The Spanish Stroke Group published the “Plan for stroke healthcare delivery” in 2006 with the aim that all stroke patients could receive the same degree of specialized healthcare according to the stage of their disease, independently of where they live, their age, gender or ethnicity. This Plan needs to be updated in order to introduce new developments in acute stroke.
Material and methodsA committee of 19 neurologists specialized in neurovascular diseases representing different regions of Spain evaluated previous experience with this Plan and the available scientific evidence according to published literature.
Results and conclusionsThe new organized healthcare system must place emphasis on the characteristics of the different care levels with promotion of reference stroke hospitals, set up less restrictive stroke code activation criteria that include new therapeutic options, establish new standard measures for endovascular treatment and develop tele-medicine stroke networks.
El Grupo de Estudio de Enfermedades Cerebrovasculares de la Sociedad Española de Neurología publicó en el año 2006 el Plan de Atención Sanitaria del Ictus (PASI) con el objetivo de elaborar un sistema organizado de atención al ictus que de respuesta a las necesidades de cada enfermo y optimice la utilización de los recursos sanitarios. Este plan pretendía garantizar la equidad en la atención sanitaria del paciente con ictus. La Estrategia Nacional en Ictus del Sistema Nacional de Salud aprobada en el año 2008, recogió en gran medida el tipo de modelo organizativo sanitario del PASI. Sin embargo, en el tiempo transcurrido desde su publicación, han aparecido nuevos avances en el tratamiento de la fase aguda del infarto cerebral que obligan a realizar una revisión del mismo.
FuentesUn comité de 19 neurólogos especialistas en patología neurovascular y representativos de las diferentes comunidades autónomas han revisado el PASI con el objetivo de incorporar los nuevos avances del tratamiento en la fase aguda del infarto cerebral. Esta revisión se ha basado en una revisión de la literatura científica y en la experiencia acumulada con el plan anterior.
DesarrolloEl nuevo modelo organizativo propuesto debe hacer hincapié en las características de los diferentes niveles asistenciales con la potenciación de Hospitales de Referencia, establecer nuevos criterios de activación del Código Ictus menos restrictivos que contemplen las nuevas posibilidades terapéuticas, establecer medidas organizativas para la implantación del intervencionismo neurovascular y permitir la utilización del recurso técnico de la Telemedicina.
Cerebrovascular disease is a leading social and health-care problem in Spain. It is the number one cause of mortality in women and the number two cause of death overall, as well as the leading cause of disability in adults and the second leading cause of dementia. This will clearly be aggravated within a few years time due to the gradual ageing of the Spanish population. Approximately 75% of all strokes affect patients over the age of 65 years and it has been calculated that by 2025, 1,200,000 Spaniards will have survived a stroke, of whom, more than 500,000 will have some kind of disability.
Stroke is a complex disease requiring immediate care and benefiting from specialized care. The intervention of neurologists with experience in cerebrovascular disease improves the evolution of stroke patients and lowers the costs related to the process.1,2 One study conducted in Spanish hospitals revealed that the evaluation of stroke patients by a neurologist within the first 6h is associated with 5 times less risk of a poor evolution.3 The PRACTIC study4,5 confirmed that neurological care entails a statistically significant decrease in mortality and intrahospital complications among stroke patients, increases the percentage of independent patients, and reduces the probability of suffering vascular recurrence. The findings of these studies carried out in Spain are similar to those obtained in other countries.6,7
In addition to the clear benefit of early, specialized neurological care for people who have suffered a stroke, the advantages of organizing medical and nursing care in a Stroke Unit (SU) have also been proven. Care in an SU is associated with a lower probability of death or disability in all patient subgroups, except in patients with a lowered level of consciousness8,9; these benefits are maintained in the long term.
In the light of scientific evidence presented previously, it is clear that the ideal objective pursued by stroke care is for all patients to receive early care by a neurologist with experience in cerebrovascular disease, and those that so require can benefit from being admitted to an SU. However, at present, most stroke patients are still cared for at hospitals that do not have neurologists on-call and are therefore deprived of the best medical practice for their ailment.10
Part of the benefit early care currently provides in stroke patients is due to the administration of thrombolytic treatment. Intravenous thrombolysis is a highly effective treatment when administered within the first few hours of ischaemic stroke.11–13 However, fewer than 5% of patients currently receive this therapy.14 Although there are several causes that account for these low percentages, undoubtedly the delay in arriving at the hospital is the limiting factor in most cases. At present, there is an official regulation for the administration of thrombolysis in the setting of stroke that prevents it from being administered routinely after the 3-h time point, and while the limit has been extended to 4.5h, this increase has failed to make a marked improvement in the percentages of patients treated if health-care systems do not work at decreasing the delay in providing stroke care. Despite the fact that the stroke code (SC) has done much to reduce this delay,15 experience has shown that it can be reduced even further.14 Moreover, thrombolysis is more effective the earlier it is administered; the likelihood of recovery is threefold if initiated within the first 90min. Therefore, if one only considers the issue of time-saving, patients with a clinical diagnosis of stroke should be transferred to a hospital with an SU as soon as possible. The administration of thrombolysis by physicians who have not been previously trained to do so, as well as having scant experience, is related to higher complication rates and mortality.16–18 In light of this evidence, health-care systems have made efforts to improve the emergency transportation of patients to appropriate hospitals for stroke treatment. However, these measures do not always reach everyone (especially in geographically large regions or those with difficult terrain), they increase the delay in treatment, and give rise to a high proportion (48%) of unnecessary transfers.19 Telemedicine is a technical resource that has demonstrated it efficacy in improving these data.
In 2006, the Cerebrovascular Disease Study Group (GEECV) of the Spanish Society of Neurology (SEN) published a Stroke Health-care Plan (PASI, for its Spanish acronym) with the aim of elaborating an organized system of care for stroke patients that responds to the needs of each person and makes the best use of health-care resources. This plan seeks to guarantee equality in the health care received by stroke patients regardless of place and time of day. To a large extent, the National Stroke Strategy of the National Health System approved in 2008 sets forth the type of organizational model for health-care under the PASI.
However, the treatment of stroke during the acute phase has integrated new advances that have made it necessary to update former organizational models. This updated organizational model should emphasize the characteristics of the different levels of care with the promotion of Reference Hospitals (RH), establishing new, less restrictive criteria for activating the SC contemplated by new treatment possibilities, setting forth organizational measures to implement neurovascular interventionism (NVI) and allowing for the use of the technical resource of telemedicine. Furthermore, previously defined, co-ordinated transfer circuits must be facilitated with non-hospital Emergency Services.2,20
Levels of careAcknowledging stroke as a major public health problem21,22 demands efficient organization of health-care resources so as to guarantee accessibility to treatment and the correct application of said treatments. The organizational system must ensure equal, continued, quality care for all patients during the process of their disease. Hence, it must incorporate the scientific and technical advances achieved in the management of cerebrovascular disease with the implementation of appropriate organizational structures.
Hospitals that treat stroke in its acute phase must be qualified to care for these patients and have a pre-defined transfer circuit that is co-ordinated with Emergency Services outside the hospital. All individuals presenting with symptoms suggestive of permanent or transient stroke must be immediately directed to an acute care hospital prepared to treat stroke.
With these objectives in place, depending on the characteristics of each centre, there are three levels of care in the hospital network2,20:
- -
Hospital with a Stroke Team (ST).
- -
Hospital with a Stroke Unit (SU).
- -
Stroke Reference Hospital.
The group of hospitals of different level in charge of the health care of stroke patients belonging to a certain geographical area should have an interhospital protocol available to determine the joint and orderly use of health-care resources, as well as procedures for the exchange of patients when indicated. The health-care system and the non-hospital emergency systems must be co-ordinated so that those individuals who so require can be referred directly or, when suitable, transferred to the most appropriate hospitals for each type of patient.
The system of care for stroke must identify the functions each type of hospital must carry out and define the responsibilities inherent to each type of hospital.
Hospitals with stroke teamsThe ST is the basic level of care for stroke patients. It is defined as a multidisciplinary team of specialists who collaborate in the diagnosis and treatment of stroke by means of a protocol, under the co-ordination of a neurologist. This team is based on its organization and does not have a fixed physical location; its number one aim is to provide an integral and rapid service to the persons suffering from acute stroke. STs are an alternative to SUs for those hospital centres that include care services for stroke patients during both the acute phase and during their hospitalization.23
In accordance with the indications of the expert committee Brain Attack Coalition,24 there should be a system that is organized well enough to notify and activate the team as quickly as possible, so that a member of the team is at the patient's bedside in a matter of 15min. What this urgent activation seeks to do is initiate those measures that demand shorter windows of action or that must be performed at higher level centres.
Among the recommendations put forth by the American Stroke Association for the organization of stroke care services in each region25 is that each hospital must recognize its level of stroke care and there must be circuits and protocols to be able to refer patients from one level to another, depending on their needs. Hence, hospitals that have an ST should have joint protocols with hospitals that have SUs and RH for the referral of those patients who can benefit from them.26 In hospitals with STs, a neurologist may not be physically present 24h of the day. Therefore, the action protocols must state the procedure to be followed for intravenous thrombolysis. The recommended options consist of referral to a centre with an SU or the organization of co-ordinated telemedicine systems from an RH.
In a study conducted in European hospitals, it was found that less than 10% of all hospitals who admit stroke patients in Europe do so in optimal conditions with respect to their care level and that 40% do not meet the minimum conditions desired.27 That is why it is important to define exactly what the minimum requirements are that a hospital must satisfy in order to care for stroke patients at this most basic level. With the objective of identifying these essential elements, experts in cerebrovascular disease from our country were asked to complete a survey to define what conditions must be met by each centre if it is to be considered to have a given level of care.28 Bearing this study in mind, as well as what was stated in the previous edition of the PASI2, the requirements presented in Table 1 can be considered to be indispensable.
Indispensable components for the organization of a Stroke Team.
| Existence of an emergency medicine service |
| Laboratory and computerized tomography available 24h every day of the week (24×7) |
| A multidisciplinary team, directed by a neurologist |
| Action protocols in writing |
| Established referral circuits to hospitals with Stroke Units or to Reference Hospitals |
In the survey just mentioned, the experts defined the existence of the following as being important requirements in this type of hospital: rehabilitation services, social workers, a stroke registry, Intensive Care Unit, patient education programme, echocardiography, professional education programme, neurological monitoring, and community education.
In a similar survey carried out among European experts,29 the elements deemed essential for a hospital at the most elementary level were: emergency service with trained Emergency Department personnel, computed tomography (CT) available 24h a day with priority given to performing these scans on patients with acute stroke, collaboration with an external rehabilitation centre, established levels of care for stroke victims in each community with defined referral circuits, and a prevention programme. As we can see, the requirements are quite similar to ours.
Hospitals with a Stroke UnitThe SU is the most efficient resource for the treatment during the acute phase of stroke. This care device has demonstrated that it decreases mortality, dependence, and the need for institutional care in stroke patients, with a level of evidence I. The benefit observed is independent of age, gender, and severity of the neurologic deficit at the time of admission30 and is similar for the various aetiological sub-types of stroke.31 This level of care in SUs has been proven in trials, can be extrapolated to daily clinical practice,8,32 is maintained over time,33 and is cost-effective.34,35 With these data in hand, different consensus committees, such as the American Heart Association,25,36 the 2006 Declaration of Helsinborg,37 and the Spanish Society of Neurology,2,38 have recommended that all patients in the acute phase of stroke must be guaranteed access to an SU, due to the fact that it is the resource that has proven to be the most efficacious for the treatment of their ailment. In order to meet this objective, the stroke care network considers the level of Hospital with an SU.
This type of hospital is equipped with the personnel, infrastructure, and programmes necessary to stabilize and treat the majority of stroke patients during the acute phase. Its distinctive characteristic is that it has an SU. As yet, we still do not have any recommendations or guidelines regarding the criteria that must be applied in the territorial distribution of SUs. In European countries, population criteria or geographical planning criteria have been used indistinctively, in an attempt to determine the number of SUs there must be in order to achieve the purpose of guaranteeing that patients with acute stroke will have a bed in the SU.39–41 Translating these criteria to the characteristics of our health-care system, together with the known epidemiological data as to the incidence and prevalence of stroke, the recommendation for the distribution of SUs would be as follows:
- -
Population-based distribution: 1 bed with monitoring in an SU for every 100,000 inhabitants.
- -
Geographical distribution: the area defined by a 60-min isochrone must have an SU.
Thus, the mean number of patients observed in studies of 155 strokes/100,000 inhabitants/year,22,42 with mean stays in SU of 3–4 days for those patients can be cared for. On the other hand, in geographical distribution planning it is also advisable that we bear in mind a distribution following distances that do not give rise to delays of care in therapies aimed at re-channelling blood flow (transfer times not to exceed 1h). The use of telemedicine would be a complementary system to territorial organization, since it would make it possible to provide thrombolytic treatment in hospitals that are not prepared for this type of therapy, and in situations where the transfer distances would lead to exclusions due to times in excess of the time stated in the treatment window criteria.
The SU within a hospital is the geographically delimited structure for the care of stroke patients with personnel and diagnostic services available around the clock. The SU must belong to the Neurology Service in terms of its organization, and its management and co-ordination must be the responsibility of the SU Co-ordinator. Ideally, it should be located on the Neurology floor and patients meeting admission criteria for the neurology service are transferred there directly from the Emergency Department. Most stroke patients, whether transient or established, with less than 24h of evolution should be admitted to the ICU.38,43 The SU model of critical intermediate care with continuous, non-invasive monitoring is being implemented as the most appropriate for the clinical control of patients in the acute phase of cerebral ischaemia.36,44,45 The development of new technical and therapeutic resources conditions the convenience of extending the model of monitorized SUs.46 At present, continuous monitoring equipment in an SU is the most advisable for adequate control of the acute phase of stroke.
The components of an SU have been established, as knowledge has been obtained from studies and its transcendence has been determined following the opinions of experts evaluated by means of surveys.28,29 Two categories are contemplated, indispensable and important. The category of indispensable resources (Table 2) comprehends all elements (human resources, infrastructures, protocols, and technical elements) that are fundamental for an SU to carry out its functions.
Indispensable components for a Stroke Unit.
| Personnel resources |
| In the Stroke Unit |
| Co-ordinating Neurologist (member of the Neurology Department, expert in stroke care) |
| Neurologist on-duty 24×7 |
| Nursing staff (ratio 1 nurse/4–6 beds; in 8-h shifts) |
| In the Hospital |
| Neurorradiologists (diagnosis) |
| Access to a neurosugeon |
| Specialists in Intensive Care |
| Rehabilitation Service |
| Social Workers |
| Infrastructure |
| Emergency Service |
| Existence of specific beds for stroke patients with non-invasive, multiparameter monitoring (ECG, oxymetry, blood pressure) |
| Neurological monitoring |
| Intensive Care Unit |
| Protocols |
| Programme of work co-ordinated with other specialists |
| Clinical pathways and diagnostic-treatment protocols |
| Nursing protocols |
| Protocols of rapid, preferential access to high-tech hospitals for highly specific diagnostic and/or treatment techniques |
| Diagnostic techniques |
| Cerebral computed tomography 24×7 |
| Ultrasound 24h/7d |
| Emergency laboratory service 24×7 |
| Echocardiography |
| Treatment techniques |
| Intravenous thrombolysis 24×7 |
| Ventricular drainage 24×7 |
| Surgery for intracranial hypertension 24×7 |
| Physical therapy |
Other components that can be useful in organizing the SU would be deemed important: the capacity to have magnetic resonance imaging (MRI) available 24h a day, 7 days a week, co-ordination with vascular surgery for carotid endarterectomy, availability of occupational therapy, telemedicine, health-care training or education programmes, and the use of computerized patient registry in databases.28
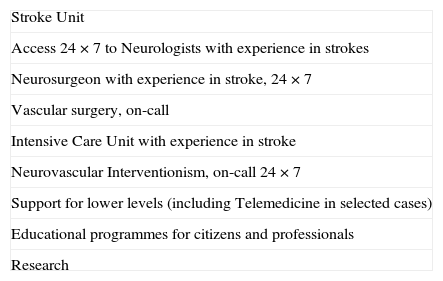
Stroke Reference HospitalsAlthough most strokes can be resolved in terms of diagnosis and treatment at centres that have an SU, as defined above, there are some patients who, given their complexity, severity, or because they require advanced technical monitoring or treatment, must be treated at the highest level hospitals, known as RH. The characteristics of these centres were set out by the Brain Attack Coalition47 with all their requirements: neurosurgery, vascular surgery, Intensive Care Unit,47,48 and neurovascular interventionism. These devices should be guaranteed without any kind of deadline or schedule (Table 3). Recent surveys28,29 have established the requirements for this level of care according to expert opinion. Others have revealed interesting differences of perception among professionals and hospital medical directors as regards stroke care.49 It is convenient for these RH to have a neurologist with experience in stroke available who should participate in supporting lower levels through tools such as telemedicine, as well as organize education and training programmes targeting both citizens and professionals. Whether it is indispensable that they be involved in research or not is a matter of debate, although it is advisable.47 Be that as it may, the most important consideration in their definition and accreditation is their capacity for patient care.
Indispensable components for a Stroke Reference Hospital.
| Stroke Unit |
| Access 24×7 to Neurologists with experience in strokes |
| Neurosurgeon with experience in stroke, 24×7 |
| Vascular surgery, on-call |
| Intensive Care Unit with experience in stroke |
| Neurovascular Interventionism, on-call 24×7 |
| Support for lower levels (including Telemedicine in selected cases) |
| Educational programmes for citizens and professionals |
| Research |
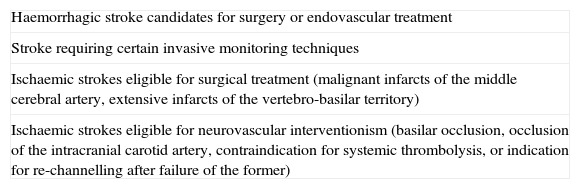
At this highest level, all individuals suffering from haemorrhagic stroke who are candidates for surgical or endovascular treatment and all those requiring certain intensive care techniques, such as invasive neurological monitoring; ischaemic stroke victims eligible for surgery (malignant infarcts of the middle cerebral artery [MCA], extensive infarcts affecting the vertebrobasilar territory), and those who are candidates for NVI (thrombosis of the basilar artery [BA], terminal occlusion of the internal carotid artery [ICA], those who present contraindications for systemic thrombolysis, or after failure of systemic thrombolysis)45,50–52 (Table 4). There should be agreements and protocols that address the transfer of these cases from lower levels, including priority criteria.26,53Unlike the more basic levels, the efficacy, efficiency, and cost-effectiveness of these centres are not well defined, largely because these considerations have not been defined for the elements characterizing these Reference Hospitals; hence, their assessment is as yet an open process and parallel to that of the evaluation of the specific measures they should offer, an evaluation in which they should participate actively through registries and clinical trials.27,54–56
Patients eligible for treatment at a Stroke Reference Hospital.
| Haemorrhagic stroke candidates for surgery or endovascular treatment |
| Stroke requiring certain invasive monitoring techniques |
| Ischaemic strokes eligible for surgical treatment (malignant infarcts of the middle cerebral artery, extensive infarcts of the vertebro-basilar territory) |
| Ischaemic strokes eligible for neurovascular interventionism (basilar occlusion, occlusion of the intracranial carotid artery, contraindication for systemic thrombolysis, or indication for re-channelling after failure of the former) |
The territorial or population distribution of these RH is hard to establish, given that different factors intervene that should be considered: the number of stroke patients cared for, the reference population, characteristics of the area, and portfolio of services at each hospital. From a geographical organization perspective, a recommended distribution model would be that of having one RH for each catchment area that cares for more than 1000 strokes per year. In any case, there should be at least one RH in each Region.
Stroke codeClassically speaking, the stroke code (SC) denotes a procedure of pre-hospital action based on the early recognition of the signs and symptoms of stroke, possibly ischaemic in nature, with the resulting prioritization of care and immediate transfer to a hospital with an SU or RH of those patients who can benefit from reperfusion therapy and from special care in an SU.57 The SC activation criteria were fairly restrictive given that the criteria for intravenous thrombolytic treatment were those of the SITS-MOST Registry (Safe Implementation of Thrombolysis in Stroke: a Multinational Multicentre Monitoring Study of Safety and Efficacy of Thrombolysis in Stroke).58
At present, the treatment possibilities for acute stroke have been enlarged and diversified, with intravenous thrombolysis a main pillar, but not the only one. It has been proved that the treatment that benefits the greatest number of stroke patients is admission into an SU. Close to 80–90% of all stroke patients can be admitted to this type of unit, which is of benefit not only in cases of cerebral infarcts but also in transient ischaemic attacks (TIA) or brain haemorrhage.31,43
Intravenous thrombolytic treatment in Spain started to become more general practice in 2003 with the beginning of the SITS-MOST Registry that was subsequently expanded to the SITS-ISTR Registry. Close to two thousand patients (1914) were included and the safety and efficacy data were comparable to the global outcomes of the registry, as well as a the data from clinical trials.59 Over the course of time since then, the existing information about the safety and efficacy of this treatment have grown enormously. The experience accumulated in thrombolysis in Spanish SUs has, on the one hand, made it possible to increase the number of patients treated and, on the other hand, cast doubt on some of the exclusion criteria. The increase in the number of thrombolysis treatments performed has also been a consequence of the enhanced organization of care during the acute phase with the generalization of the SC in almost all regions and the increase in the number of centres with a thrombolysis programme. The results of the ECASS-3 study have shown that t-PA is safe and efficacious for up to 4.5h after the onset of symptoms.60 Several studies have shown that thrombolysis is as safe in patients over the age of 80 as it is in people under the age of 80. Other also controversial criteria are patients on anti-coagulant treatment with INR levels <1.7 or a history of diabetes and prior stroke in whom thrombolysis applied by expert neurologists has been seen to be both safe and efficacious.61,62 As will be discussed later on in this work, NVI or telemedicine are other alternatives that can be considered. Therefore, the conclusion we arrive at in light of these data is that treatment for acute stroke is a highly complex matter. This demands that the neurologist on-call individualize treatment; as a result, the SC activation criteria should be less restrictive.The main objective of the SC is to stabilize and transfer the patient to an appropriate centre in the shortest time possible. The benefits of the SC have been amply proven for improving timing and the number of patients treated with thrombolysis.63–65
Characteristics of the stroke codeAs the first link in the chain of care of any presentation compatible with stroke, the SC must comprise a series of characteristics66:
- (1)
Consideration of stroke as a medical emergency: under the SC, priority is given to those patients who may be eligible for acute treatment and for rechannelling therapy and, by extension, those who end up not being candidates for such therapies, but who can benefit from other specific treatments in an SU.
- (2)
Early recognition of a possible stroke: through specific training for health-care personnel.
- (3)
Specific care in dealing with stroke patients, keeping them in an appropriate clinical situation as to make it possible to administer the ideal treatment to them upon arrival at the hospital.
- (4)
Prioritization in transfer, making the most advanced resource available as quickly as possible.
- (5)
Co-ordination with the rest of the links in the chain of care, in accordance with the operational procedures of the emergency services outside the hospital.
The activation of the SC requires a procedure establishing the organization and rationalization of the existing resources to enable patients suffering a stroke to be cared for in hospitals with an SU within less than 2h from the onset of symptoms (Tables 5 and 6).
Criteria for stroke code activation.
| Independent patient (that is, capable of walking, personal hygiene and getting dressed) |
| Time of onset of symptoms less than 8h or unknown time of onset |
| Neurological focality currently present at the time of diagnosis: presence of any of the symptoms of acute onset stroke alarm. |
| 1. Sudden numbness, weakness, or paralysis of the face, arm, or leg on one side of the body |
| 2. Difficulty in speaking or understanding |
| 3. Sudden onset of blurry vision in one or both eyes |
| 4. Sudden, intense headache without any apparent cause associated with nausea and vomiting (not attributable to other causes) |
| 5. Difficulty in walking, loss of balance or co-ordination |
If these aims of this procedure are to be met, the following are needed:
- (1)
The availability of the existing resources must be co-ordinated: continuously and permanently centralizing information with respect to the availability or saturation of the various centres by means of the Co-ordinating Emergency Centre.
- (2)
The information from the different peripheral units as to the detection of a possible SC candidate must be centralized: as health-care in Spain is structured, the units that can carry out early detection of these patients are:
- (a)
Primary Care Units.
- (b)
Co-ordinating Emergency Centres.
- (c)
Emergency Rooms and emergency outpatient facilities.
- (d)
Others (Geriatric Residences, etc.).
- (a)
- (3)
When the units listed above detect a patient with signs and symptoms of stroke who meet the activation criteria for SC, this information will be made known immediately to the Co-ordinating Emergency Centre. In any case, the patient who is suspected of suffering a stroke must be transferred immediately to the centre with an SU without first going to other health-care centres. It is wise to have a family member accompany the patient, especially if the individual affected is not able to provide informed consent.
- (4)
Distribution of patients and activation of hospitals with an SU: the Co-ordinating Emergency Centre will designate the closest receiving centre, unless it is already saturated, and will take care of activating the code, informing the centre of the patient's characteristics and approximate time of arrival. The mechanisms ensuring direct and proper reception of the patient will be established from the receiving centre.
- (5)
Deactivation of the SC: if during transport, the patient presents any of the exclusion criteria or if the patient or family refuses to be transferred to the reference centre.
The intrahospital SC is the operational system that puts a specific team of physicians into motion with the aim of prioritizing stroke patients through the implementation of pre-defined actions and procedures in the hospital. Its activation takes place as a result of the detection of a stroke patient in the Emergency Department. Among the standardized procedures that are generally part of the clinical pathway at the centre, we can highlight direct assessment by the neurologists on-call and emergency neuroimaging studies, as well as the application of thrombolysis when indicated.20
Special mention should be made of the intrahospital SC that is activated for hospitalized patients who have been admitted to the hospital for reasons other than stroke. Strokes in this type of patient display special characteristics that are more often cardioembolic and associated with a higher mortality rate, but most of these cases also benefit from admission into an SU and from intravenous reperfusion therapies or NVI. Hospital health-care professionals require training campaigns to enable them to recognize and act when facing suspected stroke.67,68
Neurovascular interventionismCurrent evidenceThrombolytic treatment of ischaemic stroke seeks to achieve early rechannelling of the occluded cerebral arteries, so that perfusion to the brain is restored in time to save the ischaemic brain tissue that has not been irreversibly damaged. At present, we know that t-PA is safe and efficacious in the first 4.5h following cerebral infarct. Many recent studies have demonstrated that ischaemic cerebral tissue can remain viable and can be salvaged even after 4.5h have transpired since the onset of symptoms. MRI and multiparametric CT are capable of discriminating tissue that has been irreversibly damaged from hypoperfused but salvageable tissue69,70 and, in this way, it is possible to select patients who will benefit from receiving thrombolytic treatment after this time period or those in whom the time of onset of symptoms is not known.71,72
In a high percentage of patients, intravenous thrombolysis is not able to induce arterial rechannelling in time to save the brain tissue at risk. In the case of occlusions of the proximal MCA, approximately 30% of patients will achieve full, early, arterial rechannelling. The percentage decreases dramatically in patients with occlusions of the terminal ICA, of whom only one in 10 present early arterial rechannelling.73 Moreover, many individuals present contraindications to systemic administration of t-PA because they are in situations of high risk for bleeding. Therefore, more efficacious and safer rechannelling treatment strategies must be designed, such as those provided by NVI.
The PROACT study74 demonstrated the efficacy and safety of intra-arterial thrombolysis in occlusions of the MCA within the first 6h since the onset of symptoms. In addition, the MELT study,75 with a similar design, obtained favourable results with intra-arterial urokinase and the meta-analysis of both studies confirmed the favourable effect of intra-arterial thrombolysis in MCA occlusion in the first 6h.76 Despite the fact that there are controlled studies comparing intravenous thrombolysis with intra-arterial thrombolysis, in indirect comparisons the percentage of rechannelling achieved with intra-arterial thrombolysis (70%) is greater than that of intravenous thrombolysis (34%), especially when large intracranial vessels are assessed.12,77,78 The risk of symptomatic ICH is somewhat greater with intra-arterial thrombolysis (10%), although this increase may be attributable to the greater severity of the stroke and to the longer time elapsed between the onset of symptoms until treatment initiation.
In order to decrease the latency until treatment that intra-arterial thrombolysis entails, and given the existence of patients who are resistant to systemic thrombolysis, studies of combined reperfusion therapy have been performed. In patients under the age of 80 and with moderate to major neurological impairment (NIHSS≥10), the combination of intravenous t-PA in the first 3h (0.6mg/kg, with a bolus of 15% and the rest over the course of 30min) followed by intra-arterial t-PA between 3 and 6h (bolus of 2mg followed by infusion of 22mg over the course of the following 2h or until rechannelled) is safe (6% of symptomatic ICH)79,80 and achieves a higher percentage of rechannelling (73%), albeit without significant differences in the functional prognosis after three months in comparison with the NINDS study.
The occlusion of the BA is associated with an 85–95% mortality rate in the absence of rechannelling. Two treatment windows have been defined for thrombolytic treatment on the basis of the form of presentation of symptoms: 48h since the onset of symptoms when said symptoms are progressive or fluctuating, and 12h when onset is sudden.81 Non-controlled observational studies of intravenous thrombolytic treatment in basilar thrombosis have attained similar outcomes as those achieved with NVI.82 Intravenous thrombolytic therapy in the first 6h, followed by mechanical thrombolysis has obtained satisfactory results.83
Special situationsIn patients who have recently undergone surgery, NVI appears to be a safer technique than systemic thrombolysis. The largest series included 36 stroke patients affecting different arterial territories between 1 and 120h after undergoing different surgical procedures.84 NVI was completed within a mean of 4.5(range 1–8)h, with full or partial rechannelling in 44% of the cases and 25% of the patients with bleeding at the surgical site, 3 of which were fatal. The total mortality rate for the series was 25%, and 38% of the patients achieved good functional recovery.84
Anticoagulant treatment prior to the stroke is a contraindication for the administration of intravenous thrombolysis regardless of the patient's INR according to European regulations, and in patients with an INR<1.7 according to North American guidelines. NVI is a safe treatment option in patients with acute stroke who have previously undergone anticoagulant therapy.85–87 In clinical situations with a high risk of haemorrhage, for instance in anticoagulated patients with an INR>1.9, platelet count <100,000/mm3, recent craniectomy or active systemic bleeding, among others,88 mechanical thrombolysis is the only treatment option. The MERCI89,90 and Multi MERCI91 studies were designed to assess the efficacy and safety of mechanical thrombectomy in resistant patients (15.7%) or patients with contraindications to systemic thrombolysis. Revascularization multiplied the probability of functional independence at 3 months by 20 and reduced mortality by 72%.87 Mechanical thrombectomy after the administration of systemic thrombolysis was as safe as mechanical thrombectomy alone. The recent results coming out of the Penumbra study, whose mechanism of action is based on aspiration in addition to extraction of the thrombus, have shown an 82% rate of rechannelling, 11% of symptomatic intracranial haemorrhage, 33% mortality, and a score on the modified Rankin scale (mRS) δ 2 at 3 months in 25% of the cases. It must be remembered that the patients in the mechanical thrombolysis studies presented a high level of expected morbi-mortality prior to the procedure, since the median for the baseline NIHSS was 19, and more than 40% displayed occlusion of the terminal ICA or of the BA.92
On the basis of the studies named above, different scientific societies recommend intra-arterial pharmacological thrombolysis as an option in patients with occlusions of the MCA within the 6-h time window, or in patients with contraindications to intravenous thrombolysis. Mechanical thrombectomy is mentioned as a reasonable intervention for the extraction of intra-arterial thrombi in selected patients up to 8h.45,93–95 Initial experience with thrombectomy devices following systemic or intra-arterial thrombolysis has shown the safety and high rate of rechannelling.96
Criteria for referral to a Reference Hospital for interventionist neurovascular or neurosurgical treatmentThe objective is to identify patients who are candidates for this treatment at the first level of care, with the purpose of being directly referred to the RH and to shorten intervention times, or at the second level of care, taking into account the results of the complementary testing and response to treatments administered. The warning criteria for neurovascular interventionist treatment must therefore combine both easily identified clinical variables and the results of accessible diagnostic tests. Activation of the transfer protocol must always be carried out by the neurologist.
Referral criteria are broader as regards the window of treatment (up to 8h, including stroke of unknown onset), but more restrictive with respect to severity (NIHSS≥10) and in relation to the characteristics of baseline diagnostic tests. No age limit has been set, but patients with relevant co-morbidity and reduced life expectancy would not be candidates, nor would dependent patients (disability for walking, personal hygiene, or getting dressed).
Generally speaking, three circumstances are recognized in which NVI would be indicated: patients in whom systemic thrombolysis is contraindicated; those in whom NVI would be initially indicated (occlusion of terminal ICA or of the BA), or in those in whom systemic thrombolysis has failed.
Hence, the possibility of referring a patient to an RH for endovascular treatment will be considered in the following situations when the patient can reach the centre in question within the treatment window:
- (1)
Stroke upon waking up or stroke with unknown time of onset.
- (2)
Thrombosis of BA if:
- (a)
Time of evolution is less than 12h since the onset of symptoms or up to 48h if the course is progressive, fluctuating, or preceded by TIA.
- (b)
Excluded if brain stem reflexes are absent or in the presence of extensive hypodensity on the CT or of an extensive lesion in the MRI diffusion sequence.
- (a)
- (3)
In patients treated with IV t-PA in <4.5h since the onset of symptoms, if any of the following conditions is met:
- (a)
There is no improvement of the NIHSS during thrombolytic infusion (transfer should be activated at 30min if no response).
- (b)
Rechannelling cannot be confirmed at 30min by means of transcranial doppler (TCD) monitoring or by means of other non-invasive angiographic examinations, such as angio-CT.
- (c)
An occlusion of the terminal ICA, proximal MCA, or BA is suspected. In these cases, transfer should be activated immediately.
- (a)
- (4)
Patients in whom systemic thrombolysis is contraindicated:
- (a)
Anti-coagulation (if INR>1.7 or prolonged APTT).
- (b)
Platelets<100,000/mm3.
- (c)
Treatment with low molecular weight heparins at anticoagulant doses.
- (d)
Major surgery in the preceding 3 months.
- (e)
Disease or condition entailing a high risk of bleeding.
- (f)
Stroke in the last 3 months.
- (g)
History of brain haemorrhage.
- (a)
- (5)
An NIHSS score≥10, in the absence of complementary tests providing a reliable vascular diagnosis, predicts a poor response to intravenous thrombolysis. In these cases, intravenous treatment must necessarily be initiated and, if vascular monitoring methods are not available, the patient must be referred immediately to the RH. The attitude will be the same when major arterial occlusion (terminal ICA, MCA, or BA) is confirmed by means of a vascular diagnostic technique (TCD, angio-CT, or angio-MRI) independently of neurological severity (NIHSS). In any case, a cranial CT is mandatory to rule out the existence of signs of extensive infarct (clear hypodensity in more than one third of the MCA territory or ASPECTS score<797).
Moreover, patients with spontaneous subarachnoid haemorrhage, lobar haemorrhages, and malignant infarct of the MCA will be candidates for transfer to the RH for NVI or neurosurgical treatment.
Be that as it may, there must be protocols to refer the patient to their hospital of origin, after a prudent time of observation in the RH.
TelemedicineBroadly defined, “telemedicine” can be considered as the application of telecommunications techniques to medical information and services. Telemedicine covers all aspects of remote medical practice, carried out with the help of the telephone, fax, e-mail, or video-conference. The term, “telestroke” was coined in 1993 to describe the application of telemedicine to facilitate remote consultation of stroke patients to experts in cerebral vascular pathology.98 Over time, much experience has been gained worldwide in telestroke systems and it is currently a growing technology that also exists in Spain.19 Telestroke systems bring specialized consultation to regional centres and have managed to double the number of stroke patients who received emergency neurological care, multiplying the number of thrombolytic treatments by two, significantly reducing by some 50min the time elapsed until thrombolysis is initiated and increasing the number of patients treated within the first 3h; furthermore, they reduce the number of interhospital transfers by more than one third.99 The development of telestroke systems, together with educating and training health-care professionals about this pathology has been seen to increase the use of t-PA in community hospitals that do not have access to experts specialized in this treatment.19 However, we must bear in mind that telemedicine is not a substitute and does not achieve the level of quality of the service provided in specialized centres.
Telemedicine has made use of telephone and interactive systems via the World Wide Web for teleconferencing, with high quality, two-way communications systems that enable the evaluation of patients and their CT or MRI images (Tables 7 and 8). Remote interpretation of neuroimaging studies has proved to be reliable100; likewise, it has also been confirmed that video-conferencing systems enable the neurologist to examine the patient remotely, improve the percentage of correct decisions regarding thrombolytic treatment indications in comparison to the systems that rely solely on the telephone (without images).101–103 In fact, telemedicine has demonstrated its reliability in the remote application of the NIH rating scale in stroke patients and is comparable to the score obtained “in person”.101,104–106 The orientation, language, motor dysarthria, gaze, facial palsy, sensory, extinction and inattention items show greater concordance than do ataxia and obeying commands.107 Moreover, the telestroke systems have also shown their usefulness when guiding ultrasound studies with TCD being carried out by inexperienced observers, although it takes more time to perform the examination.108
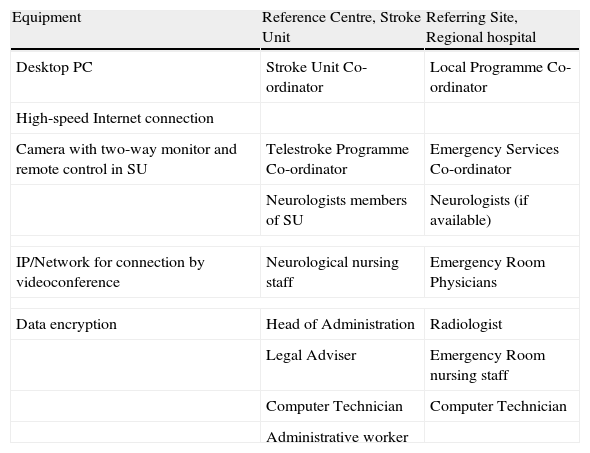
Basic equipment and professionals for a telestroke system.
| Equipment | Reference Centre, Stroke Unit | Referring Site, Regional hospital |
| Desktop PC | Stroke Unit Co-ordinator | Local Programme Co-ordinator |
| High-speed Internet connection | ||
| Camera with two-way monitor and remote control in SU | Telestroke Programme Co-ordinator | Emergency Services Co-ordinator |
| Neurologists members of SU | Neurologists (if available) | |
| IP/Network for connection by videoconference | Neurological nursing staff | Emergency Room Physicians |
| Data encryption | Head of Administration | Radiologist |
| Legal Adviser | Emergency Room nursing staff | |
| Computer Technician | Computer Technician | |
| Administrative worker | ||
IP: Internet protocol; PC: personal computer; SU: Stroke Unit.
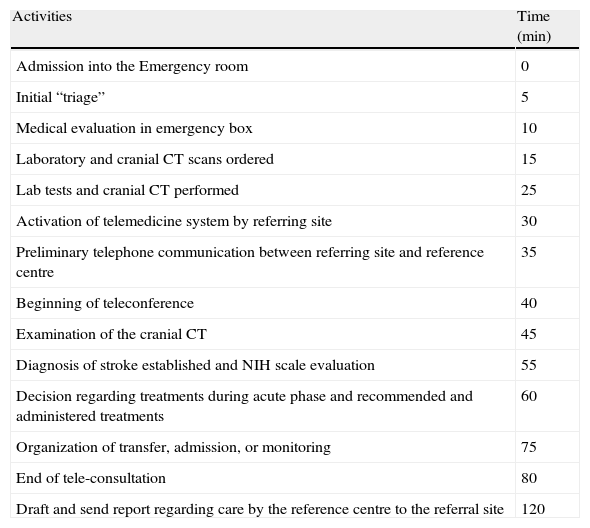
Approximate time intervals for the different activities involved in a telestroke consultation.
| Activities | Time (min) |
| Admission into the Emergency room | 0 |
| Initial “triage” | 5 |
| Medical evaluation in emergency box | 10 |
| Laboratory and cranial CT scans ordered | 15 |
| Lab tests and cranial CT performed | 25 |
| Activation of telemedicine system by referring site | 30 |
| Preliminary telephone communication between referring site and reference centre | 35 |
| Beginning of teleconference | 40 |
| Examination of the cranial CT | 45 |
| Diagnosis of stroke established and NIH scale evaluation | 55 |
| Decision regarding treatments during acute phase and recommended and administered treatments | 60 |
| Organization of transfer, admission, or monitoring | 75 |
| End of tele-consultation | 80 |
| Draft and send report regarding care by the reference centre to the referral site | 120 |
NIH: National Institutes of Health; CT: Computed Tomography.
In addition to saving time in the administration of thrombolysis in the setting of a stroke, telemedicine systems have been proven to be safe.109–111 In fact, intravenous thrombolysis conducted by means of telemedicine, simply by virtue of the fact that it shortens the time needed until treatment, enhances the possibilities that a particular patient can benefit to full recovery without sequelae.112 This benefit has even been seen in series including a high percentage of patients over the age of 80113 and is maintained in the long term.114 The experiences of various health-care catchment areas that already have telestroke systems available have demonstrated that these systems make it possible to increase the number of patients treated with t-PA and, by improving the overall degree of disability, they shorten hospital stays and reduce the need for institutional care following the patient's discharge from hospital.115 Additionally, telestroke systems are cost-effective in two senses as they eliminate unnecessary transfers109 or make it possible to delay them until after intravenous treatment, thereby avoiding more expensive transfers, such as airlifts (by helicopter).116
Another use for telemedicine is that it establishes a continuous system of education, familiarizing health-care personnel in the early diagnosis and protocoled management of stroke. This has been illustrated by the German experience, obtaining measures of improved prognosis similar to those seen in randomized SU trials.113,117 The assessment of occupational disability, language or physical impairment can also be of use when it is impossible to perform these assessments on site, in order to implement rehabilitation measures.118,119
A good example of telemedicine's usefulness is the TEMPIS project that integrates up to 12 regional hospitals in the German region of Bavaria with two RH and that has enabled more than 9000 patients to be to evaluated.108,113 In this project, the evaluation time was 15min including the physical examination and CT interpretation, with 140min elapsing between onset of symptoms and start of treatment. A full or nearly full recovery (mRS<2) was seen in 38% and 34% of the patients treated at the regional centres or RH, respectively. The mortality rate in both groups was similar, close to 11% at the three-month point. The 7.8% rate of symptomatic haemorrhagic transformation was very much in line with the NINDS study (6.4%). A recent study shows the long-term benefit to participants in the study, although it is greater in the RH insofar as the percentage of patients treated is concerned (5.8 versus 2.4%) and in terms of the door-needle time (57 versus 65min).113
In Spain, telemedicine has been implemented in the Balearic Islands and the Tele-Ictus Catalonia project. Other regions in Spain are pending the start-up of telestroke systems.
Telemedicine opens up new avenues for relationship between health-care professionals and facilitates access to thrombolytic treatment. Nevertheless, the legal aspects, such as confidentiality, or aspects such as the evaluation of the procedure or quality assurance may be subject to controversy.120 The legal framework in which this procedure is carried out will have to be revised in order to protect both patients and professionals.120,121 Given the complexity of the procedure, its implementation in each health-care catchment area demands a very high degree of co-ordination among hospitals and the non-hospital emergency services, as well as the existence of a well-defined plan of action that has the approval of the various clinical and health-care authorities’ ethics review boards.
Telemedicine is an efficient measure in the care of stroke patients; it contributes to geographical equality in health-care provision and to enhancing the quality of care in stroke patients who may go to the emergency room at a hospital that does not have a neurologist with expertise in cerebrovascular pathology. This system provides an access to reperfusion therapies during the acute phase; it saves time in the evaluation, diagnosis, and treatment of patients, and facilitates the selection of those individuals requiring referral to a reference hospital. In addition, telestroke systems enable a broader interrelationship among health-care professionals and improve their continuous training. In the light of the evidence currently available and in order to guarantee territorial equality in the best care for stroke patients, the development of telemedicine systems is recommended in those health-care catchment areas with geographical characteristics that hinder the prompt arrival of patient at a hospital with an SU.
Conflict of interestThe authors have no conflict of interests to declare.
Consensus document drafted by an ad hoc Committee of the Cerebrovascular Disease Study Group (GEECV, for its acronym in Spanish) of the Spanish Society of Neurology (SEN, for its acronym in Spanish).
Please cite this article as: Masjuan J, et al. Plan de asistencia sanitaria al ICTUS II. 2010. Neurología. 2011;26:383–96.