Paraneoplastic neurological syndromes (PNS) occur in approximately 1% of patients with solid tumours, and are thought to be rarer in haematological cancers.1 The PNS most commonly associated with Hodgkin lymphoma (HL) is cerebellar degeneration, whereas peripheral nervous system involvement is more infrequent.2 Motor neuronopathy (MN) is a rarely reported symptom in patients with HL. We present a case of this association, as well as a brief literature review.
The patient was an 82-year-old man with no relevant medical history who was diagnosed with classical HL after biopsy of cervical adenopathies. He presented no B symptoms. A tumour staging study returned results compatible with stage Ia. He started treatment with doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) at 2 months, with neutropaenia and skin lesions appearing as adverse effects. Remission was achieved after 2 months and 2 full cycles of treatment.
The patient was transferred from the emergency department due to rapidly progressive paraparesis, which within 8 days prevented him from walking independently. The patient reported no pain, sensory symptoms, or sphincter dysfunction. The neurological examination revealed predominantly left-sided, distal, asymmetrical paraparesis, with patellar tendon and Achilles tendon areflexia. Stretch reflexes were normal in the upper limbs and plantar reflexes were flexor. Sensory examination showed normal results.
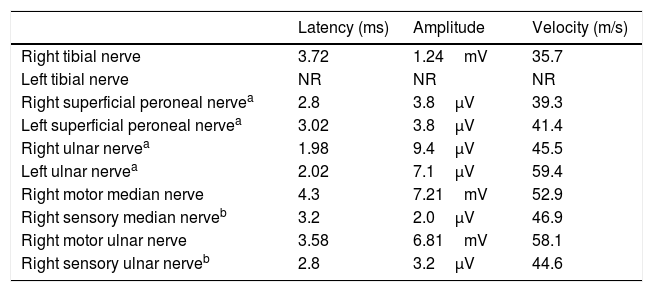
Serology (herpesvirus family, HIV, Lyme disease), immunology (antinuclear antibodies, anti-neutrophil cytoplasmic antibodies, antiganglioside antibodies), and laboratory analysis (including total protein test) yielded normal results. Results from brain and spinal cord MRI scans were normal. A lumbar puncture revealed normal cerebrospinal fluid (CSF) (glucose, 63mg/dL; proteins, 40mg/dL; 0leukocytes/mm3; no oligloclonal bands). No onconeuronal antibodies targeting synaptic antigens were detected in the serum or the CSF. CSF cytology results were normal. A nerve conduction study (Table 1) performed 2 days after admission revealed severely decreased motor potential amplitudes in the lower limbs, with normal distal latencies. No conduction block was detected. Findings from the sensory conduction study were normal. An electromyography recorded increased insertion activity, with many diffuse fibrillations and fasciculations in the quadriceps, tibialis anterior, and gastrocnemius muscles. Motor action potentials presented markedly increased amplitudes, mild increases in the percentage of polyphasia, and prolonged duration. Recruitment was decreased. Based on these results, we diagnosed severe axonal motor neuropathy with significant signs of denervation and reinnervation. The presence of these 2 signs suggests more prolonged progression than that reported by the patient, and justifies using the term subacute neuronopathy.
Summary of the neurography study results.
| Latency (ms) | Amplitude | Velocity (m/s) | |
|---|---|---|---|
| Right tibial nerve | 3.72 | 1.24mV | 35.7 |
| Left tibial nerve | NR | NR | NR |
| Right superficial peroneal nervea | 2.8 | 3.8μV | 39.3 |
| Left superficial peroneal nervea | 3.02 | 3.8μV | 41.4 |
| Right ulnar nervea | 1.98 | 9.4μV | 45.5 |
| Left ulnar nervea | 2.02 | 7.1μV | 59.4 |
| Right motor median nerve | 4.3 | 7.21mV | 52.9 |
| Right sensory median nerveb | 3.2 | 2.0μV | 46.9 |
| Right motor ulnar nerve | 3.58 | 6.81mV | 58.1 |
| Right sensory ulnar nerveb | 2.8 | 3.2μV | 44.6 |
NR: no response.
The patient's condition improved spontaneously during hospitalisation. He continued on ABVD treatment with no radiotherapy. At one month, he showed a slight neurological deterioration, and a therapeutic trial was conducted with intravenous immunoglobulins, which obtained no response. He remained neurologically stable for the rest of the follow-up period. After 4 cycles and a half of ABVD, the patient presented pancytopaenia with severe neutropaenia, septic shock, and bilateral pulmonary thromboembolism, leading to his death.
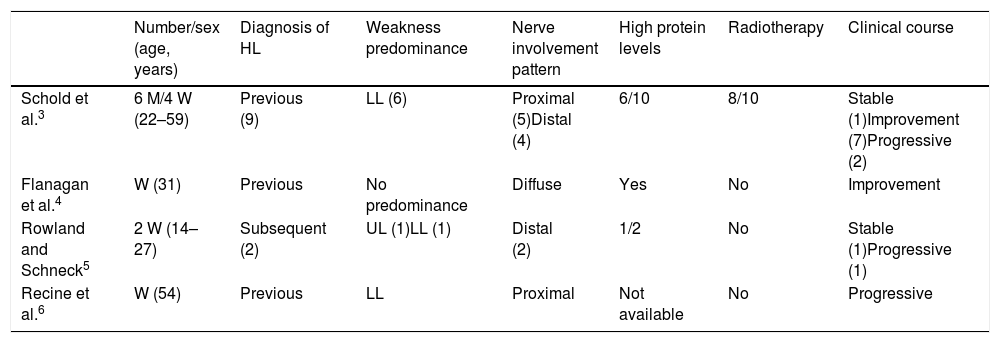
MN associated with HL is an infrequent entity and is not included in the European registry of PNS in patients with lymphoma.2 Most of the reported data are from isolated case reports or small series (Table 2).3–6 It generally manifests after diagnosis of HL, or even during remission.3 In general, it predominantly affects proximal or distal areas of the lower limbs. It tends to be mild and to stabilise or even improve without specific treatment,3 although a favourable response to intravenous immunoglobulins has recently been reported.4 CSF findings vary, and MRI scans reveal hyperintensities in the anterior horns and contrast uptake in the anterior roots.4 Anatomical pathology studies show degeneration in the anterior horn of the spinal cord, with variable presence of inflammatory elements.3,5,6 All these characteristics suggest that the main lesion affects the cell bodies of motor neurons and not the peripheral nerves, hence the term neuronopathy. No associated antibodies have been described.
Main characteristics of reported patients with motor neuronopathy associated with Hodgkin lymphoma.
| Number/sex (age, years) | Diagnosis of HL | Weakness predominance | Nerve involvement pattern | High protein levels | Radiotherapy | Clinical course | |
|---|---|---|---|---|---|---|---|
| Schold et al.3 | 6 M/4 W (22–59) | Previous (9) | LL (6) | Proximal (5)Distal (4) | 6/10 | 8/10 | Stable (1)Improvement (7)Progressive (2) |
| Flanagan et al.4 | W (31) | Previous | No predominance | Diffuse | Yes | No | Improvement |
| Rowland and Schneck5 | 2 W (14–27) | Subsequent (2) | UL (1)LL (1) | Distal (2) | 1/2 | No | Stable (1)Progressive (1) |
| Recine et al.6 | W (54) | Previous | LL | Proximal | Not available | No | Progressive |
HL: Hodgkin lymphoma; LL: lower limbs; M: man; UL: upper limbs; W: woman.
The aetiopathogenesis of MN associated with HL remains unknown. Exposure to radiotherapy in some of the published cases3 means we cannot rule out a toxic effect of this treatment. Regarding the possible causal role of ABVD, only vinblastine has been associated with small-fibre sensory neuropathy, and never with motor neuropaties.7 It is also likely that patients with this diagnosis actually present different entities, considering its clinical variability and the limitations on the complementary studies conducted in older series. Our patient was not treated with radiotherapy, which excludes this aetiological possibility. His progression, which was initially favourable, suggests a potentially reversible functional disorder rather than a degenerative or inflammatory process involving the death of motor neurons. In any case, adequate differential diagnosis is necessary to exclude conditions that are potentially treatable or that may influence the therapeutic approach to HL.
Please cite this article as: Muñiz-Castrillo S, Martín H, Ballesteros L, Salama P. Neuronopatía motora subaguda asociada a linfoma de Hodgkin. Neurología. 2020;35:517–518.