Interferon beta (IFNβ) is considered a safe treatment for relapsing-remitting multiple sclerosis (RRMS). However, hepatotoxicity due to IFNβ is not uncommon.1,2
Aloe vera (AV) has also been associated with liver damage.3,4 Some patients with RRMS use AV to alleviate constipation.
No cases of hepatitis in patients taking IFNβ and AV have previously been described in the literature. We present the case of a patient with RRMS who developed toxic hepatitis following long-term treatment with IFNβ and AV.
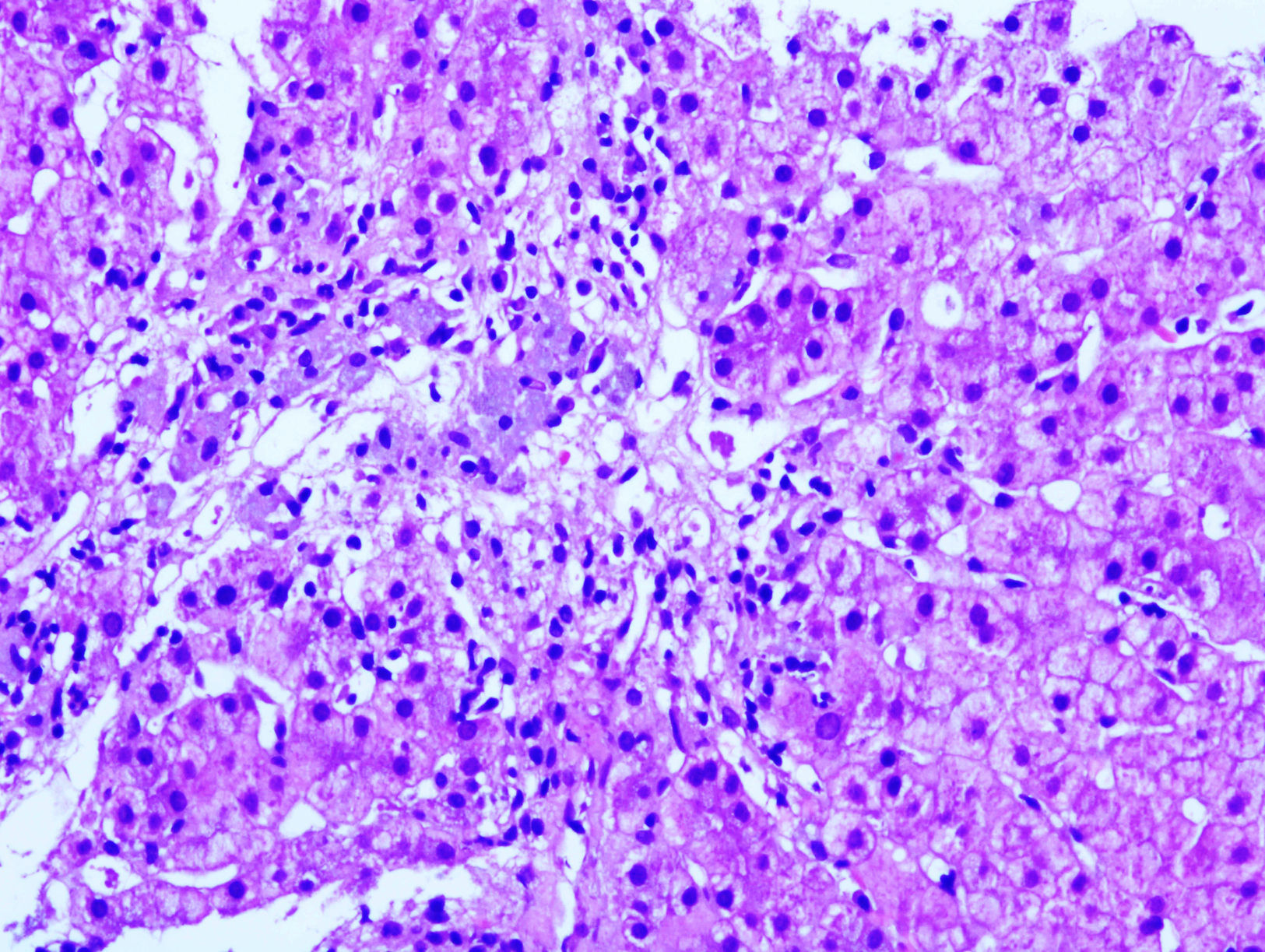
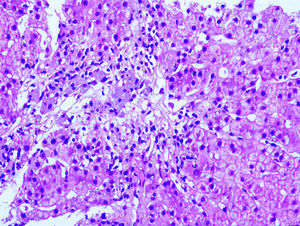
Our patient was a 61-year-old woman with RRMS who had been receiving intramuscular IFNβ-1a dosed at 30μg per week for 10 years (Avonex®) and LX_Trem® (182mg AV) for 3 years to prevent constipation. She attended the emergency department complaining of asthenia and abdominal pain. Examination revealed jaundice and pain upon palpation of the right hypochondriac region. A blood analysis revealed AST 1236 U/L, ALT 824 U/L, GGT 169 U/L, ALP 121 U/L, and prothrombin activity 73%. Results from serology tests (hepatitis A, B, and C) and an autoimmune study (ANA, AMA, LKM, and SMA) were negative. A liver ultrasound and a thoracic-abdominal CT scan revealed a fatty liver with no associated hepatomegaly or space-occupying lesions; liver biopsy results (Fig. 1) were compatible with toxic hepatitis. Intramuscular IFNβ-1a and AV were discontinued due to clinical suspicion of hepatotoxicity. An analysis performed 3 weeks later showed improved liver function, which normalised after 6 months.
Our patient scored 7 points (probable causality) on the Roussel Uclaf Causality Assessment Method (RUCAM).5 Our patient's drug reaction was reported to the Catalan pharmacovigilance authorities.
Asymptomatic transaminase elevation constitutes the most frequent liver alteration associated with IFNβ; this is usually mild and transient and does not normally require drug discontinuation or dose adjustment.1 In the study published by Francis et al.,1 IFNβ was discontinued due to toxicity in only 0.4% of the patients taking it, and the rate of severe hepatotoxicity was one case in 2300 patients. Severe, late-onset hepatotoxicity due to IFNβ, as in our case, is exceptional6,7; drug-induced liver damage usually occurs during the first year of treatment, and is uncommon after the first 6 months.1 Regarding type of IFNβ, toxicity increases with higher doses and frequency of administration and rarely occurs in patients receiving weekly doses.2
Hepatotoxicity due to AV is common during the first months of treatment. According to our literature search, only one case of late-onset toxic hepatitis (>5 years from treatment onset) has been reported to date.3
The pathophysiological mechanisms of IFNβ- and AV-induced liver damage are not known. IFNβ is thought to cause hepatotoxicity by directly damaging hepatocytes or by inducing autoimmunity against the liver1,8; the drug's immunomodulatory properties may trigger immunological alterations such as autoimmune hepatitis. AV-induced hepatotoxicity, in turn, may be due to direct toxicity or to hypersensitivity.
Hepatotoxicity is more frequent in men but tends to be more severe in women. The reason for these sex-related differences is unknown; it has been hypothesised that a lower body mass index and a higher level of treatment adherence may predispose to hepatotoxicity.6
Our patient developed toxic hepatitis after several years of treatment with IFNβ and AV; hepatotoxicity resolved with treatment discontinuation. In our case, we cannot rule out an association between hepatotoxicity and either of the 2 drugs, given that both IFNβ and AV can cause delayed hepatotoxicity and that symptoms improved after discontinuation of both drugs. As for the pathophysiology of the disease, the autoimmune study yielded negative results, which rules out autoimmune hepatitis and points to drug toxicity as the most likely cause.
In conclusion, the risk of IFNβ-induced hepatotoxicity increases when this drug is combined with other drugs or herbal products.9 Doctors administering IFNβ to patients with RRMS should monitor use of concomitant treatments. Although the risk of hepatotoxicity is greater during the first months of treatment, liver damage may also occur at later stages.
FundingThe authors have received no funding for this study.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Hervás-García JV, Montané E, Serrado-Iglesias A, Ramo-Tello C. Hepatitis tóxica tras tratamiento concomitante con interferón beta y aloe vera en un paciente con esclerosis múltiple: a propósito de un caso. Neurología. 2017;32:546–547.