The specific diagnosis of toxic encephalopathy (TE) by chronic exposure to neurotoxics presents difficulties, mainly due to lack of consensus of clinical diagnostic criteria. The EUROQUEST (EQ) is a multicultural tool proposed for using in epidemiological studies on neurotoxicity. The aim of this study was to validate the Spanish version of this questionnaire for using as a diagnostic and prevention tool in the workplace.
MethodsAfter translation and cultural adaptation, leading to a final questionnaire in Spanish, validation was performed by asking a total of 759 people to complete the questionnaire, of whom 292 were workers exposed to neurotoxic solvents, 391 non-exposed workers, and 22 patients diagnosed with chronic alcoholism.
ResultsIn the analysis of the reliability, the Cronbach α value for the questionnaire was 0.94, indicating very high internal consistency. The test-retest reproducibility analysis was highly significant (r=0.91, P<.001). In the analysis of the validity, comparing the three study groups, the mean scores of the questions included in each of the dimensions of the test (ANOVA) detected major differences in the dimensions that assess cognitive symptoms, depressive disorders, sleep and psychopathological symptoms. After factor analysis obtained a total of nine axes, allowing a clear distinction between the three study groups.
El diagnóstico específico de la encefalopatía tóxica (TE) por exposición crónica a neurotóxicos presenta dificultades fundamentalmente por la carencia de criterios clínicos de diagnóstico consensuados. El EUROQUEST (EQ) es un instrumento multicultural propuesto para su uso en estudios epidemiológicos sobre la neurotoxicidad. El objetivo de este estudio ha sido la validación de la versión española de este cuestionario para su uso como instrumento de diagnóstico y prevención en el ámbito laboral.
MétodosTras la traducción y adaptación transcultural se ha generado un cuestionario definitivo en español y se ha realizado la validación mediante el pase del cuestionario a un total de 759 personas: 292 trabajadores expuestos a disolventes neurotóxicos, 391 trabajadores no expuestos y 22 pacientes diagnosticados de alcoholismo crónico.
ResultadosEn el análisis de la fiabilidad el valour del α de Cronbach para la totalidad del cuestionario fue de 0,94, lo que indica una consistencia interna muy elevada. La prueba test-retest para el análisis de la reproducibilidad fue muy significativa (r=0,91, p<0,001). En el análisis de la validez la comparación para los 3 grupos de estudio de las puntuaciones medias de las preguntas incluidas en cada una de las dimensiones del test (ANOVA) detectó mayores diferencias en las dimensiones que valoran los síntomas cognitivos, depresivos, alteraciones del sueño y síntomas psicopatológicos. Tras el análisis factorial se han obtenido un total de 9 ejes, que permiten diferenciar claramente entre los 3 grupos de estudio.
Long-term exposure to neurotoxins, solvents, metals, and pesticides in the workplace affects the central and peripheral nervous systems and causes cognitive and neuropsychiatric alterations, a syndrome known by the generic name of toxic encephalopathy (TE).1–5 Specific diagnosis of TE is difficult, primarily because no consensus on clinical diagnostic criteria has been reached.6 The diagnostic process is currently based on measuring cognitive function using psychological tests.7,8
A network of researchers in neurotoxicology, EURONEST,9 began to develop tools and created a battery of standardised evaluation instruments, including the European Questionnaire or EUROQUEST (EQ).
EQ is a multicultural tool designed for epidemiological studies of neurotoxicity in the workplace. Its distinguishing feature is that it includes items from earlier questionnaires and also screens for neuropsychiatric neurotoxin-related symptoms.
EQ has been used in several European countries following validation of the translated versions. In addition to the original English-language version, EQ is now available in French, Italian, Swedish, and Dutch, but not in Spanish.8 Several validation studies of this instrument have been published10–12; these studies proposed a 10-dimension evaluation structure and concluded that the test shows good convergent validity and a high internal consistency.
This study was designed to validate the Spanish-language version of the EQ, a questionnaire which we believe will fulfil 2 major objectives: to enable early identification of neuropsychiatric alterations in preclinical states, and contribute to occupational hazard protocols as a prevention strategy for long-term exposure to neurotoxins.
Although the EQ was designed as a multicultural instrument, we have detected differences between the translated versions, and therefore decided to translate and adapt the EQ transculturally before validating the new Spanish-language version. The sensitivity and specificity of the structure have also been evaluated.
Subjects and methodsOur study consisted of 2 phases: (1) translation and transcultural adaptation, and (2) validation to measure the reliability, validity, and usefulness of the questionnaire.
We studied a total population of 759 subjects, comprising 3 groups: 292 workers exposed to neurotoxins employed by different companies and industry sectors, 391 workers not exposed to neurotoxins who served as controls, and 22 patients diagnosed with long-term alcoholism as representative cases of CNS disease caused by neurotoxin exposure.
Participants were selected randomly from among workers undergoing protocolised health tests in risk prevention services in collaboration with Universidad Miguel Hernández in Elche, and patients being treated in the substance abuse unit at Hospital Universitario San Juan de Alicante.
All participants signed an informed consent form to be included in the study. Exclusion criteria were prior diagnosis of any type of diseases causing neuropsychiatric alterations or central and/or peripheral neurological symptoms, and long-term consumption of drugs affecting the central or peripheral nervous systems. Current alcohol consumption was also an exclusion criterion for the patient group.
Workers and patients were assessed with the EQ, which is self-administered; we also drew up medical and occupational histories containing patients’ sociodemographic characteristics, employment history, family and personal medical history, drug habits, and hobbies.
Three months later a small group of 15 workers exposed to neurotoxins repeated the questionnaire for the test-retest reproducibility study.
EUROQUESTThe original questionnaire includes 83 items and is structured in 10 domains or dimensions grouped in 3 main categories: symptoms associated with long-term exposure to solvents (6 dimensions), symptoms associated with acute exposure to solvents (1 dimension), and aspects related to personal traits (3 dimensions).13
Translation and transcultural adaptationWe translated and adapted the French version of the EQ after requesting permission from the authors. The study design was based on the translation–back translation model: native Spanish-speaking translators translated the questionnaire, which was later administered to healthy subjects and patients for transcultural adaptation.
The original questionnaire was translated into Spanish by 2 Spanish-dominant bilinguals working independently. These translators were familiar with the areas of medicine, sociology, and questionnaire validation methods, had been informed of the purpose of the present study, and were aware that the translation would be reviewed jointly between translators and the researchers. This first stage resulted in a questionnaire approved by consensus. In the second stage, 2 independent bilingual translators back-translated the questionnaire; these translators were not the same ones as in the first stage and they were not aware of the existing French version. The version of the questionnaire generated in the second stage was then administered to healthy subjects and patients during the transcultural adaptation stage. A member of our research group (the same in all cases) presented the items on the questionnaire and then asked the subjects the following questions:
- -
Do you find it difficult to understand this question?
- -
If you find it difficult, how would you express it?
- -
What do you think this question means? Explain it in your own words.
- -
Do you think the response scale is clear and appropriate for the question? If not, explain why not and suggest alternatives.
This phase produced the third provisional version of the questionnaire, which was evaluated by the research group. The result was the final Spanish version of the EQ that was used in the validation process.
Data processing and statistical treatmentValidation of the final Spanish-language version involved analysing the psychometric properties of the questionnaire: reliability and validity.
Reliability was evaluated in 2 ways. We first determined internal consistency as a measure of homogeneity of items on the questionnaire, using the Cronbach α coefficient, with values ranging from 0 to 1. Values equal to or greater than 0.7 were considered acceptable. Secondly, we assessed test-retest reproducibility as an index of temporal stability of the measures in situations in which measurement conditions or the measured concept do not change. For the test-retest analysis, the questionnaire was administered on 2 occasions, 3 months apart. To obtain the level of agreement, intraclass correlation coefficient was calculated by repeated measures analysis of variance and breakdown of sources of variation (intrasubject, intersubject, error, and total). We calculated the Pearson correlation coefficient or its non-parametric alternative, the Spearman rank correlation coefficient, whose values range between 0 and 1. Values equal to or greater than 0.7 were considered acceptable.
The following aspects of validity were evaluated: (a) content validity, which was understood to be demonstrated since the questionnaire design was based on expert consensus, and (b) construct validity, evaluated by administering the questionnaire to extreme groups (patients, exposed workers, and non-exposed workers). Mean scores for different groups were compared using analysis of variance for independent samples (ANOVA), principal component analysis, and Varimax rotation.
In the principal component analysis, the correlation coefficients for each component and global satisfaction were calculated using a multivariate procedure.
The references used when establishing axes were the questions whose correlation coefficient was higher than 0.2. Items with a correlation coefficient higher than 0.7 were identified, since this value means that the items measure the same area and one of them can therefore be excluded from that axis. For items showing a correlation coefficient higher than 0.7, we identified redundant questions by detecting associations between a specific item and the exposure groups in the association study using the Pearson chi-square test.
Data quality control was performed by pre-establishing the possible input values in each field. PASW version 2.0 for Windows was used for statistical analysis.
Our study complies with the standards established by the experimental research ethics committee of the Universidad Miguel Hernández and the Declaration of Helsinki of 1975, whose current, revised version can be accessed at http://www.wma.net/s/policy/b3.htm.
Confidentiality was guaranteed in 2 ways. Firstly, the members of the research team were the only ones with access to the password-protected electronic databases, and secondly, workers were identified with codes only in order to protect their identities.
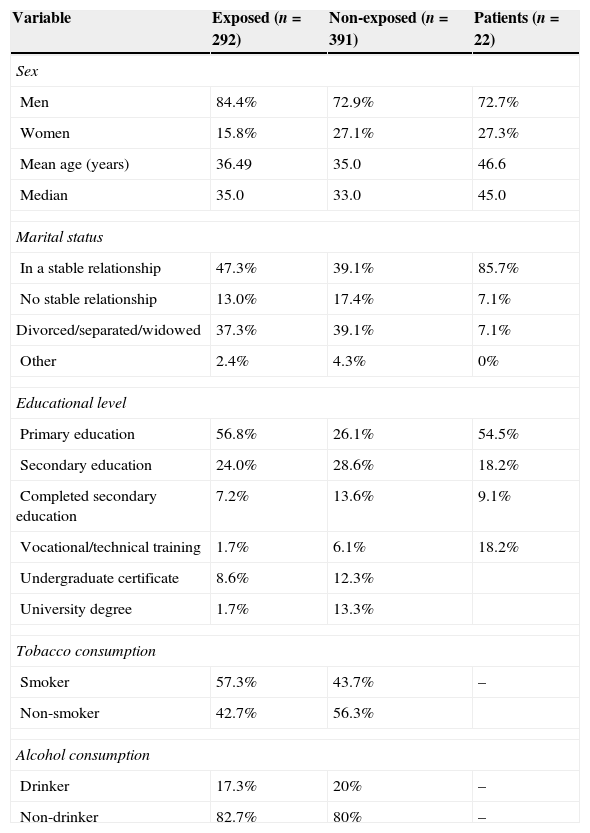
ResultsTable 1 summarises the sociodemographic characteristics of all patients included in the 3 study groups. The variables associated with drug habits are not shown in the patient group since these participants by definition did not consume alcohol. Some of the patients had also stopped using tobacco since they associated that habit with alcohol consumption.
Sociodemographic characteristics of the study groups
| Variable | Exposed (n=292) | Non-exposed (n=391) | Patients (n=22) |
|---|---|---|---|
| Sex | |||
| Men | 84.4% | 72.9% | 72.7% |
| Women | 15.8% | 27.1% | 27.3% |
| Mean age (years) | 36.49 | 35.0 | 46.6 |
| Median | 35.0 | 33.0 | 45.0 |
| Marital status | |||
| In a stable relationship | 47.3% | 39.1% | 85.7% |
| No stable relationship | 13.0% | 17.4% | 7.1% |
| Divorced/separated/widowed | 37.3% | 39.1% | 7.1% |
| Other | 2.4% | 4.3% | 0% |
| Educational level | |||
| Primary education | 56.8% | 26.1% | 54.5% |
| Secondary education | 24.0% | 28.6% | 18.2% |
| Completed secondary education | 7.2% | 13.6% | 9.1% |
| Vocational/technical training | 1.7% | 6.1% | 18.2% |
| Undergraduate certificate | 8.6% | 12.3% | |
| University degree | 1.7% | 13.3% | |
| Tobacco consumption | |||
| Smoker | 57.3% | 43.7% | – |
| Non-smoker | 42.7% | 56.3% | |
| Alcohol consumption | |||
| Drinker | 17.3% | 20% | – |
| Non-drinker | 82.7% | 80% | – |
The final Spanish-language version of the EQ consists of 82 questions: transcultural adaptation evidenced that 2 items were regarded as the same question by both healthy subjects and patients, and one of them was therefore eliminated (the validated questionnaire is available in Appendix A, additional on-line material). The questionnaire is scored on a scale from 1 to 4.
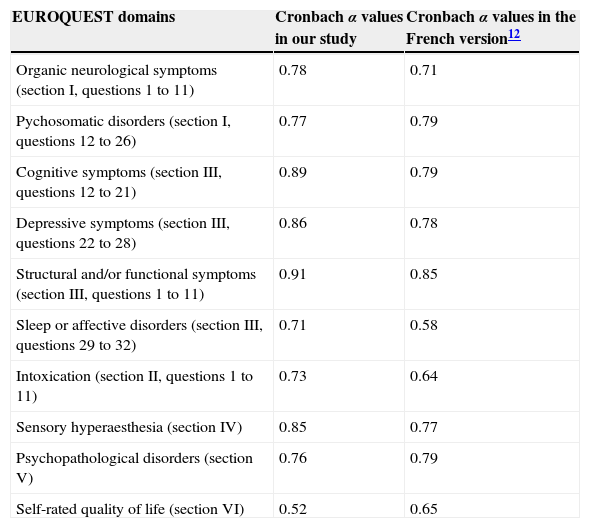
The different dimensions assessed by the questionnaire are structured according to the EQ domains. Table 2 shows the items included in each dimension.
Cronbach α values for EUROQUEST items
| EUROQUEST domains | Cronbach α values in our study | Cronbach α values in the French version12 |
|---|---|---|
| Organic neurological symptoms (section I, questions 1 to 11) | 0.78 | 0.71 |
| Pychosomatic disorders (section I, questions 12 to 26) | 0.77 | 0.79 |
| Cognitive symptoms (section III, questions 12 to 21) | 0.89 | 0.79 |
| Depressive symptoms (section III, questions 22 to 28) | 0.86 | 0.78 |
| Structural and/or functional symptoms (section III, questions 1 to 11) | 0.91 | 0.85 |
| Sleep or affective disorders (section III, questions 29 to 32) | 0.71 | 0.58 |
| Intoxication (section II, questions 1 to 11) | 0.73 | 0.64 |
| Sensory hyperaesthesia (section IV) | 0.85 | 0.77 |
| Psychopathological disorders (section V) | 0.76 | 0.79 |
| Self-rated quality of life (section VI) | 0.52 | 0.65 |
The Cronbach α value for the full questionnaire is 0.94. Table 2 shows the α value for each EQ domain. Regarding the test-retest reproducibility analysis (n=15), the correlation between global scores for both questionnaire administrations was both high and significant (r=0.97, P<.01).
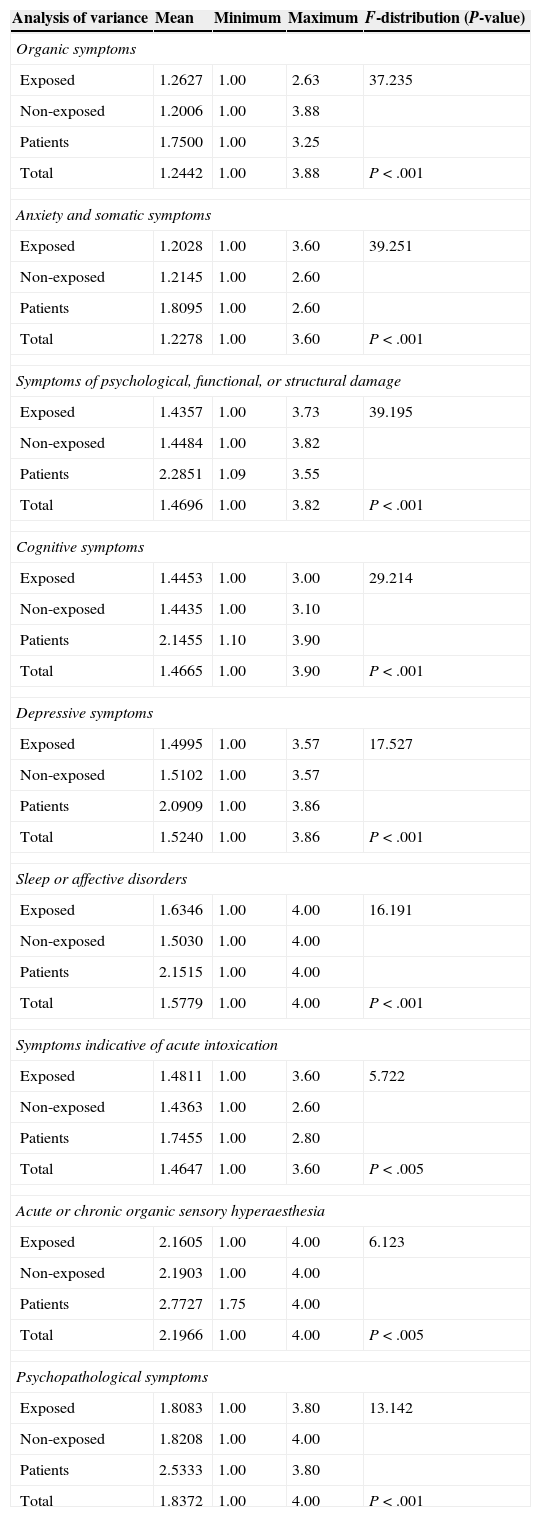
ValidityWe compared mean scores of the questions included in each of the EQ dimensions between the 3 study groups (exposed workers, non-exposed workers, and patients) using analysis of variance (ANOVA), and found a very high statistical significance in general. The greatest differences between means were seen in the domains assessing sleep disorders and cognitive, depressive, and psychopathological symptoms (Table 3).
Analysis of variance of mean scores for items from each domain (P>.05) (ANOVA)
| Analysis of variance | Mean | Minimum | Maximum | F-distribution (P-value) |
|---|---|---|---|---|
| Organic symptoms | ||||
| Exposed | 1.2627 | 1.00 | 2.63 | 37.235 |
| Non-exposed | 1.2006 | 1.00 | 3.88 | |
| Patients | 1.7500 | 1.00 | 3.25 | |
| Total | 1.2442 | 1.00 | 3.88 | P<.001 |
| Anxiety and somatic symptoms | ||||
| Exposed | 1.2028 | 1.00 | 3.60 | 39.251 |
| Non-exposed | 1.2145 | 1.00 | 2.60 | |
| Patients | 1.8095 | 1.00 | 2.60 | |
| Total | 1.2278 | 1.00 | 3.60 | P<.001 |
| Symptoms of psychological, functional, or structural damage | ||||
| Exposed | 1.4357 | 1.00 | 3.73 | 39.195 |
| Non-exposed | 1.4484 | 1.00 | 3.82 | |
| Patients | 2.2851 | 1.09 | 3.55 | |
| Total | 1.4696 | 1.00 | 3.82 | P<.001 |
| Cognitive symptoms | ||||
| Exposed | 1.4453 | 1.00 | 3.00 | 29.214 |
| Non-exposed | 1.4435 | 1.00 | 3.10 | |
| Patients | 2.1455 | 1.10 | 3.90 | |
| Total | 1.4665 | 1.00 | 3.90 | P<.001 |
| Depressive symptoms | ||||
| Exposed | 1.4995 | 1.00 | 3.57 | 17.527 |
| Non-exposed | 1.5102 | 1.00 | 3.57 | |
| Patients | 2.0909 | 1.00 | 3.86 | |
| Total | 1.5240 | 1.00 | 3.86 | P<.001 |
| Sleep or affective disorders | ||||
| Exposed | 1.6346 | 1.00 | 4.00 | 16.191 |
| Non-exposed | 1.5030 | 1.00 | 4.00 | |
| Patients | 2.1515 | 1.00 | 4.00 | |
| Total | 1.5779 | 1.00 | 4.00 | P<.001 |
| Symptoms indicative of acute intoxication | ||||
| Exposed | 1.4811 | 1.00 | 3.60 | 5.722 |
| Non-exposed | 1.4363 | 1.00 | 2.60 | |
| Patients | 1.7455 | 1.00 | 2.80 | |
| Total | 1.4647 | 1.00 | 3.60 | P<.005 |
| Acute or chronic organic sensory hyperaesthesia | ||||
| Exposed | 2.1605 | 1.00 | 4.00 | 6.123 |
| Non-exposed | 2.1903 | 1.00 | 4.00 | |
| Patients | 2.7727 | 1.75 | 4.00 | |
| Total | 2.1966 | 1.00 | 4.00 | P<.005 |
| Psychopathological symptoms | ||||
| Exposed | 1.8083 | 1.00 | 3.80 | 13.142 |
| Non-exposed | 1.8208 | 1.00 | 4.00 | |
| Patients | 2.5333 | 1.00 | 3.80 | |
| Total | 1.8372 | 1.00 | 4.00 | P<.001 |
The factor analysis yields a total of 9 axes which let us differentiate between the 3 established groups (exposed workers, non-exposed workers, and patients). The correlation coefficient obtained in the factor analysis was used to check that the questions making up each of these axes either affect only on one specific axis, or else have a greater explanatory weight on one axis.
DiscussionThere is no consensus on diagnostic criteria for neuropsychiatric problems associated with long-term occupational exposure to neurotoxins. However, authors do agree that a series of specific dimensions are affected,12–14 and the EQ aims to evaluate those dimensions.
The Cronbach α value obtained shows that the Spanish version of the EQ has a very high internal consistency. Furthermore, the Cronbach α value for each of the EQ domains was high except in the case of self-rated quality of life, which showed a value below 0.7. This value is in line with that obtained by the authors of the French version,12 who reported a Cronbach α value of 0.6 (Table 2). We can therefore state that compared to the French version, our results for reliability are comparable for some dimensions and better for most of the dimensions. In addition to this, the test-retest reproducibility analysis in the sample of 15 patients shows good temporal stability for these measures.
The 9 axes yielded by the factor analysis (Table 3) correlate to the dimensions or blocks of questions in which the original version of the EQ is structured.8
From a practical perspective, these axes can be used to orient suspected diagnoses of neuropsychological impairment in solvent-exposed workers since they let us differentiate between the 3 study groups (exposed workers, non-exposed workers, and patients), especially in the dimensions assessing sleep disorders and cognitive, depressive, and psychopathological symptoms (Table 3).
The factor analysis revealed correlations for the same item on different axes. This finding may seem contradictory, but it is not: although the original EQ was designed according to specific dimensions, some questions have explanatory influence on more than one axis. Most of the questions included in the different blocks of items must be evaluated within the group in which they originally appear, and not individually. A positive answer to most of the questions on an axis points to a problem in a specific dimension. We agree with the authors of the original EQ on the relevance of the questionnaire, and on its structure and distribution in 9 blocks of questions,8 as well as on the questions with explanatory weight on each dimension.
Several studies have compared the EQ, especially the Swedish version, with similar questionnaires. Karlson et al. compared the EQ with the Symptom Checklist-90 (SCL-90) and the General Health Questionnaire-30 (GHQ-30), and concluded that the EQ has several advantages, essentially its high sensitivity and specificity. These authors also state that the items included in the EQ effectively detect the effects of exposure to neurotoxins.11 Carter et al. compared the EQ with the Q16, a widely used questionnaire for screening symptoms of exposure to organic solvents, and found that the EQ subset of questions evaluating memory and concentration was more sensitive than the Q16. However, we agree with these authors that neither of these detection tools alone can replace current clinical diagnostic procedures.13 Recent studies conducted by the Finnish Institute of Occupational Health also support this view. Furthermore, these studies have suggested that memory and concentration symptoms may be regarded as threshold symptoms for chronic solvent encephalopathy, and assessing them may reduce under-detection of this entity.15–17
ConclusionsWe have validated the Spanish version of the EUROQUEST, which is considered a reliable, valid, and useful tool for toxicity diagnosis and prevention. Our purpose was to provide specialists in occupational medicine with a questionnaire to help them identify workers with potential neuropsychiatric alterations caused by neurotoxin exposure who will need a referral to clinical neurologists for thorough evaluation and treatment.
Conflict of interestThe authors have no conflicts of interest to declare.
FundingThis study received financial support from the Institute of Health Carlos III (health research fund 04/1169).
We would like to thank Prof. Chouanière and the psychiatry department at Hospital Universitario San Juan de Alicante (substance abuse unit).
Please cite this article as: Marhuenda D, Prieto MJ, Cardona A, Roel JM, Oliveras MA. Adaptación transcultural y validación de la versión española del EUROQUEST. Neurología. 2015;30:201–207.
This study has been partially presented at the 7th International Symposium on Biological Monitoring in Occupational and Environmental Health.