQR) | 41.7 (16) | 52 (19) | 47 (17) | .008 |
CNS: central nervous system; IQR: interquartile range; MS: multiple sclerosis; NMOSD: neuromyelitis optica spectrum disorder; OCRE: ocrelizumab; RTX: rituximab.
Statistically significant differences were found between the OCRE and the RTX groups for age (47 vs 52 years; P = .008) and the type of CNS disease (MS in 100% of patients in the OCRE group vs 61% in the RTX group; P = .002).
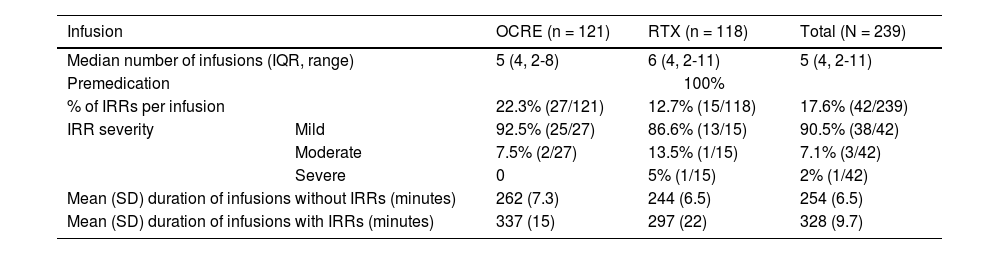
Characteristics of the infusion and infusion-related reactionsThe 48 patients received a total of 239 infusions over a median of 27 months (IQR: 24; range, 6-63). All infusions were preceded by intravenous medication in accordance with our centre’s protocol. The median number of infusions received per patient was 5 (IQR: 4; range 2-8) in the OCRE group and 6 (IQR: 4; range 2-11) in the RTX group. IRRs occurred in 17.6% (42/239) of infusions, with an incidence of 22.3% (27/121) in the OCRE group and 12.7% (15/118) in the RTX group. Overall, 40% of patients (19/48) experienced at least one IRR, with an incidence of 41% (11/27) in the OCRE group and 38% (8/21) in the RTX group. We observed no statistically significant differences between the 2 groups (Table 2).
Characteristics of the infusions and reactions in each treatment group.
| Infusion | OCRE (n = 121) | RTX (n = 118) | Total (N = 239) | |
|---|---|---|---|---|
| Median number of infusions (IQR, range) | 5 (4, 2-8) | 6 (4, 2-11) | 5 (4, 2-11) | |
| Premedication | 100% | |||
| % of IRRs per infusion | 22.3% (27/121) | 12.7% (15/118) | 17.6% (42/239) | |
| IRR severity | Mild | 92.5% (25/27) | 86.6% (13/15) | 90.5% (38/42) |
| Moderate | 7.5% (2/27) | 13.5% (1/15) | 7.1% (3/42) | |
| Severe | 0 | 5% (1/15) | 2% (1/42) | |
| Mean (SD) duration of infusions without IRRs (minutes) | 262 (7.3) | 244 (6.5) | 254 (6.5) | |
| Mean (SD) duration of infusions with IRRs (minutes) | 337 (15) | 297 (22) | 328 (9.7) | |
IQR: interquartile range; IRRs: infusion reactions; OCRE: ocrelizumab; RTX: rituximab; SD: standard deviation.
IRRs were mild in 90% of cases (38/42), moderate in 7% (3/42), and severe in 2.3% (1/42). Only one patient (2%) in the RTX group experienced a severe IRR. No IRR caused the death of a patient or transfer to a critical care unit.
Statistically significant differences in the duration of the infusion were found between treatment groups, with a mean of 280 minutes in the OCRE group and 246 minutes in the RTX group (P < .05). Infusions with IRR had significantly longer durations than those without IRR (328 vs 254 minutes, P < .05). This difference was significant for OCRE infusions (337 vs 262 minutes, P < .05), but not for RTX infusions (Table 2).
Patients with IRRs presented a median of 2 different symptoms per episode (range, 1-7). Oropharyngeal symptoms (itching of throat and ears, nasal congestion, sensation of oropharyngeal obstruction) were the most frequent, occurring in 75% of IRRs.
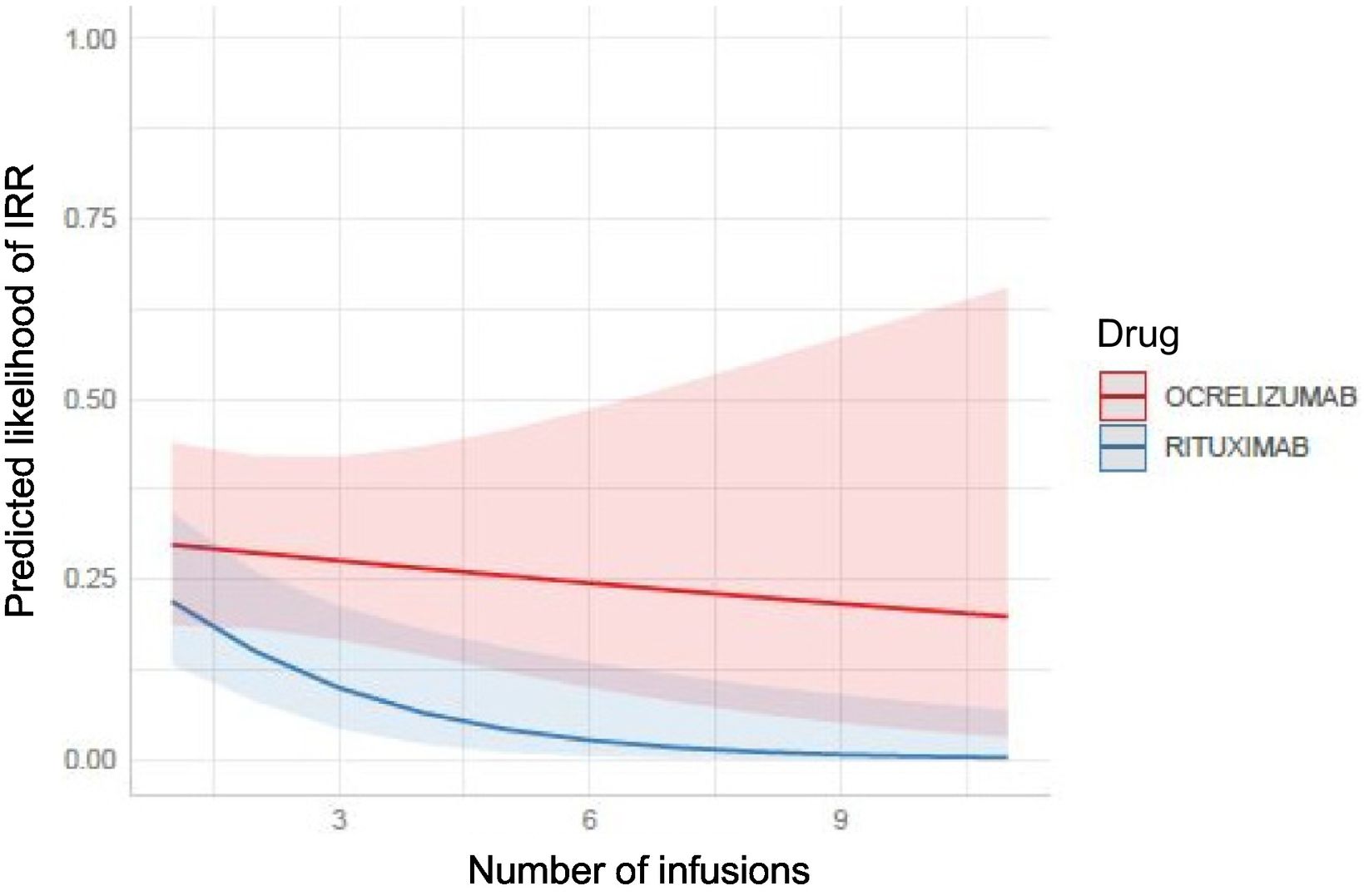
Risk factors for infusion-related reactionsAssociation between infusion-related reactions and the number of infusionsThe probability of developing an IRR was higher in OCRE infusions compared to RTX, especially as the number of infusions increased. However, no significant differences were found between the 2 treatments (Fig. 1).
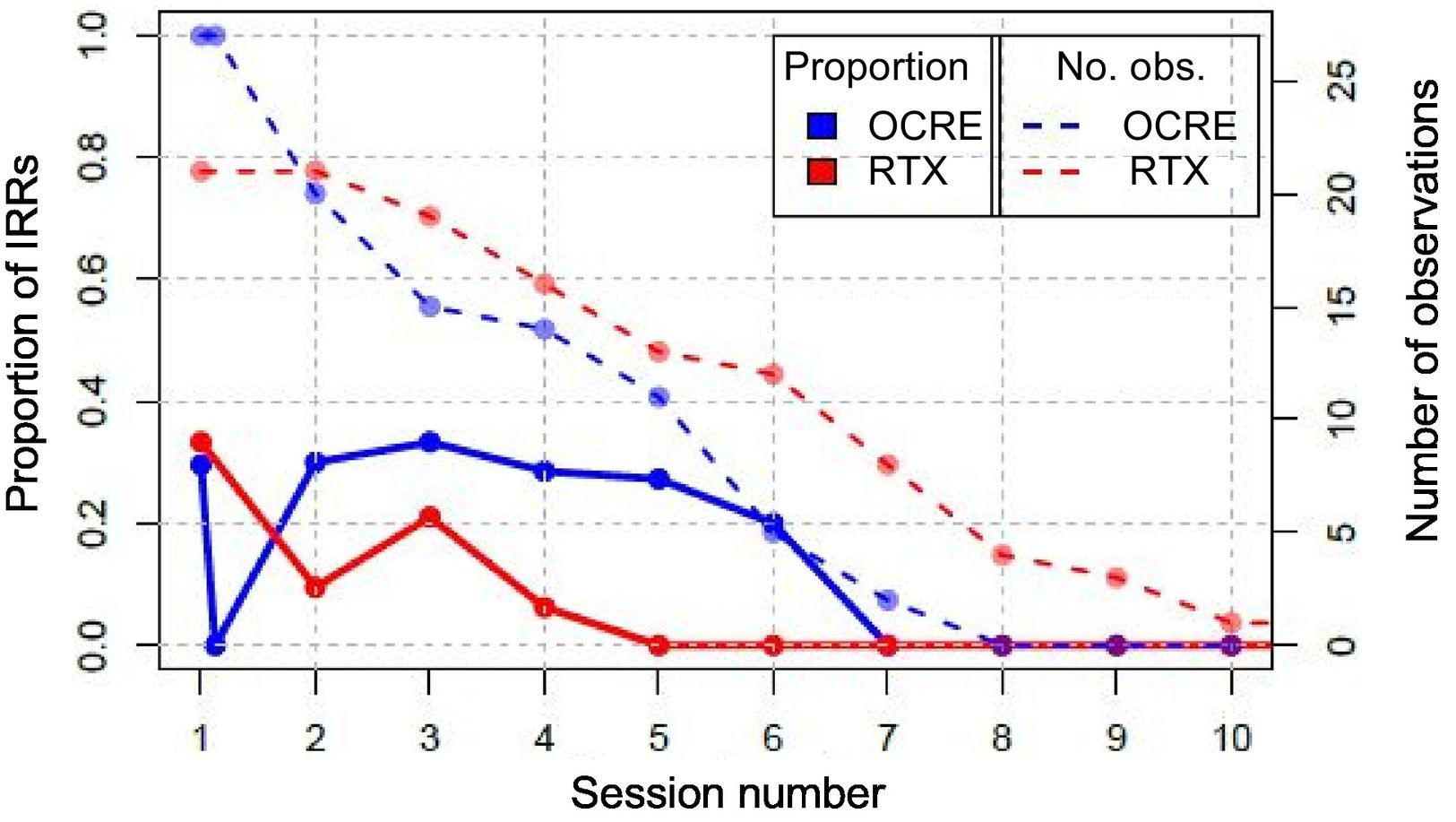
The proportion of IRR is prolonged in the case of treatment with OCRE compared to treatment with RTX, where no IRR occurs after the fifth infusion. Given that the sample size decreases as the number of infusions increases, the results presented in Fig. 2 become progressively less reliable.
Association between infusion-related reactions and history of infusion reactionThe logistic regression model revealed that patients who presented an IRR in previous infusions were more likely to develop new IRRs in subsequent infusions, with this risk being significantly higher in the group treated with OCRE (48%) than in the RTX group (16%). In contrast, the probability of developing IRR with the infusion of OCRE without having previously presented IRR was 9%, vs 2% in the case of RTX.
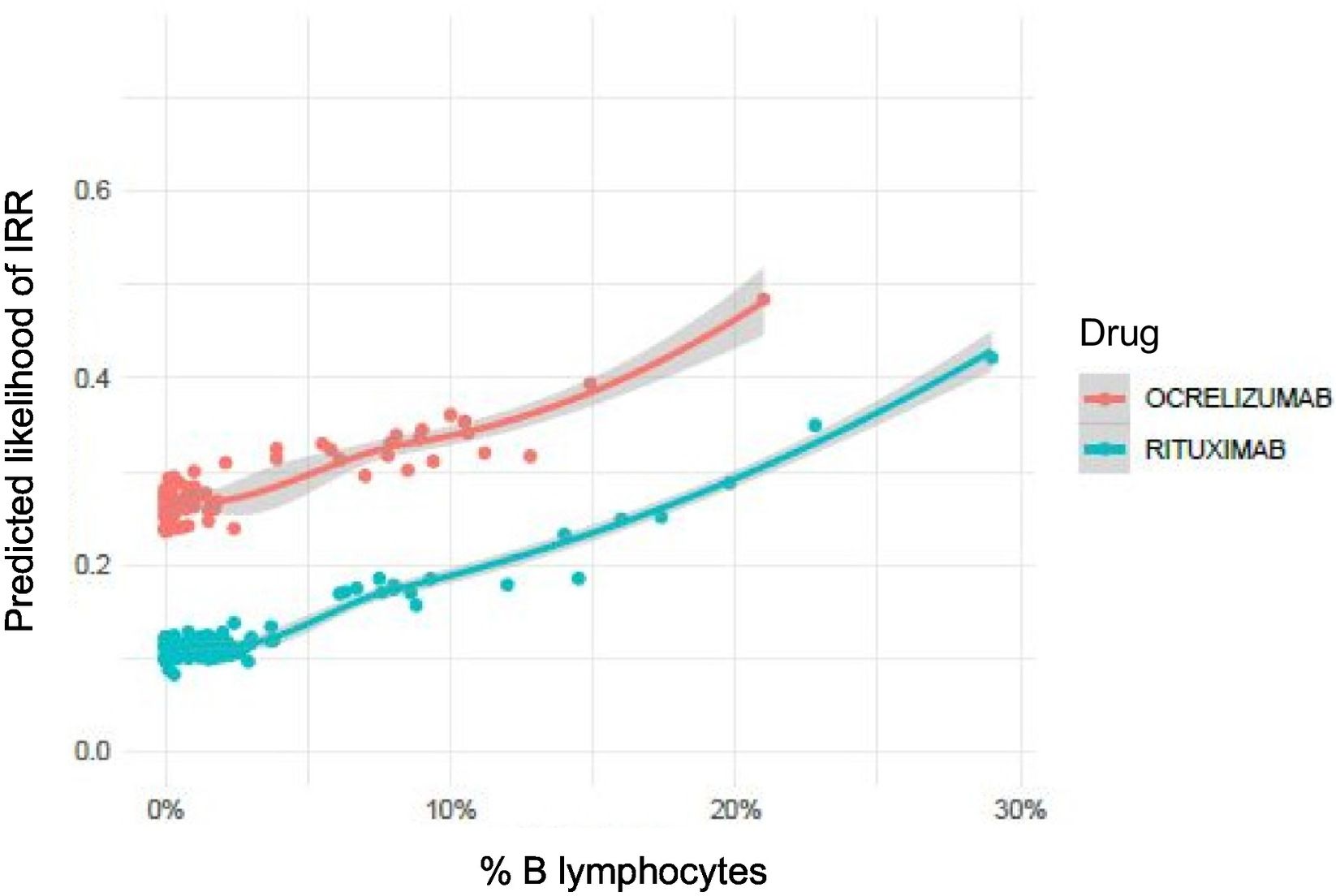
Association between infusion-related reactions and percentage of B lymphocytes prior to administrationThe mean (SD) percentage of B lymphocytes before the first infusion was 9% (4%) in the OCRE group and 10% (7%) in the RTX group, with no statistically significant differences between the 2 treatments. This trend was maintained in all subsequent infusions, with no significant differences between the 2 groups (Appendix 1). An increase of one unit in the percentage of B lymphocytes prior to the administration of the anti-CD20 Ab was found to increase the risk of developing an IRR by 5.5% (OR = 1.055). Although the graph in Fig. 3 shows differences between the 2 drugs, B lymphocyte values prior to the administration of the treatments that triggered IRR did not show statistically significant differences between treatment groups (P = .08) (Appendix 2).
Infusion-related reaction perception questionnaireAll patients answered the IRR perceptions questionnaire. A total of 62.5% of patients (30/48) reported experiencing symptoms compatible with IRR during previous infusions. Of these, 60% (18/30) had documented evidence of infusion reactions in their medical records, while the remaining 40% (12/30) did not. One patient (2%) who reported no clinical IRRs in previous infusions did have at least one episode documented in the medical record, suggesting a possible discrepancy between subjective perception and clinical documentation of the events.
DiscussionFrom a sociodemographic perspective, differences were observed between patients who received one drug or the other, both in age and in the neuroimmunological disease of the CNS. This would be explained by the indication of OCRE as the drug of first choice in RRMS and PPMS, whereas RTX is mainly used in other CNS diseases with an older age of onset. More than a third of the infusions administered triggered IRRs (40% of treated patients), a percentage similar to the highest described in the literature.23–25 The main symptoms reported by patients were oropharyngeal discomfort, which was mostly mild, despite the fact that oropharyngeal symptoms are considered moderate by some authors, given their potential to trigger upper airway oedema.24,32 Our results are consistent with the study by Hauser et al.,25 in which the majority of IRRs were classified as mild.
The risk of presenting IRR is greater in patients with a history of IRR in previous infusions, particularly in those receiving OCRE. However, this trend was also identified in RTX infusions. Only one study has suggested that a history of IRR may be a risk factor for its recurrence in patients treated with monoclonal Ab for cancer.33 On the other hand, patients without a previous IRR were much less likely to develop an IRR during infusion of OCRE or RTX. In our study, no other sociodemographic risk factors or associated comorbidities, including BMI, were identified, unlike other studies, which have suggested a higher probability of IRR in patients with high BMI.23 On the other hand, we observed long-term persistence of the risk of IRR in OCRE treatment, compared to RTX; this has not previously been described and would be an interesting subject for more detailed analysis in a more extensive study.
It is important to note that there are 2 differences in the administration of the 2 drugs, which may explain a greater tendency towards IRRs with OCRE than with RTX. Firstly, the medications administered prior to the infusion of RTX but not OCRE include famotidine, which acts by blocking the anti-H2 histamine receptors. We believe that famotidine would not explain the lower likelihood of IRRs in RTX treatment, since it is not currently a routine drug in the guidelines for the management of anaphylaxis.34 Secondly, the doses of both drugs are different. In the case of OCRE, the first dose is divided into 2 administrations, whereas for RTX, an induction at double the dose is administered (2 weeks between one dose and the other). Although our analysis did not demonstrate this fact, it may be responsible for greater depletion of B lymphocytes and consequently reduce the occurrence of IRR. OCRE is also known to present greater affinity than RTX for the FcγRIII immunoglobulin receptor, responsible for the Ab-dependent cytotoxicity mechanism, causing the release of cytokines that would be responsible for type 2 HS reactions. This could explain the greater tendency to IRRs in OCRE infusions than in RTX treatment.
It is worth noting that the percentage of B lymphocytes prior to infusion was the only blood analysis variable identified as a risk factor for IRR. The probability of developing IRR increases in line with the percentage of B lymphocytes. Though graphical differences were observed between the 2 drugs, the values did not show statistically significant differences; therefore, further studies would be needed to confirm this trend. These results would suggest that IRR would be more related to the release of cellular products due to the depletion of the patient’s B lymphocytes, rather than with a type 1 HS reaction.
Another important finding is that infusions triggering IRR presented significantly longer durations than infusions that did not present them (especially in the group treated with OCRE); this entails an increase in the length of hospital stay and higher healthcare costs.
The individualised questionnaire allows us to conclude that patients’ perception of IRRs does not always coincide with the record in their medical history; this may suggest underreporting, especially in mild cases, which could be due to the trivialisation of IRR among trained healthcare staff. It is worth noting that some other studies report a lower incidence of IRR than our own; this may be explained by underreporting in other areas.19,23,24,35 To improve clinical practice, it is necessary to work on the identification of symptoms compatible with mild IRR and to optimise their recording in patient medical records.
It is important to recognise the limitations of our study. This is a retrospective, single-centre study with a small sample of patients, especially those who had received more than 5 infusions of each drug. In this sense, although the data suggest a trend towards a greater likelihood of long-term IRR on OCRE treatment, further research is needed to confirm these findings. Another limitation is the underreporting of IRR, as observed in our results on patient perceptions. In terms of strengths, this is a study that includes the entire population with neuroimmunological diseases of the CNS attended over a specific period at a reference unit with a cohesive, interdisciplinary team trained in the homogeneous collection of data, including patient perceptions.
In conclusion, this is the first study to comparatively evaluate the incidence and characteristics of IRRs in real-world data on OCRE and RTX treatment in patients with neuroimmunological diseases of the CNS. A trend towards a higher incidence of IRR was observed in the OCRE group, especially in the long term, and an association was identified with a history of IRR in previous infusions and elevated B lymphocyte levels. The presence of IRR results in a longer duration of the infusion.
The potential severity of clinical manifestations, especially oropharyngeal symptoms, requires specialised monitoring of these patients. Additionally, the prolongation of infusion times due to the appearance of IRR leads to an increase in healthcare costs for patients who present or are at risk of presenting IRR. Therefore, it is essential to improve the recording of IRRs and to conduct additional prospective studies to confirm our findings, to identify predisposing factors, and to develop preventive strategies that can reduce hospital stays, minimise healthcare costs, and improve the quality of life of these patients.
Ethical considerationsThe authors declare that they have followed their centre’s protocols regarding the publication of patient data.
FundingThis work has not received any funding from any public or private sources.
The authors declare no conflict of interest or personal relationship that could have influenced this study.
We would like to thank Thermo Fisher Scientific for their invaluable support in the performance of this work. Their collaboration has been essential for this research, and their commitment to science and innovation has been key to the success of this study.