CGRP, a neuropeptide involved in migraine pathophysiology, is also known to play a role in the respiratory system and in immunological conditions such as sepsis. We analyzed the impact of the use of CGRP antagonists in patients with migraine during the COVID-19 pandemic, caused by the SARS-CoV-2 coronavirus.
MethodsThis is a multicentre cross-sectional study. From May to November 2020, through a national survey distributed by the Spanish Society of Neurology, we collected data about the presence of COVID-19 symptoms including headache and their characteristics and severity in patients with migraine treated with anti-CGRP monoclonal antibodies (mAb), and compared them with patients with migraine not receiving this treatment. We also conducted a subanalysis of patients with COVID-19 symptoms.
ResultsWe recruited 300 patients with migraine: 51.7% (155/300) were taking anti-CGRP mAbs; 87.3% were women (262/300). Mean age (standard deviation) was 47.1 years (11.6). Forty-one patients (13.7%) met diagnostic criteria for COVID-19, with no statistically significant difference between patients with and without anti-CGRP mAb treatment (16.1% vs 11.0%, respectively; P=.320). Of the patients with COVID-19, 48.8% (20/41) visited the emergency department and 12.2% (5/41) were hospitalised. Likewise, no clinical differences were found between the groups of patients with and without anti-CGRP mAb treatment.
ConclusionAnti-CGRP mAbs may be safe in clinical practice, presenting no association with increased risk of COVID-19.
El péptido relacionado con el gen de la calcitonina (CGRP, por sus siglas en inglés), es un neuropéptido involucrado en la fisiopatología de la migraña, que también es conocido por participar en la regulación del sistema respiratorio y en algunas enfermedades inmunológicas como la sepsis. Hemos analizado el impacto del uso de los antagonistas de CGRP en pacientes con migraña durante la pandemia de COVID-19, causada por el coronavirus SARS-CoV-2.
MétodosEstudio transversal multicéntrico desarrollado entre mayo y noviembre de 2020, en el que la Sociedad Española de Neurología distribuyó a nivel nacional una encuesta de la que recogimos datos sobre la presencia, las características y la gravedad de síntomas de COVID-19, entre los que se encontraba la cefalea, en pacientes con migraña tratados con anticuerpos monoclonales (AcM) anti-CGRP, y los comparamos con los de pacientes con migraña que no recibían dicho tratamiento. También realizamos un subanálisis de los pacientes con síntomas de COVID-19.
ResultadosIdentificamos 300 pacientes con migraña: 51,7% (155/300) recibían AcM anti-CGRP; el 87,3% eran mujeres (262/300) y la edad media (desviación estándar) de la muestra fue de 47,1 (11,6) años. Un total de 41 pacientes (13,7%) cumplían los criterios diagnósticos de COVID-19, sin diferencias estadísticamente significativas entre los pacientes que recibían tratamiento con AcM anti-CGRP y los que no (16,1% y 11,0%, respectivamente; p=0,320). De los pacientes con COVID-19, el 48,8% (20/41) acudieron a urgencias y el 12,2% (5/41) fueron hospitalizados. Igualmente, no se detectaron diferencias clínicas entre los pacientes que recibían dicho tratamiento y los que no.
ConclusiónEl tratamiento con AcM anti-CGRP parece un recurso seguro en la práctica clínica, y no se asocia a un mayor riesgo de COVID-19.
Calcitonin Gene-Related Peptide (CGRP) is a 37-amino acid peptide with strong vasodilating properties, that has a fundamental role in migraine pathophysiology.1 Its antagonism is effective in treating migraine and monoclonal antibodies targeting CGRP (MAbs) are currently one of the available anti-CGRP therapies,2 but the only one available in Spain. However, CGRP has several other functions in the human body.3 For example, by producing vasodilation and modifying vascular permeability, potentially allows the recruitment of inflammatory cells to the local area,4 being involved in tissue inflammation and sepsis. However, CGRP, depending on the situation, may promote inflammation or protect from it, through its ability to increase cAMP.5 Another function of CGRP is the regulation of the peripheral resistance of the cardiovascular and pulmonary systems during infection, and in this context CGRP antagonism may alter the ability to cope with acute lung injury.6
At present the world is facing the Coronavirus Disease 2019 (COVID-19) pandemic, caused by the Severe-Acute-Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) that is able to produce a respiratory infection associated with a severe inflammatory response.7 Under the hypothesis that CGRP may play a role in the evolution of COVID-19, it was necessary to assess the impact of drugs promoting its antagonism. Therefore, we decided to investigate the impact of the use of anti-CGRP MAbs in migraine patients during the COVID-19 pandemic, specifically analyzing their safety profile since new waves of the disease may be approaching.
MethodsThis is a Spanish multicenter cross-sectional study, conducted between May and November 2020. The following represents the primary analysis of these data. Outpatients with migraine, who were under treatment with anti-CGRP MAbs, were invited to fill in an online survey available on the website of the Spanish Neurological Society. The same questionnaire was filled in, at the same time, to age- and sex-matched random outpatients with migraine but without anti-CGRP treatment. Demographic data, presence of symptoms suggestive of COVID-19 and headache, including its characteristics, acute medication intake, type of anti-CGRP MAb and other preventive treatment were collected through the survey. COVID-19 symptoms were selected according to the list of symptoms reported by the World Health Organization (WHO).8 We also collected data about healthcare resource utilization (outpatient visits, ER admission, hospitalization) in relation to COVID-19 as indicators of disease severity. Then, we compared participants with and without anti-CGRP MAbs and conducted a subanalysis in those patients that had a confirmed diagnosis of COVID-19 or represented suspected cases of COVID-19. We defined confirmed cases as those participants who reported a SARS-CoV-2 positive real-time reverse transcriptase polymerase-chain reaction (RT-PCR) assay by nasopharyngeal swabs. We defined suspected cases as those with three or more of the COVID-19 symptoms reported by WHO either with negative RT-PCR assay or if no confirmatory test had been performed, following the definition used in a national epidemiological study.9
Statistical analysisWe obtained descriptive and frequency statistics and made comparisons using the SPSS, version 21.0 for Windows. We reported nominal (categorical) variables as frequencies (percentages) and continuous variables as mean±standard deviation (age). We checked normality assumption of quantitative variables through visual methods (Q–Q plots) and normality tests (Kolmogorov–Smirnov test). For our preplanned analyses, we assessed statistical significance for intergroup variables by Pearson's chi-square when comparing categorical variables. In the case of having an expected count less than 5 in more than 20% of cells in the contingency table, we used Fisher's exact test. We used linear trend chi-square for ordinal variables and the independent t-test for the continuous variable that followed a normal distribution (age). The false discovery rate with Benjamini–Hochberg procedure was used to correct P values for multiple comparisons.
We did not conduct a statistical power calculation prior to the study because the sample size was based on the available data. All patients recruited completed the survey and there were no missing data.
P values presented are for a two-tailed test and we considered P values<0.05 statistically significant.
Ethics approval and patients’ consentThe study was approved by the Vall d’Hebron Ethics Committee (PR(AG)317/2020). All patients gave informed consent for the analysis of patients’ data which was collected according to Spanish regulation on clinical trials. All patients consented to publication of anonymous individual data.
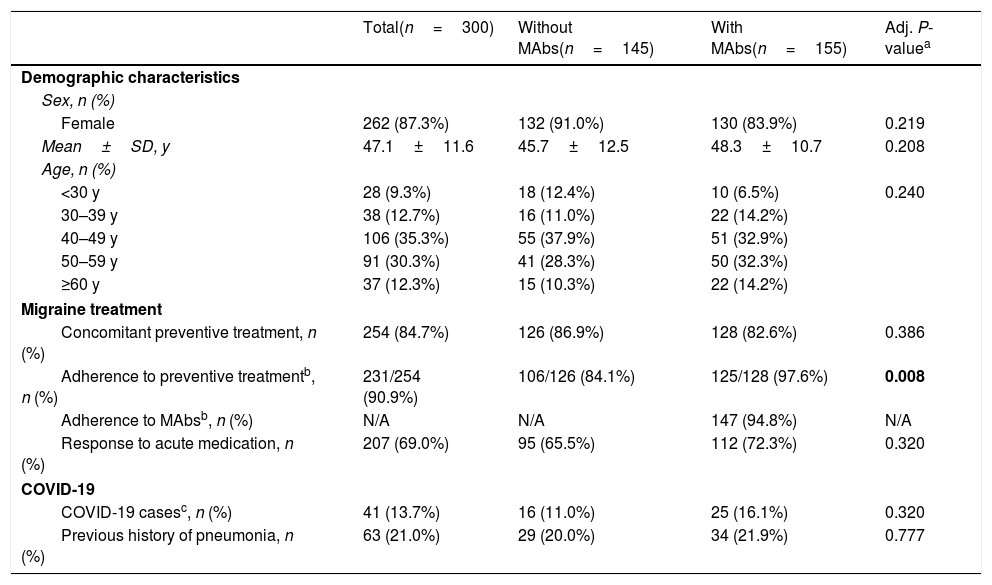
ResultsOf the 300 participants with migraine who answered the survey, 51.7% (155/300) were treated with MAbs. 87.3% were women and mean age was 47.1±11.6 years old (Table 1).
Characteristics of the study cohort.
| Total(n=300) | Without MAbs(n=145) | With MAbs(n=155) | Adj. P-valuea | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Sex, n (%) | ||||
| Female | 262 (87.3%) | 132 (91.0%) | 130 (83.9%) | 0.219 |
| Mean±SD, y | 47.1±11.6 | 45.7±12.5 | 48.3±10.7 | 0.208 |
| Age, n (%) | ||||
| <30 y | 28 (9.3%) | 18 (12.4%) | 10 (6.5%) | 0.240 |
| 30–39 y | 38 (12.7%) | 16 (11.0%) | 22 (14.2%) | |
| 40–49 y | 106 (35.3%) | 55 (37.9%) | 51 (32.9%) | |
| 50–59 y | 91 (30.3%) | 41 (28.3%) | 50 (32.3%) | |
| ≥60 y | 37 (12.3%) | 15 (10.3%) | 22 (14.2%) | |
| Migraine treatment | ||||
| Concomitant preventive treatment, n (%) | 254 (84.7%) | 126 (86.9%) | 128 (82.6%) | 0.386 |
| Adherence to preventive treatmentb, n (%) | 231/254 (90.9%) | 106/126 (84.1%) | 125/128 (97.6%) | 0.008 |
| Adherence to MAbsb, n (%) | N/A | N/A | 147 (94.8%) | N/A |
| Response to acute medication, n (%) | 207 (69.0%) | 95 (65.5%) | 112 (72.3%) | 0.320 |
| COVID-19 | ||||
| COVID-19 casesc, n (%) | 41 (13.7%) | 16 (11.0%) | 25 (16.1%) | 0.320 |
| Previous history of pneumonia, n (%) | 63 (21.0%) | 29 (20.0%) | 34 (21.9%) | 0.777 |
In bold are marked statistically significant variables (P value≤0.05).
Participants with confirmed SARS-CoV-2 real-time reverse transcriptase polymerase-chain reaction (RT-PCR) assay by nasopharyngeal swabs or with >2 of the COVID-19 symptoms either in the absence of a confirmatory test or with negative RT-PCR assay.
MAbs=anti-CGRP monoclonal antibodies; SD=standard deviation; N/A=not applicable.
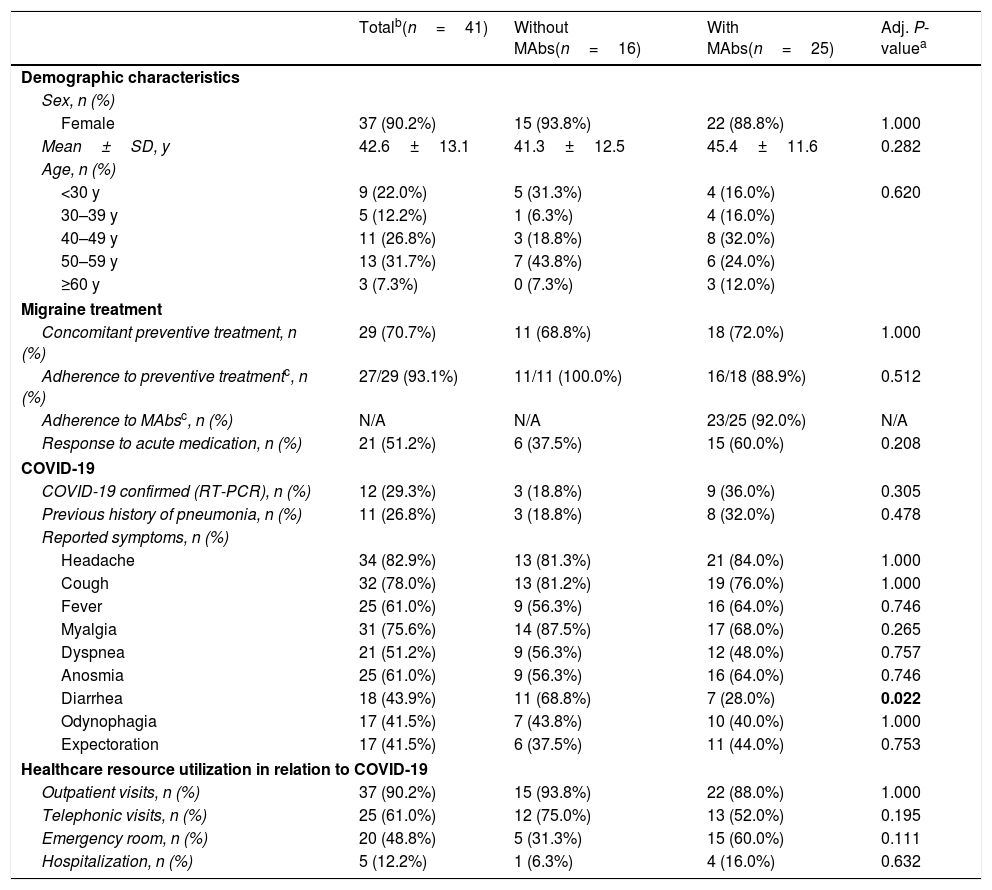
In our cohort, 13.7% (41/300) met the criteria for either confirmed or suspected case of COVID-19, 5 of them required hospital admission (Table 2). Headache was the most frequent symptom in 82.9% of patients (34/41) (Table 2). 47.1% (16/34) of patients with headache associated with COVID-19 reported that it had worsened compared to their usual migraine.
Characteristics of the COVID-19 subgroup.
| Totalb(n=41) | Without MAbs(n=16) | With MAbs(n=25) | Adj. P-valuea | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Sex, n (%) | ||||
| Female | 37 (90.2%) | 15 (93.8%) | 22 (88.8%) | 1.000 |
| Mean±SD, y | 42.6±13.1 | 41.3±12.5 | 45.4±11.6 | 0.282 |
| Age, n (%) | ||||
| <30 y | 9 (22.0%) | 5 (31.3%) | 4 (16.0%) | 0.620 |
| 30–39 y | 5 (12.2%) | 1 (6.3%) | 4 (16.0%) | |
| 40–49 y | 11 (26.8%) | 3 (18.8%) | 8 (32.0%) | |
| 50–59 y | 13 (31.7%) | 7 (43.8%) | 6 (24.0%) | |
| ≥60 y | 3 (7.3%) | 0 (7.3%) | 3 (12.0%) | |
| Migraine treatment | ||||
| Concomitant preventive treatment, n (%) | 29 (70.7%) | 11 (68.8%) | 18 (72.0%) | 1.000 |
| Adherence to preventive treatmentc, n (%) | 27/29 (93.1%) | 11/11 (100.0%) | 16/18 (88.9%) | 0.512 |
| Adherence to MAbsc, n (%) | N/A | N/A | 23/25 (92.0%) | N/A |
| Response to acute medication, n (%) | 21 (51.2%) | 6 (37.5%) | 15 (60.0%) | 0.208 |
| COVID-19 | ||||
| COVID-19 confirmed (RT-PCR), n (%) | 12 (29.3%) | 3 (18.8%) | 9 (36.0%) | 0.305 |
| Previous history of pneumonia, n (%) | 11 (26.8%) | 3 (18.8%) | 8 (32.0%) | 0.478 |
| Reported symptoms, n (%) | ||||
| Headache | 34 (82.9%) | 13 (81.3%) | 21 (84.0%) | 1.000 |
| Cough | 32 (78.0%) | 13 (81.2%) | 19 (76.0%) | 1.000 |
| Fever | 25 (61.0%) | 9 (56.3%) | 16 (64.0%) | 0.746 |
| Myalgia | 31 (75.6%) | 14 (87.5%) | 17 (68.0%) | 0.265 |
| Dyspnea | 21 (51.2%) | 9 (56.3%) | 12 (48.0%) | 0.757 |
| Anosmia | 25 (61.0%) | 9 (56.3%) | 16 (64.0%) | 0.746 |
| Diarrhea | 18 (43.9%) | 11 (68.8%) | 7 (28.0%) | 0.022 |
| Odynophagia | 17 (41.5%) | 7 (43.8%) | 10 (40.0%) | 1.000 |
| Expectoration | 17 (41.5%) | 6 (37.5%) | 11 (44.0%) | 0.753 |
| Healthcare resource utilization in relation to COVID-19 | ||||
| Outpatient visits, n (%) | 37 (90.2%) | 15 (93.8%) | 22 (88.0%) | 1.000 |
| Telephonic visits, n (%) | 25 (61.0%) | 12 (75.0%) | 13 (52.0%) | 0.195 |
| Emergency room, n (%) | 20 (48.8%) | 5 (31.3%) | 15 (60.0%) | 0.111 |
| Hospitalization, n (%) | 5 (12.2%) | 1 (6.3%) | 4 (16.0%) | 0.632 |
In bold are marked statistically significant variables (P value≤0.05).
Comparing patients with and without MAbs, no differences were found in terms of baseline characteristics or proportion of COVID-19 cases, with the exception of adherence to migraine preventive treatment: patients with MAbs presented a statistically significant higher adherence than patients without MAbs (97.6% vs. 84.1%; P=0.008) (Table 1). Discontinuation was due to lack of efficacy, impossibility in administering or collecting the medication.
In the subgroup of COVID-19 cases, there were no differences in COVID-19 symptoms except for diarrhea (without MAbs: 68.8% vs. with MAbs: 28.0%; P=0.022). The proportions of patients with headache as a COVID-19 symptom as well as with worsening with respect to the usual migraine (47.1%, 16/34) were not different between the group with and without MAb. Healthcare resource utilization related to COVID-19 and adherence to other preventive medications was similar between the two groups (Table 2). Two patients in this group discontinued MAbs, reporting that it was for fear of possible interactions with the concomitant COVID-19 infection. We finally performed a sensitivity analysis of confirmed COVID-19 patients (12/41) and we also found no statistically significant differences between patients with MAbs vs. without MAbs.
DiscussionIn our study we decided to evaluate de potential impact of CGRP antagonism through MAbs in patients with migraine during COVID-19 pandemic, considering that, at present, there are no clear data assessing the effects of this treatment during a viral infection.
Our findings are relevant for three main reasons:
First, we observed that prevalence of COVID-19 in people with migraine is similar to the general population, suggesting that they have not an increased risk on the basis of their migraine history. In our cohort, 13.7% of participants had confirmed or suspected COVID-19 in line with national epidemiological study in Spain conducted in the same period on the general population, showing COVID-19 prevalence around 13.69–18.6%.10
Second, prevalence of COVID-19 is similar in migraine patients with and without MAbs, suggesting that anti-CGRP MAbs may be safe, not predisposing to COVID-19.
Third, patients with confirmed or suspected COVID-19 under MAb treatment do not seem to have a worse course of the disease compared with migraine patients under other preventive treatments.
Yet, what are the implications, in particular, of these latter findings?
Present implication: migraineFrom the migraine standpoint, since new waves of the pandemic may be approaching, neurologists can keep prescribing anti-CGRP MAbs, reassuring migraine patients on their use in order to avoid unnecessary discontinuation, as it does not seem to be associated with an increased susceptibility to SARS-CoV-2 infection.
Future implication: COVID-19Extremely interesting could be the COVID-19 perspective. In this context, new studies are hypothesizing that a neuro-immunological cross-talk within the lungs takes place through the activation of the vagus nerve during infections. CGRP may be involved in this pathway and could alter inflammatory responses.11 In support to this concept, a study on animal models of Staphylococcus aureus pneumonia, for example, has observed that antagonizing CGRP improves survival in infected mice.12 So far, the role of CGRP had not been studied in viral infections, but recently a clinical trial has started to investigate intranasal vazegepant, a new anti-CGRP molecule for migraine therapy, specifically to treat COVID-19.13
In our study, we have observed that anti-CGRP MAbs have no major negative effects during COVID-19, however according to this neuro-immunological hypothesis, they could even represent a new option to treat infectious diseases such as COVID-19. Therefore, further studies must be designed to specifically address this relevant question, while the results of the previously mentioned clinical trial are awaited.
As for the main limitations of our study, we are aware of the potential selection bias related to patient self-reported symptoms without possibility of a laboratory confirmation for SARS-CoV-2 in most of the cases. However, we have to consider that at the time of the peak of the pandemic, almost exclusively patients admitted at the hospital were tested to confirm COVID-19, being such bias inevitable in many online-based questionnaires. In addition, our findings should be examined carefully since the size of the COVID-19 group in our cohort is limited and participants had a mild-moderate course of the disease, being more severe patients unlikely to answer an online survey. Nevertheless, our work represents a useful exploratory study on the impact of anti-CGRP in patients with migraine. Our real-life data should be confirmed by further and ampler population-based studies with the objective of clarifying not only the absence of negative effects but also the potential therapeutic activity of these drugs in COVID-19.
ConclusionsThe prevalence of COVID-19 cases in people with migraine is similar to the one of the general population. The use of monoclonal antibodies against CGRP may be safe in clinical practice, not being associated with an increased risk or worse prognosis of COVID-19.
Authors’ contributionsPPR and EC made substantial contributions to conception and study design. EC, VJG, AA, MTF, NMSM, JVR, ACLV, ALB, MFR, MDGB, SMGS, MMB, MPA, CS, CV, BCC, CVE, PPJ, CTP, LGV, AGV, PIS, JPE, SSL, PPR worked for acquisition of data. VJG and EC contributed to analysis and interpretation of data. EC and VJG wrote first draft. PPR, MTF, AA critically revised and finally approved the version to be published. All authors fully comply with and approve the version to be published.
Consent to publishAll patients consented to publication of anonymous individual data.
Data availability statementAll data are available and any anonymized data will be shared by request from any qualified investigator.
FundingNo funding was received for this study.
Conflict of interestDr. Caronna reports no disclosures relevant to the manuscript.
Mr. Gallardo reports no disclosures relevant to the manuscript.
Dr. Alpuente has received honoraria from Allergan plc, Novartis, Chiesi.
Dr. Torres-Ferrus has received honoraria from Allergan plc, Novartis, Chiesi.
Dr. Morollón Sánchez-Mateo reports no disclosures relevant to the manuscript.
Dr. Viguera-Romero has received honoraria from Allergan plc, Novartis, Chiesi.
Dr. López-Veloso reports no disclosures relevant to the manuscript.
Dr. López-Bravo reports no disclosures relevant to the manuscript.
Dr. Gago-Veiga has received honoraria from Allergan plc, Novartis, Chiesi.
Dr. Irimia Sieira has received honoraria from Allergan, Novartis and Teva Pharmaceuticals.
Dr. Porta-Etessam has received honoraria from Allergan, Chiesi, Eli Lilly, Novartis and Teva.
Dr. Santos-Lasaosa has received honoraria from Allergan, Chiesi, Eli Lilly, Novartis and Teva.
Dr. Pozo-Rosich has received honoraria as a consultant and speaker for: Allergan, Almirall, Biohaven, Chiesi, Eli Lilly, Novartis and Teva. Her research group has received research grants from Allergan and Novartis, and has received funding for clinical trials from Alder, Electrocore, Eli Lilly, Novartis, Teva. She is in the editorial board of Revista de Neurologia. She is an associate editor for Cephalalgia, Headache, Neurologia and Frontiers of Neurology. She is the founder of www.midolordecabeza.org. PP-R does not own stocks from any pharmaceutical company.
Spanish CGRP-COVID Study Group: María Fernández-Recio; Maria Dolores García-Bargo; Sonia María García-Sánchez; María Martín Bujanda; Manuel Peinazo-Arias; Cristina Soriano; Carlos Vilar; Berta Claramonte-Clausell; Cecile Van Eendenburg; Patricia Perea-Justicia; Cristina Treviño-Peinado; Lidia Gómez-Vicente.