Cerebrovascular disease is the third-leading cause of death and the second-leading cause of disability and dementia.
ObjectiveDetermine stroke incidence and risk factors in a population of adults aged 65 and over in Cuba (Havana and Matanzas).
Material and methodsThis prospective longitudinal study, completed between April 2008 and April 2011, re-evaluated 2916 elderly adults with an average follow-up time of 4 years. Cases included 2316 living subjects and 600 verbal autopsies. Study variables were age, sex, educational level, self-reported health, and description of chronic diseases and substance abuse. Laboratory tests included genotyping APOE. Stroke was diagnosed based on the World Health Organization definition. We calculated the global incidence rate for stroke, broken down by sex, age group, and risk factors for incident stroke.
ResultsStroke incidence was 786.2 in 100000 persons/year (95% CI, 672.3-906.4). History of alcohol consumption (HR, 3.5; 95% CI, 3.3-3.7), dementia (HR, 3.0; 95% CI, 1.6-5.5) and male sex (HR, 1.8; 95% CI, 1.2-2.8) were shown to be risk factors for incident stroke.
ConclusionsStroke incidence was similar to rates reported in developed countries and lower than that in low- to middle-income countries. Given that diabetes mellitus, heart disease, arterial hypertension, smoking, APOE4, etc. are associated with higher mortality rates, they will require separate analysis in a study of stroke risk factors.
La enfermedad cerebrovascular constituye la tercera causa de muerte y la segunda de discapacidad y demencia.
ObjetivoDeterminar la incidencia y los factores de riesgo de ictus en adultos de 65 años y más en La Habana y Matanzas, Cuba.
Material y métodoSe realizó un estudio prospectivo longitudinal, entre abril del 2008 y abril del 2011, que reevaluó a 2.916 adultos mayores, con una media de seguimiento de 4 años, incluidos 2.316 adultos vivos y 600 autopsias verbales. Las variables utilizadas fueron: edad, sexo, nivel educacional, autorreporte y descripción de enfermedades crónicas y hábitos tóxicos. Se realizaron exámenes de laboratorio, incluido el genotipo de la APOE. El diagnóstico de ictus se basó en la definición de la Organización Mundial de la Salud. Se calculó la tasa de incidencia de ictus global, por sexos y grupos de edad, y los factores de riesgo de ictus incidente.
ResultadosLa incidencia de ictus fue de 786,2 por 100.000 personas/año (IC del 95%, 672,3-906,4). El antecedente de consumo de alcohol (HR: 3,5; IC del 95%, 3,3-3,7) y la demencia (HR: 3,0; IC del 95%, 1,6-5,5) y el sexo masculino (HR: 1,8; IC del 95%, 1,2-2,8) constituyeron factores de riesgo de ictus incidente.
ConclusionesLa incidencia de ictus es similar a la reportada en países desarrollados y menor que la reportada en otros países de bajos y medianos ingresos. Como la diabetes mellitus, enfermedad cardiaca, la hipertensión arterial, el hábito de fumar y APOE4, entre otros, se asocian con una mayor mortalidad requieren un análisis diferente en el estudio de factores de riesgo de ictus.
Stroke is the third leading cause of death in the world, with most deaths occurring in low- and middle-income countries.1,2
In the last 4 decades, global stroke incidence has decreased by 42% in high-income countries and increased more than 100% in low- and middle-income countries. In 2008, stroke incidence rates in low- and middle-income countries exceeded stroke incidence rates in high-income countries for the first time.2
Epidemiological data on stroke in Cuba are extracted from medical records held by the Cuban statistics division and from a disease-specific self-reported survey conducted in 2002.3 To date, no population-based epidemiological studies of cerebrovascular disease that meet criteria for an ideal stroke study have been conducted.4
We decided to perform this study to determine stroke incidence and risk factors in the population aged 65 or older in Havana and Matazanas, Cuba.
Material and methodsStudy designThis is a prospective longitudinal study of a cohort of 3000 patients older than 65 years. It includes 2 clearly defined stages: a cross-sectional study performed between June 2003 and May 2007,5 followed by a 4-year prospective longitudinal study (June 2008-July 2011) aimed at determining stroke incidence and risk factors in residents of Havana and Matanzas aged 65 and older.
The total study sample comprised 2944 patients, of whom 608 died (20.7%) and 28 declined to participate (0.95%; 20 subjects declined to be interviewed and 8 relatives refused to participate in a verbal autopsy). We interviewed 2316 patients (78.7%) and conducted 600 verbal autopsies. The cohort for analysis of stroke incidence was defined as all patients who were stroke-free during the first study, amounting to 2731 patients. We excluded 28 cases who declined to participate in the study; 2703 patients remained for the incidence analysis.
Stroke diagnosisDiagnosis was based on the definition proposed by the World Health Organization. Patients and reliable informants provided information regarding the following: (a) sudden or rapid onset of focal (or global) neurological dysfunction signs lasting more than 24h, with no apparent non-vascular cause (head trauma, neoplasm, coma attributable to metabolic disorder or water-electrolyte imbalance, vasculitis, central nervous system infection, or peripheral neuropathy).6
All patients surveyed underwent a structured physical and neurological examination (NEUROEX) to objectively and quantitatively measure focal signs, parkinsonism, ataxia, apraxia, and primitive reflexes.7 This examination pinpoints stroke symptoms and signs using the National Institutes of Health Stroke Scale (NIHSS).7
This study was conducted as part of the international project 10/66, whose full description has been published elsewhere.7
For the longitudinal study, we applied a protocol of interviews and a diagnostic algorithm similar to that used in the baseline study (clinical evaluation with cognitive tests; clinical interview and neurological evaluation; complementary tests, including APOE genotyping and interviewing a family member, caregiver or other informant). In a second phase, patients with suspected cerebrovascular disease were visited once more by a vascular neurologist.
The second stage consisted of additional interviews of all participants who:
- 1.
reported having suffered a stroke during follow-up, after the initial evaluation (with or without a clinical diagnosis).
- 2.
might have shown signs of having suffered a stroke according to the NIHSS in the follow-up interview (i.e., a score of 4 or more on the NIHSS included in NEUROEX).
The interview was structured but also included blank spaces for clinical notes so that investigators could provide more in-depth evaluations. At the end of the interview, we determined whether the patient had suffered a stroke after the baseline evaluation and listed other characteristics. The interview and clinical notes were provided to a second neurologist, who performed the same evaluation. When there were discrepancies, both sources were consulted to aid in reaching a consensus.
For patients with an existing imaging study (computed tomography or magnetic resonance), we indicated if there were images suggesting stroke and copied any neuroimaging findings mentioned in patients’ reports.
IncidenceWe report stroke incidence by person-year, calculated for the time elapsed between the baseline evaluation and the follow-up study. Global stroke incidence was estimated with its 95% confidence interval (CI), along with incidence by sex, per age group by dividing the number of cases by the number of persons-year in each age group, and by place of residence.
The Cox proportional hazards model was used to estimate hazard ratios.
We calculated the annual incidence rate of stroke per 1000 population, adjusted by age group, sex, and selected variables (education level, family history of dementia, tobacco use, APOE*4 genotype, arterial hypertension, stroke, alcohol use, obesity, diabetes, cholesterol level, and triglyceride level) using the Cox proportional hazards model. We also calculated vascular dementia incidence rate, incidence of transient ischaemic attack (TIA), and risk of incident stroke adjusted by age, sex, and level of education.
We used the Stata's stcrreg command to estimate competing risks according to the Cox proportional hazards model.8
The risk factors sex, dementia, APOE genotype (presence vs absence of 1 or 2 APOE*4 alleles), arterial hypertension, smoking habit (current or former smoker vs non-smoker), alcohol abuse, cardiovascular disease, and diabetes mellitus were each assessed as dichotomous variables. The effects of level of education and 5-year increases in age were analysed as categorical variables.
Analyses were performed using Stata software version 9.2 (StatCorp 2007, Stata Statistical Software: release 10; StataCorp, College Station, USA). For the multivariate analysis, we included only participants with unmissing data for all independent variables.
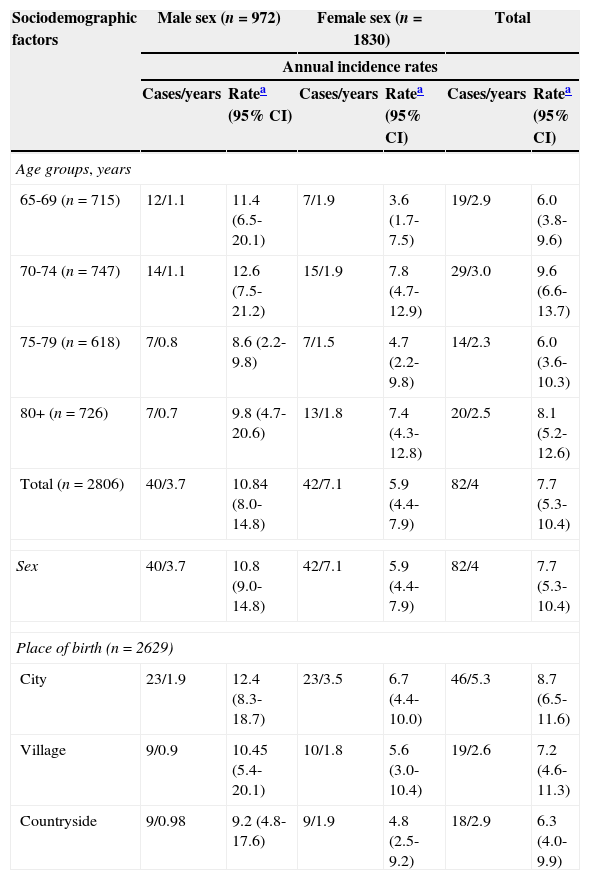
ResultsStroke incidenceGlobal stroke incidence rate among patients older than 65 years was 786.2 new cases per 100000 person-years (95% CI, 672.3-906.4), with a TIA incidence rate of 525.8 per 100000 person-years (95% CI, 416.3-676.2). Table 1 shows incidence broken down by sociodemographic characteristics. Stroke incidence increased with age, with the exception of the 75-79 age group, which showed an incidence lower than that of the 70-74 age group with 9.6 cases per 1000 person-years (95% CI, 6.6-13.7). This rate was the highest out of all age groups. The incidence was higher in men, with 10.8 cases per 1000 person-years (95% CI, 9.0-14.8). Incidence in women was 5.9 cases per 1000 person-years (95% CI, 4.4-7.9). We observed a decreasing gradient incidence for place of birth, from 8.7 cases per 1000 person-years (95% CI, 6.5-11.6) in patients born in cities, to 7.2 cases per 1000 person-years (95% CI, 4.6-11.3) in patients born in villages, to 6.3 cases per 1000 person-years (95% CI, 4.0-9.9) in patients born in the countryside.
Annual incidence rates (per 1000 person-years) broken down by sociodemographic variables. Havana and Matanzas, Cuba.
| Sociodemographic factors | Male sex (n=972) | Female sex (n=1830) | Total | |||
|---|---|---|---|---|---|---|
| Annual incidence rates | ||||||
| Cases/years | Ratea (95% CI) | Cases/years | Ratea (95% CI) | Cases/years | Ratea (95% CI) | |
| Age groups, years | ||||||
| 65-69 (n=715) | 12/1.1 | 11.4 (6.5-20.1) | 7/1.9 | 3.6 (1.7-7.5) | 19/2.9 | 6.0 (3.8-9.6) |
| 70-74 (n=747) | 14/1.1 | 12.6 (7.5-21.2) | 15/1.9 | 7.8 (4.7-12.9) | 29/3.0 | 9.6 (6.6-13.7) |
| 75-79 (n=618) | 7/0.8 | 8.6 (2.2-9.8) | 7/1.5 | 4.7 (2.2-9.8) | 14/2.3 | 6.0 (3.6-10.3) |
| 80+ (n=726) | 7/0.7 | 9.8 (4.7-20.6) | 13/1.8 | 7.4 (4.3-12.8) | 20/2.5 | 8.1 (5.2-12.6) |
| Total (n=2806) | 40/3.7 | 10.84 (8.0-14.8) | 42/7.1 | 5.9 (4.4-7.9) | 82/4 | 7.7 (5.3-10.4) |
| Sex | 40/3.7 | 10.8 (9.0-14.8) | 42/7.1 | 5.9 (4.4-7.9) | 82/4 | 7.7 (5.3-10.4) |
| Place of birth (n=2629) | ||||||
| City | 23/1.9 | 12.4 (8.3-18.7) | 23/3.5 | 6.7 (4.4-10.0) | 46/5.3 | 8.7 (6.5-11.6) |
| Village | 9/0.9 | 10.45 (5.4-20.1) | 10/1.8 | 5.6 (3.0-10.4) | 19/2.6 | 7.2 (4.6-11.3) |
| Countryside | 9/0.98 | 9.2 (4.8-17.6) | 9/1.9 | 4.8 (2.5-9.2) | 18/2.9 | 6.3 (4.0-9.9) |
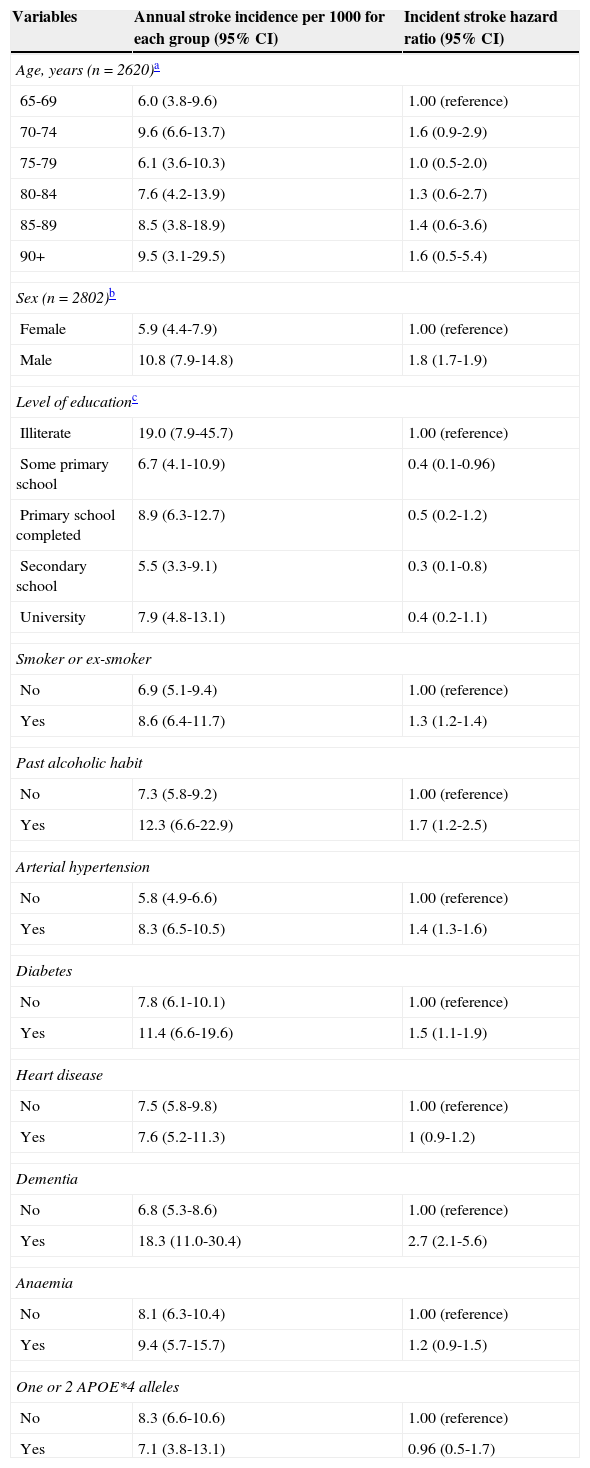
Stroke incidence was associated with older age, male sex, lower education level, smoking, personal history of alcohol abuse, arterial hypertension, diabetes mellitus, heart disease, dementia, anaemia, and being a carrier of APOE*4 allele(s) (Table 2). We observed that risk increased with age, with the exception of the 70-74 age group who presented the highest hazard ratio of all groups, similar to that displayed by the group of patients aged 90 or older. Taking female sex as our reference, we observed a hazard ratio of 1.8, meaning that risk of stroke was 1.8 times higher in men after adjusting by age and level of education. Risk of stroke was 2.7 times higher in patients with dementia, 1.7 times higher in patients with a history of alcohol abuse, 1.5 times higher in diabetic patients, and 1.4 times higher in patients with hypertension. We find it surprising that although the risk was 2.44 times higher in patients with APOE*4 alleles (1 or 2), the difference was not statistically significant.
Stroke incidence and hazard ratio according to demographic variables and risk factors. Adjusted by age, sex, and level of education (Havana and Matanzas).
| Variables | Annual stroke incidence per 1000 for each group (95% CI) | Incident stroke hazard ratio (95% CI) |
|---|---|---|
| Age, years (n=2620)a | ||
| 65-69 | 6.0 (3.8-9.6) | 1.00 (reference) |
| 70-74 | 9.6 (6.6-13.7) | 1.6 (0.9-2.9) |
| 75-79 | 6.1 (3.6-10.3) | 1.0 (0.5-2.0) |
| 80-84 | 7.6 (4.2-13.9) | 1.3 (0.6-2.7) |
| 85-89 | 8.5 (3.8-18.9) | 1.4 (0.6-3.6) |
| 90+ | 9.5 (3.1-29.5) | 1.6 (0.5-5.4) |
| Sex (n=2802)b | ||
| Female | 5.9 (4.4-7.9) | 1.00 (reference) |
| Male | 10.8 (7.9-14.8) | 1.8 (1.7-1.9) |
| Level of educationc | ||
| Illiterate | 19.0 (7.9-45.7) | 1.00 (reference) |
| Some primary school | 6.7 (4.1-10.9) | 0.4 (0.1-0.96) |
| Primary school completed | 8.9 (6.3-12.7) | 0.5 (0.2-1.2) |
| Secondary school | 5.5 (3.3-9.1) | 0.3 (0.1-0.8) |
| University | 7.9 (4.8-13.1) | 0.4 (0.2-1.1) |
| Smoker or ex-smoker | ||
| No | 6.9 (5.1-9.4) | 1.00 (reference) |
| Yes | 8.6 (6.4-11.7) | 1.3 (1.2-1.4) |
| Past alcoholic habit | ||
| No | 7.3 (5.8-9.2) | 1.00 (reference) |
| Yes | 12.3 (6.6-22.9) | 1.7 (1.2-2.5) |
| Arterial hypertension | ||
| No | 5.8 (4.9-6.6) | 1.00 (reference) |
| Yes | 8.3 (6.5-10.5) | 1.4 (1.3-1.6) |
| Diabetes | ||
| No | 7.8 (6.1-10.1) | 1.00 (reference) |
| Yes | 11.4 (6.6-19.6) | 1.5 (1.1-1.9) |
| Heart disease | ||
| No | 7.5 (5.8-9.8) | 1.00 (reference) |
| Yes | 7.6 (5.2-11.3) | 1 (0.9-1.2) |
| Dementia | ||
| No | 6.8 (5.3-8.6) | 1.00 (reference) |
| Yes | 18.3 (11.0-30.4) | 2.7 (2.1-5.6) |
| Anaemia | ||
| No | 8.1 (6.3-10.4) | 1.00 (reference) |
| Yes | 9.4 (5.7-15.7) | 1.2 (0.9-1.5) |
| One or 2 APOE*4 alleles | ||
| No | 8.3 (6.6-10.6) | 1.00 (reference) |
| Yes | 7.1 (3.8-13.1) | 0.96 (0.5-1.7) |
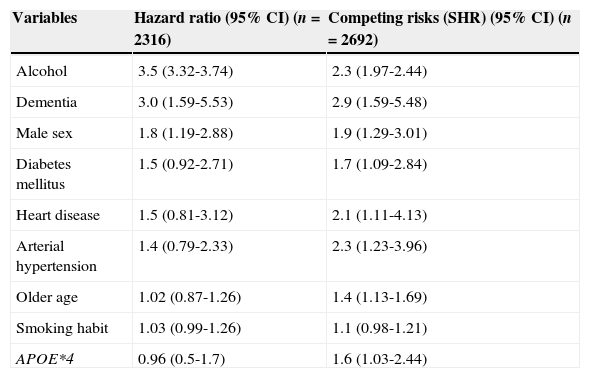
The multivariate analysis, performed using the Cox proportional hazards model and controlling for the remaining variables, showed that risk of stroke in our cohort was significantly influenced by history of alcohol abuse (HR, 3.5; 95% CI, 3.32-3.74), dementia (HR, 3.0; 95% CI, 1.59-5.53), male sex (HR, 1.8; 95% CI, 1.19-2.88), and diabetes mellitus (HR, 1.55; 95% CI, 0.92-2.71).
Patients with diabetes mellitus, heart disease, arterial hypertension, or a smoking habit, those carrying APOE*4 allele(s), older patients, and individuals with low levels of high-density lipoprotein cholesterol present a higher risk of dying before suffering a stroke. With this in mind, we conducted a competing risk analysis to verify the association of these variables with incident stroke (Table 3). The highest relative risk (RR) among dichotomous qualitative variables, a value of 3.5, was detected in patients with history of alcohol abuse. This means that risk of presenting an incident stroke is 3.5 times higher in older patients with history of harmful drinking. Relative risk for patients with dementia was 3. Men presented an RR of 1.8; diabetes mellitus and heart disease were associated with a risk of 1.5. Patients with arterial hypertension display an RR of 1.4.
Risk factors of incident stroke adjusted by age, sex, and level of education in Havana and Matanzas.
| Variables | Hazard ratio (95% CI) (n=2316) | Competing risks (SHR) (95% CI) (n=2692) |
|---|---|---|
| Alcohol | 3.5 (3.32-3.74) | 2.3 (1.97-2.44) |
| Dementia | 3.0 (1.59-5.53) | 2.9 (1.59-5.48) |
| Male sex | 1.8 (1.19-2.88) | 1.9 (1.29-3.01) |
| Diabetes mellitus | 1.5 (0.92-2.71) | 1.7 (1.09-2.84) |
| Heart disease | 1.5 (0.81-3.12) | 2.1 (1.11-4.13) |
| Arterial hypertension | 1.4 (0.79-2.33) | 2.3 (1.23-3.96) |
| Older age | 1.02 (0.87-1.26) | 1.4 (1.13-1.69) |
| Smoking habit | 1.03 (0.99-1.26) | 1.1 (0.98-1.21) |
| APOE*4 | 0.96 (0.5-1.7) | 1.6 (1.03-2.44) |
The recurrence rate of stroke in elderly patients in the incidence study was high at 29.4%. Incidence of recurrent stroke was estimated at 231.2 cases per 100000 person-years. We should highlight that the recurrence rate is calculated to consider presence of a new stroke only, and did not include patients who suffered more than one stroke after the incident stroke. Therefore, actual recurrence should have been much higher.
DiscussionThis study is one of the first studies of stroke incidence and risk factors in a Cuban population. Our study was based on the 10/66 population-based study.5 Our strategy of interviewing the entire population from the selected area increases the response rate, thereby making it easier to conduct longitudinal follow-up.
Stroke diagnosis was based on the patient's own report and that of a reliable informant, a structured questionnaire aiming to identify focal symptoms and signs of neurological dysfunction, and a structured physical and neurological examination conducted by a specialist. The final diagnosis was established by two independent physicians, which increased the accuracy of the clinical diagnosis. In 56% of the cases, diagnosis was confirmed by computed tomography or magnetic resonance imaging findings in the baseline study, and in 64.7% by the follow-up study. This represents a limitation since a high percentage of stroke diagnoses were based on symptoms. Therefore, we may have excluded patients with silent cerebral infarcts that did not cause focal symptoms or signs.
Our study of stroke incidence in adults older than 65 reported an overall age-adjusted stroke incidence of 786.2 cases per 100000 person-years. Our rate is double that reported in Chile9 between 2000 and 2002: 340.3 cases per 100000 person-years. However, it resembles those reported for Hai (Tanzania)10 between 2003 and 2006, with 967.6 cases per 100000 person-years; and rural Trivandrum (India)11 in 2005, with 997.7 cases per 100000 person-years; and the Oxford Vascular Study,12 reporting 843 cases per 100000 person-years between 2002 and 2004. Our rate is much lower that those reported for Mashhad (Iran)13 in 2006 and 2007 with 1673.8 cases per 100000 person-years; Grodno (Belarus)14 between 2001 and 2003, with 1569 cases per 100000 person-years; Dar es Salaam (Tanzania)10 between 2003 and 2006, with 2902.8 cases per 100000 person-years; urban Trivandrum (India)11 in 2005 of 1059.7 per 100000 person-years; and the Oxford Community Stroke Project (United Kingdom)12 between 1981 and 1984, with 1157.5 cases per 100000 person-years. The study from Oyabe (Japan)15 reported incidence rates for men and women, respectively, of 3366 and 3360 cases per 100000 person-years (1977-1981), 2509 and 2384 cases per 100000 person-years (1982-1986), 2533 and 2639 cases per 100000 person-years (1987-1991). Our rate was similar to that reported in Dijon (France)16 between 1995 and 2003: 790 and 600 cases per 100000 person-years in men and women, respectively. We should highlight that stroke incidence in our study presents a similar pattern to reports for high-income countries and rural areas of low- and middle-income countries where rates are shown to be lower than in urban areas.
The high and increasing incidence of stroke in low- and medium-income countries over the last 40 years is probably due to their health and demographic transitions.17
The increased stroke incidence with older age reported by our study is in line with tendencies reported by the literature. Age is the most important non-modifiable risk factor for all stroke types, including ischaemic stroke. Stroke rates double with every decade of life after age 55 in both men and women. Studies report that between 75% and 89% of all strokes affect patients older than 65 years.18
Stroke incidence in Havana and Matanzas was higher among men in all age groups, including patients older than 85 years. Analysis of gender differences in stroke incidence in the Framingham Heart Study19 reported a higher incidence of stroke in men than in women (P<.001). However, the opposite sex effect can be seen in the oldest patients (those aged 85-94 years); that group displayed a higher incidence in women than in men. The Oxford Vascular Study12 showed similar results; it reported a higher stroke incidence among women older than 85 years.
The South London Study20 reported a higher total stroke incidence in black patients than in white patients (incidence rate ratio [IRR] 1.27, 95% CI, 1.10-1.46 in men; IRR 1.29, 95% CI, 1.11-1.50 in women). This is believed to be due to the reduction in risk factors that has been observed in the white population, which remains to be achieved in the black population.
In our study, we observed a tendency towards higher stroke incidence in patients born in the city than in those from the countryside. There are no references in the literature regarding stroke incidence by place of birth, but we do find articles mentioning stroke prevalence and incidence according to area of residence. These studies show differences between countries with high, medium, and low indexes of human development.21
The stroke risk factors listed here resemble those reported in the literature, although some traditional risk factors seemed not to be statistically significant. This could be due to the hypothesis that these particular factors affect middle-aged individuals the most.
The Longitudinal Gerontological and Geriatric Population Studies22,23 was conducted in Göteborg (Sweden) in patients older than 85. Its results showed that among traditional stroke risk factors (sex, treated hypertension, systolic blood pressure, diastolic blood pressure, diabetes mellitus, overweight, smoking habit, atrial fibrillation, acute myocardial infarction, and alcohol consumption), only systolic blood pressure (RR, 1.14; 95% CI, 1.02-1.28) and female sex (RR, 2.1; 95% CI, 1.0-4.8) were associated with a higher risk of stroke.
Stroke incidence in both Havana and Matanzas was much higher in patients with diabetes, and RR of stroke was 1.5 times higher in these patients. The Northern Sweden MONICA Stroke Study showed that stroke incidence was 5 and 8 times higher in diabetic men and women, respectively.24
Contrary to prior descriptions in the literature, stroke incidence in our study was lower in patients with abdominal obesity. A meta-analysis of 25 studies including 2274961 patients and 30757 strokes showed that, based on the pooled estimates of risk, the probability of ischaemic stroke was 22% higher in overweight individuals, and 64% higher in obese individuals, than in normal-weight subjects.25
Stroke incidence in our study was 3 times higher in patients with dementia, and stroke RR was 3 times higher in these patients, which was statistically significant. Dementia is frequent after stroke and both entities contribute to the development of disability in the elderly. Stroke multiplies risk of dementia by more than nine. However, the association between stroke and dementia is complex. Stroke-free subjects with cognitive impairment or mild dementia have been reported to present an increased risk of stroke.26,27
Our study found that stroke incidence doubled in patients with a history of depression. The Framingham study28 reported a 4-fold risk of stroke in patients with depressive symptoms. The Longitudinal Gerontological and Geriatric Population Studies in Göteborg (Sweden)22,23 reported a higher incidence of first stroke in individuals older than 85 with depression and (116.5 per 1000 person-years) than in the rest of the sample (46.1 per 1000 person-years). Incident stroke risk was similar in those with major depression (RR, 1.9; 95% CI, 0.9-3.9), and in those with dysthymia (RR, 2.5; 95% CI, 0.9-5.8) in the baseline study.
The stroke recurrence rate in our study was 29.4% and we counted only single new strokes after the incident stroke. The Rochester study reported a recurrence rate of 24.4% during the first year and 29.3% after 2 years29; the Northern Manhattan Stroke Study, a large epidemiological study by Sacco et al.,30 reported a recurrence rate of 26.5% in the first year.
ConclusionsStroke incidence was similar to that reported in developed countries and lower than that reported in low and middle-income countries. The identified risk profile displays the classic risk factors.
Conflicts of interestThe authors have no conflicts of interest to declare.
This study analysing a Cuban population is part of the 10/66 research project. It is the result of a cooperative agreement between the Institute of Psychiatry, Psychology and Neuroscience in London and the Universidad de Ciencias Médicas in Havana, promoted by the Wellcome Trust Foundation and the Cuban Ministry of Public Health. We would like to thank all who participated in our population-based study.
Please cite this article as: Llibre-Guerra JC, Valhuerdi Cepero A, Fernández Concepción O, Llibre-Guerra JJ, Gutiérrez RF, Llibre-Rodriguez JJ. Incidencia y factores de riesgo de ictus en La Habana y Matanzas, Cuba. Neurología. 2015;30:488–495.