The ventralis intermedius (VIM) nucleus of the thalamus is the usual surgical target for tremor. However, locating the structure may be difficult as it is not visible with conventional imaging methods; therefore, surgical procedures typically use indirect calculations correlated with clinical and intraoperative neurophysiological findings. Current ablative surgical procedures such as Gamma-Knife thalamotomy and magnetic resonance-guided focused ultrasound require new alternatives for locating the VIM nucleus. In this review, we compare VIM nucleus location for the treatment of tremor using stereotactic procedures versus direct location by means of tractography.
DiscussionThe most widely used cytoarchitectonic definition of the VIM nucleus is that established by Schaltenbrand and Wahren. There is a well-defined limit between the motor and the sensory thalamus; VIM neurons respond to passive joint movements and are synchronous with peripheral tremor. The most frequently used stereotactic coordinates for the VIM nucleus are based on indirect calculations referencing the mid-commissural line and third ventricle, which vary between patients. Recent studies suggest that the dentato-rubro-thalamic tract is an optimal target for controlling tremor, citing a clinical improvement; however, this has not yet been corroborated.
ConclusionsVisualisation of the cerebello-rubro-thalamic pathway by tractography may help in locating the VIM nucleus. The technique has several limitations, and the method requires standardisation to obtain more precise results. The utility of direct targeting by tractography over indirect targeting for patients with tremor remains to be demonstrated in the long-term.
La diana habitual empleada para el tratamiento quirúrgico del temblor es el núcleo ventralis intermedius (Vim) del tálamo. Su localización es compleja, ya que no se puede visualizar con métodos de imagen convencionales, por lo que para el procedimiento quirúrgico se toman clásicamente medidas indirectas y se correlacionan con la clínica y neurofisiología intraoperatorias. Sin embargo, procedimientos ablativos actuales como la talamotomía por gamma-knife o por ultrasonidos (MRgFUS) hacen que sea preciso buscar otras alternativas para su localización. El objetivo del presente trabajo es comparar la localización indirecta del Vim mediante técnica esterotáctica versus la realizada directamente por tractografía para el tratamiento del temblor.
DiscusiónLa definición citoarquitectónica más empleada del Vim es la del Atlas de Schaltenbrand-Wahren. Existe un límite claro entre el tálamo motor y el sensitivo; las neuronas del Vim responden a movimientos pasivos articulares y su actividad es sincrónica con el temblor periférico. Las coordenadas estereotácticas del Vim más frecuentemente utilizadas se basan en mediciones indirectas respecto a la línea intercomisural y el III ventrículo, las cuales dependen de variaciones interindividuales. Estudios recientes han propuesto el haz dentato-rubro-talámico (DRT) como una diana óptima para el control del temblor postulando que se asocia a una mejoría clínica, sin embargo esto no ha sido corroborado por otros autores.
ConclusionesLa visualización de la vía cerebelo-rubro-talámico por tractografía puede ayudar a definir la localización del Vim. Esta técnica tiene limitaciones inherentes y sería necesaría una estandarización del método para lograr resultados más precisos. La posible mayor utilidad de la diana por tractografía, directa, sobre la indirecta queda por ser demostrada a largo plazo en pacientes con temblor.
The ventralis intermedius nucleus (Vim) of the thalamus is typically selected as the target for surgical treatment of tremor, as both essential tremor and parkinsonism, as well as other types of tremor, significantly improve when this site is targeted with ablative lesions (eg, radiofrequency thalamotomy) or deep brain stimulation (DBS). The Vim is a complex target, as the thalamus contains several nuclei that cannot be visualised with such conventional imaging techniques as high-field magnetic resonance imaging (MRI). Locating the target by indirect procedures (such as the classic measurement of the intercommissural line [anterior commissure-posterior commissure line, AC-PC]) may be inaccurate for the new non-invasive techniques in functional surgery for refractory tremor (such as gamma-knife or magnetic resonance–guided focused ultrasound [MRgFUS]) due to individual variations. Today, with improvements in imaging techniques, locating the white matter tracts helps tractography to define the thalamic target.
It is currently unclear whether this new technology is sufficiently precise to locate the target selected and whether this target remains the same after physiological recording. In this article, we describe the localisation of the Vim by a classic indirect procedure (measuring the AC-PC line) and direct tractography method. We also briefly describe the difference between both procedures.
DiscussionMotor thalamusThe motor thalamus is defined as the territory receiving subcortical afferents from the basal ganglia (nigral and pallidal afferents) and cerebellum, and subsequently projects to the primary motor area, supplementary motor area, and premotor cortex. It is limited medially by the internal medullary lamina, posteriorly by somatosensory nuclei, and laterally by the internal capsule.1
The motor thalamus is located in the lateral and ventral third, whereas the dorsal and lateral portion receives intrathalamic fibres and no afferents from outside the thalamus; therefore, they are considered integration nuclei.
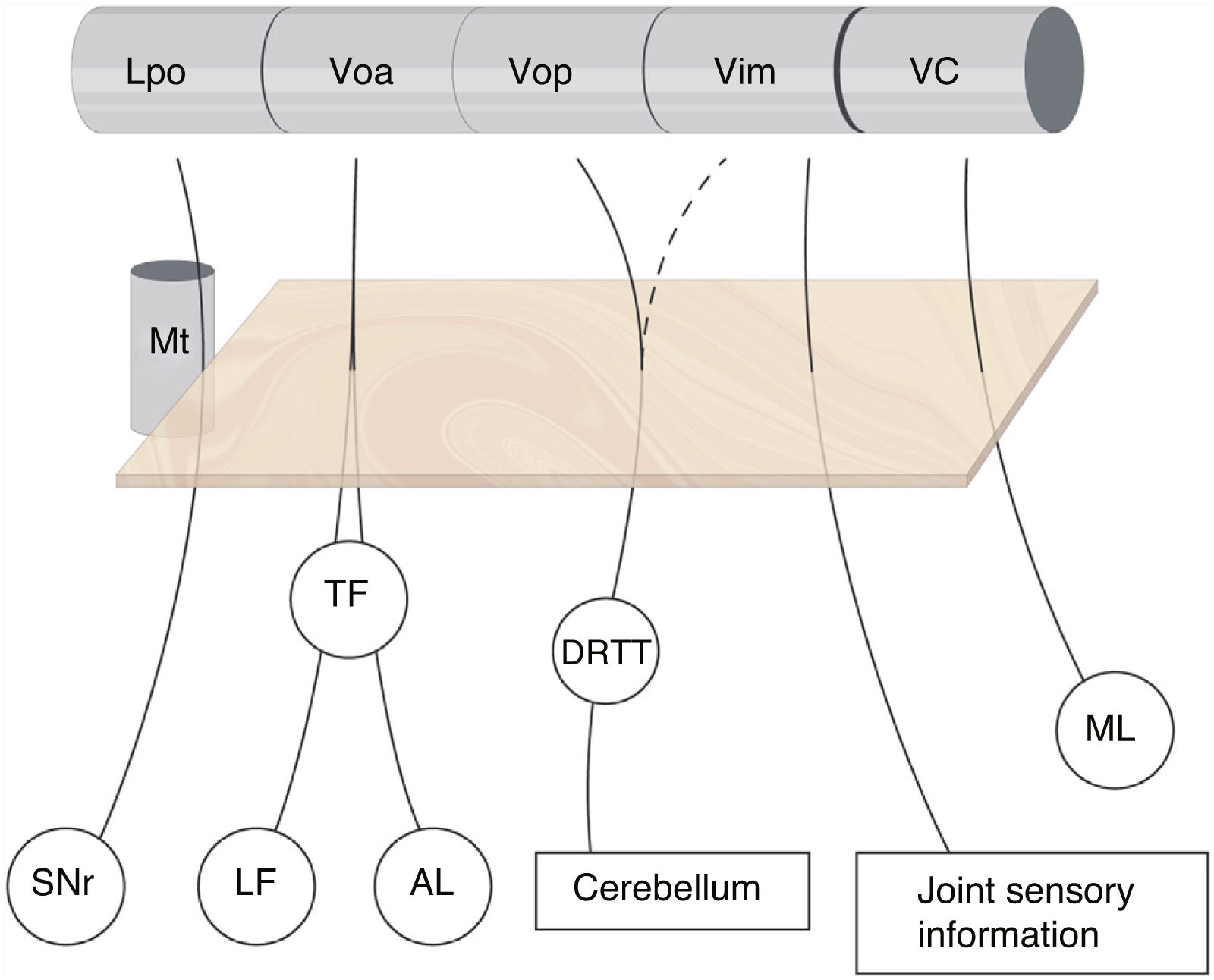
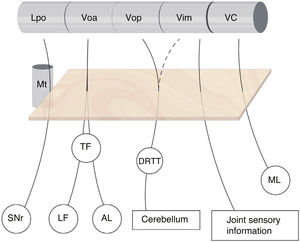
The subdivision of the human motor thalamus has always been complex, as it may vary from a cytoarchitectural perspective. This has led to a heterogeneous nomenclature for the different nuclei and afferents. The most widely used atlas in neurosurgery is that published by Schaltenbrand and Wahren,2 which uses the terminology introduced by Hassler and myelin staining. For Hassler, the most important nuclei of the motor thalamus, from the rostral to the caudal position, are the nucleus lateralis polaris (Lpo), which receives nigral afferents; the ventralis oralis anterior nucleus (Voa), which receives pallidal afferents (internal globus pallidus); the ventralis oralis posterior (Vop), which receives cerebellar afferents; the Vim, which receives deep joint afferents; and the nucleus ventro-caudalis (VC), which receives afferents from the medial and trigeminal lemniscus as well as the spinothalamic tracts (Fig. 1).3
Subdivision of the motor thalamus and its afferents. The figure shows the afferent connections with the ventral thalamus. These projections cross the posterior subthalamic area (orange square) and are closely related to the most anterior part of the mammillothalamic tract. There is no clear division of the cerebellar afferents arriving from the dentatorubrothalamic tract to the Vop and Vim, whereas the border between the motor and sensory thalamus (VC) is well delimited. AL: ansa lenticularis; DRTT: dentatorubrothalamic tract; LF: lenticular fasciculus; Lpo: lateropolaris; ML: medial lemniscus; Mt: mammillothalamic tract; SNr: substantia nigra pars reticulata; TF: thalamic fasciculus; VC: ventro caudalis; Vim: ventralis intermedius nucleus; Voa: ventralis oralis anterior; Vop: ventralis oralis posterior.
Ever since the pre-lecodopa era, the Vim has been the habitual target for tremor suppression in patients who are refractory to medical treatment. It presents a narrow parallelogram shape, with a height of 8-9 mm and rostrocaudal width of 3-3.5 mm in its most lateral section and 2 mm in the medial section. The Vim presents clear somatotopy, with the face and tongue corresponding to a medial location, the upper limbs to a more lateral position, and the lower limbs to the lateral end, close to the internal capsule.4,5
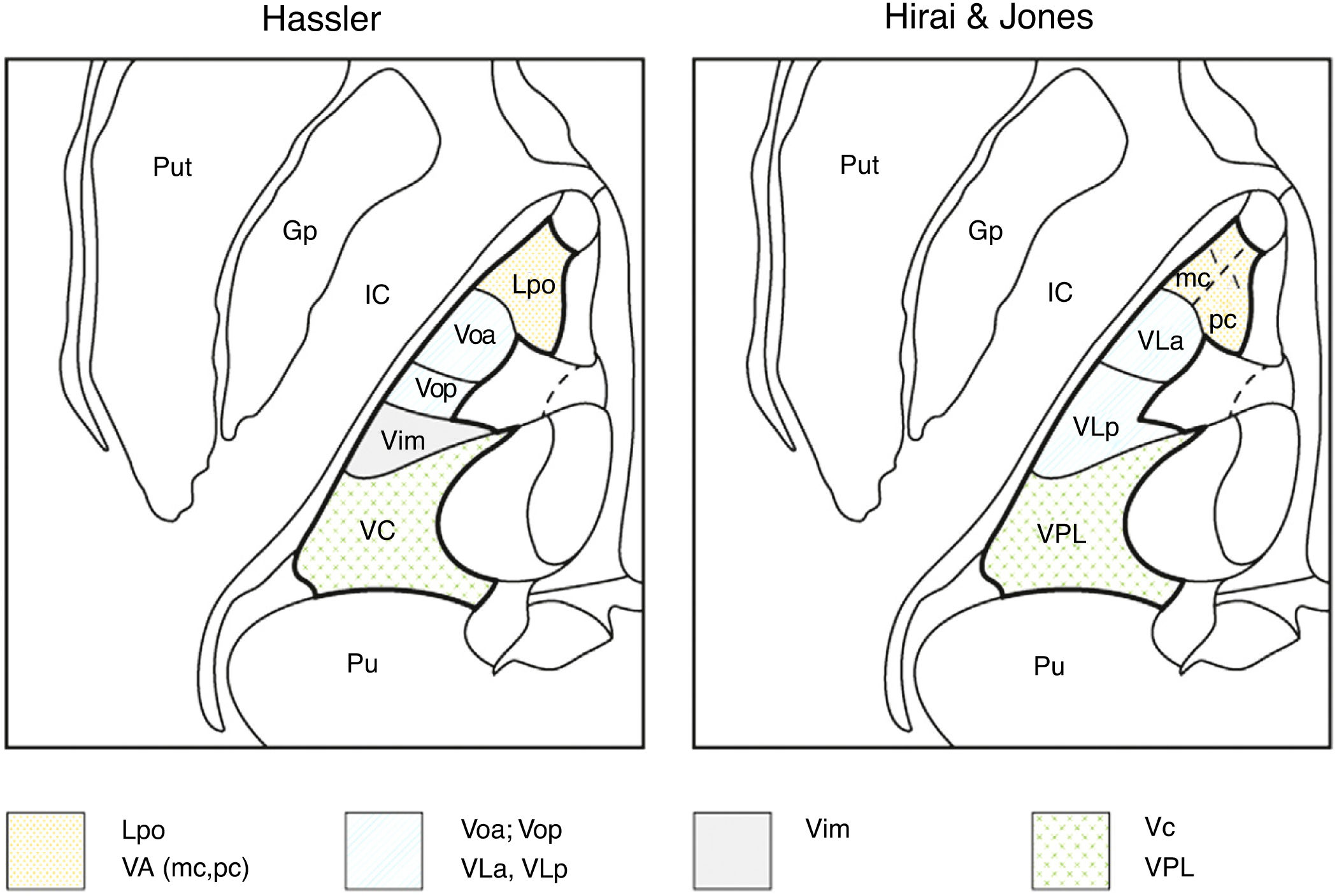
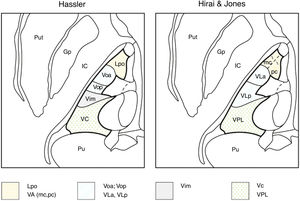
For such other anatomists as Hirai and Jones,6 who used histochemical staining with choline acetyltransferase, the ventral thalamus would be subdivided into the ventral anterior, ventral lateral, and ventral posterior thalami. The ventral anterior thalamus, in turn, is subdivided into the magnocellular portion (nigral afferents) and the parvocellular portion (pallidal afferents). Similarly, the lateral ventral thalamus is subdivided into the ventral lateral anterior thalamus, which receives pallidal afferents from the thalamic fasciculus, and the ventral lateral posterior nucleus, which receives cerebellar afferents. The anterior part of the ventral lateral posterior nucleus receives proprioceptive lemniscal afferents, and the posterior part receives cutaneous lemniscal afferents6,7 (Table 1).
Nomenclature of the human ventrolateral thalamus according to Hassler and Hirai & Jones.
| Hassler | Hirai and Jones | Afferents |
|---|---|---|
| Lpo | VA mc pca | SNr |
| Voa | VLa | GPia |
| Vop | VLp | Cerebellum |
| Vim | – | Joint sensory innervation |
| VC | VPLa VPLp | Medial lemniscus (proprioceptive) |
| Medial lemniscus (cutaneous) |
GPi: internal globus pallidus; Lpo: lateropolaris; mc: pars magnocelularis; pc: pars parvocelularis; SNr: substantia nigra pars reticulata; VA: ventral anterior; VC: ventro caudalis; Vim: ventralis intermedius; VLa: ventral lateral anterior; VLp: ventral posterior; Voa: ventralis oralis anterior; Vop: ventralis oralis posterior; VPLa: ventral posterior lateral, anterior part; VPLp: ventral posterior lateral, posterior part.
Macchi and Jones7 compare the different nomenclatures adopted by different authors and discrepancies between them. Thus, these authors consider the Voa and Vop as defined by Hassler not to be independent nuclei, as both would receive pallidal and cerebellar afferents without clear individualisation; therefore, both nuclei would be part of a single structure, the ventral lateral anterior nucleus. The Vim as defined by Hassler, which includes neurons responsive to contralateral passive joint kinematics, would be part of the ventral lateral posterior nucleus defined by Hirai and Jones,6 which would receive cerebellar afferents and not proprioceptive information from joints (spindle afferents), whereas Hassler considered cerebellar afferents to project to the Vop.7 There are also differences between both authors regarding the lemniscal terminals with kinaesthetic or proprioceptive afferents, as these terminals would project to the anterior part of the ventral lateral posterior nucleus (medial lemniscal fibres) and the posterior part of the ventral lateral posterior nucleus would receive tactile inputs, whereas for Hassler, all these structures would be included in the VC (Table 1, Fig. 2).
Nomenclatures for the motor thalamus. The nomenclature of Hassler (left) and Hirai & Jones (right) are shown in an axial image of the thalamus. The authors use different names for the same nuclei in the same anatomical regions: Lpo and VA (dotted); Voa, Vop, VLa, and VLp (lines); Vim (grey); Vc and VPL (crosses). Gp: globus pallidus; IC: internal capsule; Lpo: lateropolaris; VA: ventral anterior (mc: pars magnocellularis; pc: pars parvocellularis); VC: ventro caudalis; Vim: ventralis intermedius; VLa: ventral lateral anterior; VLp: ventral lateral posterior; Voa: ventralis oralis anterior; Vop: ventralis oralis posterior; VPL: ventral posterior lateral; Pu: pulvinar; Put: putamen.
As described above, the degree of overlap between afferents is controversial, since there appears to be none between the cerebellum and the medial lemniscus, but there may be certain degree of interdigitation between pallidal and cerebellar afferents, as in the case of the ventral lateral anterior nucleus.8
For these reasons, it is difficult to establish the localisation of the human Vim. It is invariably located anterior to the area receiving tactile inputs but, in this context, it may be considered as a structure within the VC, as it would receive joint proprioceptive inputs and, therefore, may be a sensory nucleus. Alternatively, it may be considered to be the most caudal afferent of the Vop, which receives proprioceptive afferents from contralateral joints and is located at the posterior region of the cerebellar afferent.7,9,10
The border between the Vim and the VC is an essential reference between the motor and sensory thalamus in thalamic surgery, and is only observable by neurophysiological recording.
Functional surgical targets in tremorPallidotomy was the surgical technique of choice for patients with Parkinon’s disease during the 1950s, to treat both the tremor-dominant and the akinetic/rigid subtypes.11 Hassler was the first author to perform a thalamotomy of the pallidal receiving nuclei, moving the target form the internal globus pallidus, which did not significantly improve patients’ status.12 Due to the better outcomes for treating tremor, most neurosurgical procedures induced more caudally located thalamic lesions, particularly since the 1960s, when physiological recording was introduced.13,14 Researchers confirmed that tremor neurons (neurons that discharge at the same frequency as tremor of the contralateral joints) were located in the areas receiving cerebellar projections, the Vop and Vim. Since then, the Vim has been considered the best target for treating tremor in different diseases.15,16 DBS was also first applied to the Vim in patients with tremor, whether due to Parkinson’s disease or essential tremor.17 Studies revealed significantly improved clinical scores on tremor assessment scales, as well as a reduction in adverse effects with stimulation techniques, as compared to ablative procedures.18
As an alternative to targeting the Vim, other authors selected the zona incerta and the posterior subthalamic area, causing lesions that were known as campotomies,19,20 although the risk of inducing dyskinesia was higher than with thalamic surgery. The zona incerta and the posterior subthalamic area have recently been proposed as targets for DBS, as an alternative to the thalamic Vim; good clinical results have been reported for both essential and parkinsonian tremor. The posterior subthalamic area is visible on T2-weighted MRI sequences; this is not the case for the Vim, for which activity must be recorded to locate the specific target.21–24 The main projections or fasciculi crossing the posterior subthalamic area include the zona incerta, prelemniscal radiations (fibres running from the reticular formation that project to the thalamus and the cerebello-rubro-thalamic pathway, which runs from the dentate nucleus to the Vop), the lenticular fasciculus and thalamic fasciculus, with projections from the internal globus pallidus to the Voa, passing medially and dorsally to the subthalamic nuclei and anteriorly to the red nucleus.3,24–26
A recent clinical trial compared patients treated with stimulation of the 2 different targets (Vim or posterior subthalamic area), with a follow-up period of 4 years. The results revealed benefits for both patient groups (P < .05). Tremor showed statistically similar improvements with stimulation of both targets at 6 months to 2 years, but at 3-4 years of DBS, follow-up showed greater improvements in the group of patients treated with Vim stimulation (P < .01).27
Electrophysiological definition of the ventralis intermedius nucleusFrom an anatomical perspective, the Vim cannot be well defined with MRI studies, as it is not correctly visualised,28–30 but neurophysiological recording is able to locate it more clearly. Neurons mainly responding to voluntary or active movements of the contralateral joints would correspond to the Vop, whereas Vim neurons are located more posteriorly, respond to passive movements (performed by the examining physician), and are located 2 mm anteriorly to the tactile sensory neurons.5,31–35 These neurons present spontaneous high-amplitude activity with tonic or phasic discharges in response to joint movements and to pressure on the contralateral muscles, with no response to tactile stimuli. Neurons receiving afferents from the joints are known as kinaesthetic neurons, and are probably located in the Vim, although this has never been confirmed in humans. Some authors believe there to be an overlap between neurons responding to voluntary movements and those responding to passive movements (kinaesthetic neurons), especially in the Vop/Vim36; however, this is not the case with kinaesthetic and tactile neurons, which show 2 very compartmentalised areas: the motor and sensory areas. The neurons of the medial aspect present a lateral somatotopic representation of the limbs, although according to Tasker et al.,37 the somatotopy would be less precise than in the tactile sensory area corresponding to the VC.
In patients with tremor, kinaesthetic neurons are synchronically triggered at the same frequency as contralateral peripheral tremor; these neurons are known as tremor neurons. They are located in the Vim, although they may also be found to a lesser extent in the Vop and VC. These may be the same units, as when a voluntary movement is performed, the tremor activity and tremor disappear.5,32 This physiological location is anterior in patients with sensory neurons with lemniscal response, indicates the location of the Vim, and is predictive of a good response in suppressing tremor, both using ablative techniques and stimulation.33
The incidence of tremor neurons is variable, depending on tremor pathophysiology. Thus, in patients with PD and resting tremor, the number of tremor neurons recorded is 3 times higher than in patients with essential tremor and 5 times higher than in patients with tremor in the context of multiple sclerosis or cerebellar-type tremor. However, the anatomical location of neurons is found in the same physiological region, at 1-2 mm anterior to the tactile edge.38 According to Lenz et al.,39 tremor neurons create a cluster, anterior to the principal sensory nucleus, and located 3.5 mm above the ACPC line. This has not been confirmed by other authors, who found topographically specific and distinct tremor groups with local field potential for patients with PD or essential tremor in the Vop.40
Stereotactic coordinates of the ventralis intermedius nucleusThe Vim is classically located by indirect methods, through calculation of its stereotactic coordinates. Difficulty visualising the Vim with conventional imaging techniques may be explained by the absence of laminas between the different thalamic nuclei,41 which may lead to a lack of precision in its localisation. Thalamic nuclei are closely related to medial structures, such as the third ventricle and the AC-PC line, although differences in the length of the line or the width of the third ventricle may change their position.42
The most frequently used coordinates, which we may consider indirect coordinates, are: medial-laterally (X) 14-15 mm from the medial line or 11-11.5 mm from the wall of the third ventricle; anteroposteriorly (Y) 3-7 mm anterior to the PC, or 3-4 mm posterior to the mid-commissural point; and dorsoventrally (Z) at the AC-PC line. A frequently used measurement is based on ventriculographies performed by Taren et al.43 and Kelly et al.,44 who divided the AC-PC line into 12 sections, and locate the Vim in section 3 anterior to the PC. Most authors consider this anatomical location of the Vim still to be applicable in functional neurosurgery (Table 2).
Coordinates for indirect localisation of the Vim according to different authors.
| Author | X coordinate | Y coordinate | Z coordinate | Procedure |
|---|---|---|---|---|
| Benabid et al.17 | 15 mm | 1/4 AC-PC anterior to the PC | 0 | DBS |
| Ohye et al.31 | 2 mm to the border of the IC | 7-8 mm anterior to the PC | 3-4 mm superior to the AC-PC | GK |
| Brodkey et al.38 | 15/11 mm | 3-5 mm anterior to the PC | 0 | DBS |
| Koller et al.48 | 11.5 + 1/2 3rd V | AC-PC/12 × 2.5 from the PC | 0 | DBS |
| Pilitsis et al.49 | 11-12 + 1/2 3rd V | 4 mm anterior to the PC | 0 | DBS |
| Witjas et al.51 | 11 mm | 7.3 mm anterior to the PC | 2.5 mm superior to the AC-PC | GK |
| Krauss et al.50 | 12-14 mm | 3-4 posterior to the MCP | 1 mm superior to the AC-PC | DBS |
| Hyam et al.47 | 13 mm | 4 mm posterior to the MCP | 0 | DBS |
| Kondziolka et al.46 | 1/4 of the 3rd V width +11 mm | 1/4 of the AC-PC distance +1 mm anterior to the PC | 2.5 mm superior to the AC-PC | GK |
| King et al.52 | 4 mm medial to the thalamic border | PC-FX distance: 1/3 anterior to the PC | 0 | DBS |
3rd V: third ventricle; AC: anterior commissure; AC-PC: intercommissural line; DBS: deep brain stimulation; FX: fornix; GK: gamma-knife; IC: internal capsule; MCP: midcommissural point; PC: posterior commissure; X coordinate: mediolateral coordinate; Y coordinate: anteroposterior coordinate; Z coordinate: dorsoventral coordinate.
Different authors select other coordinates based on individual variations and their personal experience (Table 2).45–52 As previously discussed, physiological recording may help locate the Vim; however, this may involve greater difficulty when using exclusively imaging-guided techniques such as gamma-knife surgery or MRgFUS, as the anatomical heterogeneity of patients may mean that the target identified by imaging techniques in the thalamus is insufficient. A correlation exists between third ventricle width and the location of the internal capsule; thus, in patients with a third ventricle less than 5 mm wide, the medial border of the internal capsule would be located 16-20 mm from the midline, whereas in patients with a width greater than 6 mm, the lateral border of the thalamus would be located between 19 and 23 mm lateral to the midline.53
Lastly, other authors who compared the classic target (indirect measurement) with the target determined by a 3D deformable brain atlas in the MRI study (assessing the length of the AC-PC line and the third ventricle of each patient) found discrepancies of at least 2 mm, mainly in the lateral measurements; therefore, the medial-lateral coordinate may be underestimated.54 King et al.52 recently reported a new indirect method for locating the Vim using imaging techniques, based on the location of the fornix.
Tractography of the dentatorubrothalamic tractRecent studies select the thalamic target based on the dentatorubrothalamic tract (DRTT), which projects to the thalamus along the AC-PC line. The tract originates from the dentate, globose, and emboliform nuclei of the cerebellum, ascending towards the upper cerebellar peduncle. Its fibres decussate contralaterally in the inferior colliculus, projecting to or bordering the red nucleus, and ascending vertically towards the contralateral Vim and Vop.55,56 There is also an ipsilateral tract, whose fibres do not decussate. This tract projects from the thalamus to the primary motor area56 and, to a lesser degree, to the supplementary motor area. The DRTT is involved in movement control; therefore, lesions to this structure may cause ataxia, tremor, or dystonia.57
Diffusion-tensor imaging or tractography is a noninvasive technique enabling visualisation of white matter pathways in the brain by identifying the main direction of the fibres in each voxel of the diffusion-tensor MRI sequence.58 Yamada et al.59 delimited the cerebellothalamocortical tract using this technique, selecting regions of interest (ROI) in the ipsilateral red nucleus and primary motor cortex and considering the Vim as the point of intersection between the cerebellothalamocortical tract and the thalamus on the AC-PC line. Other authors have located targets for DBS with probabilistic tractography by segmenting the thalamus, based on connectivity studies and predefined cortical regions that project to the thalamus (Fig. 3).60
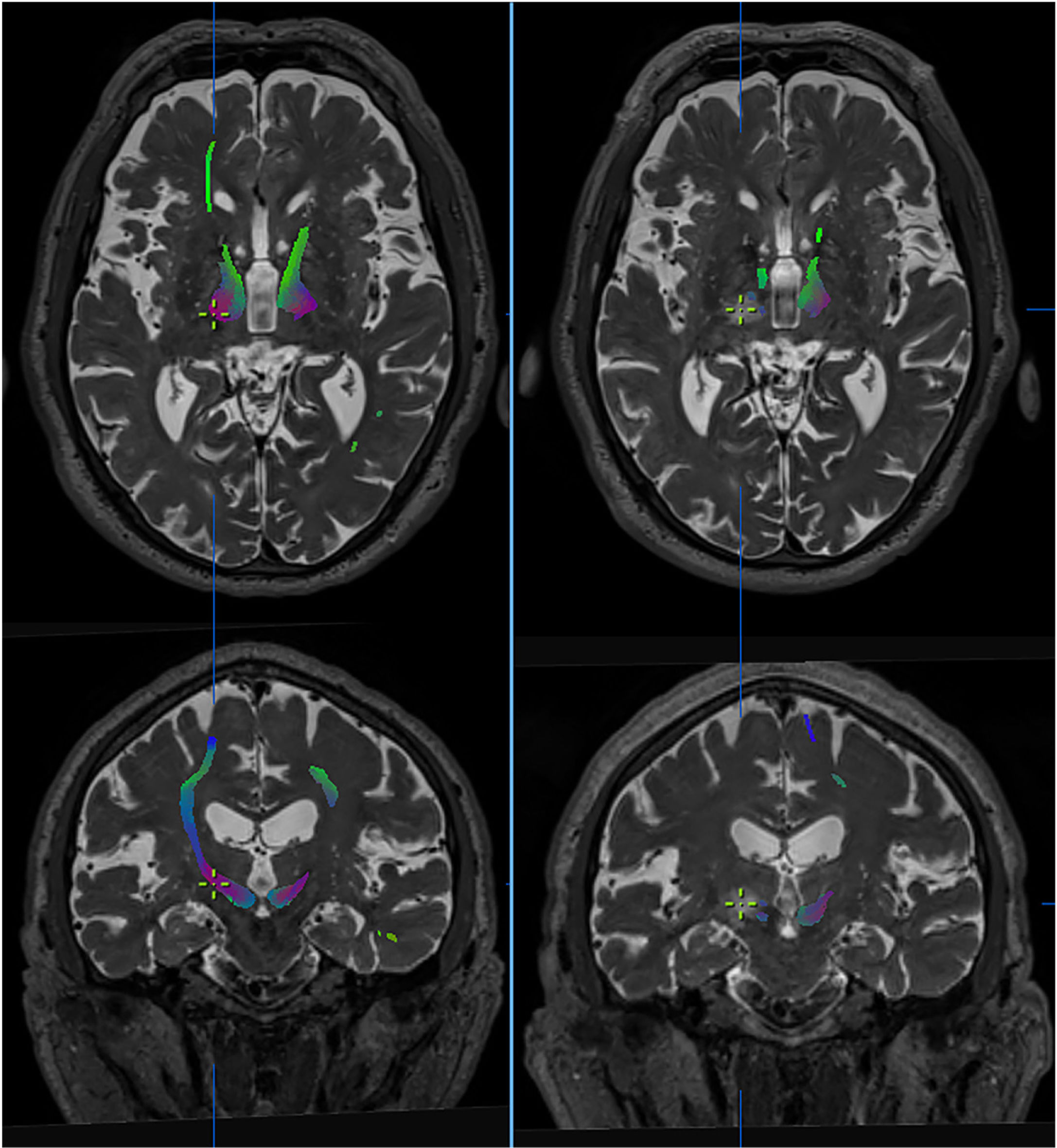
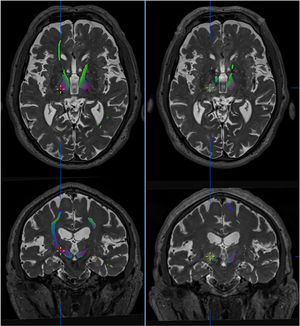
Effect of thalamotomy by ultrasound (MRgFUS) on the dentatorubrothalamic tract (DRTT). Tractography images of the DRTT from high-field diffusion tensor MRI sequences (Skyra, 3 T, Siemens), before (left) and after treatment (right). The DRTT is observed in the thalamus at the intercommissural level. Axial and coronal slices show the virtual disappearance of the tract in the post-treatment sequences, with the contralateral tract persisting. The cursor indicates the location of the lesion. Images extracted from Brainlab’s iPlan planning software.
This technique was first used by Kwon et al.61 to delimit the DRTT in 41 volunteers using probabilistic tractography, considering the dentate nucleus of the cerebellum, contralateral cerebellar peduncle, and ipsilateral red nucleus as ROIs. Thus, they were able to identify and locate the DRTT, which terminated at the ventrolateral nucleus of the thalamus.61
In 2011, Coenen et al.62 published the first surgical procedure in patients with tremor in which the target was determined by tractography. The electrode was placed using this new technique, with deterministic definition of the DRTT and locating the most effective contact for the electrode directly inside the tract; the procedure successfully resolved tremor in the patient. Coenen et al.62,63 consider the technique to be accurate to 3 mm.
Anthofer et al.64 found discrepancies between the location of the target determined by conventional indirect methods and that determined by tractography. The authors described significant diversity, as the target identified by indirect methods was inside the DRTT in 64% of cases, medial to the tract in 11%, and lateral to the tract on the medial-lateral axis in 23.5% of cases.64 In a study by the same research group,65 electrodes were placed targeting the DRTT in 5 patients with essential tremor; the authors concluded that the available evidence was insufficient to define the tract as the tremor target, as results of the stimulation were not convincing.
In 2016, Sammartino et al.66 proposed a novel method based on deterministic tractography to locate the Vim, considering the anatomical relationships of this nucleus with the internal capsule and the medial lemniscus. For determination of the DRTT, they located the ROI inside the area delimited laterally by the pyramidal tract and posteriorly by the medial lemniscus, leaving a safety margin of 3 mm with those eloquent structures. The DRTT was located 1 mm lateral and 1.8 mm anterior to the conventional target identified indirectly (classically located 1/4 anterior to the PC on the intercommissural plane). The authors also found a high degree of concordance between the localisation of the tracts using imaging techniques and intraoperative localisation by assessing clinical effects and intraoperative neurophysiology.66 Researchers have found that in the recording of patients with tremor, both kinaesthetic neurons and tremor neurons in the Vim corresponded to the target previously determined by tractography.67 Other authors have reported similar results, correlating tractography with resolution of tremor.68
Another study correlates tractography with MRgFUS thalamotomy and reports that the final distance between the localisation of the lesion by ultrasound and the target determined by direct imaging showed a discrepancy of 2.5 mm.69 This variation may be attributed to the specific characteristics of this procedure, which lead to changes in the energy of the ultrasound and therefore to changes in the response of the parenchyma to the lesion (Fig. 3).
Finally, Fenoy and Schiess70 compared the difference in tremor control between 2 cohorts of patients one year after the surgery. They found that the direct target determined using tractography controlled tremor similarly to the indirect target of the thalamic Vim in 2 groups of 20 patients. Stimulation parameters were similar in both cohorts.70
ConclusionsHaving reviewed the literature, we can conclude that identifying the DRTT may help us to directly locate the Vim. Furthermore, we should analyse whether deterministic tractography is valid and reproducible and superior to the classic indirect method of anatomical localisation.62,66 This technique presents limitations related to the software used, the length of fibres, and the fractional anisotropy, ROIs, and number of directions selected; consequently, not all authors obtain the same results with the same reliability. Therefore, standardisation of this method in MRI studies is necessary. Further studies are needed to analyse whether the direct method of locating the target by tractography is more useful than using stereotactic coordinates in patients with tremor in the long term.
FundingThis study has received no specific funding from any public, commercial, or non-profit organisation.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Parras O, Domínguez P, Tomás-Biosca A, Guridi J. El papel de la tractografía en la localización del núcleo ventralis intermedius del tálamo y el haz dentatorrubrotalámico en el tratamiento del temblor. Neurología. 2022;37:691–699.