The 90-day risk of cerebral infarction in patients with transient ischaemic attack (TIA) is estimated at between 8% and 20%. There is little consensus as to which diagnostic strategy is most effective. This study evaluates the benefits of early transthoracic echocardiography (TTE) with carotid and transcranial Doppler ultrasound in patients with TIA.
MethodsProspective study of patients with TIA in an emergency department setting. Demographic data, vascular risk factors, and ABCD2 score were analysed. TIA aetiology was classified according to TOAST criteria. All patients underwent early vascular studies (<72hours), including TTE, carotid ultrasound, and transcranial Doppler. Primary endpoints were recurrence of stroke or TIA, myocardial infarction (MI), or vascular death during the first year.
ResultsWe evaluated 92 patients enrolled over 24 months. Mean age was 68.3±13 years and 61% were male. The mean ABCD2 score was 3 points (≥5 in 30%). The distribution of TIA subtypes was as follows: 12% large-artery atherosclerosis; 30% cardioembolism; 10% small-vessel occlusion; 40% undetermined cause; and 8% rare causes. Findings from the early TTE led to a change in treatment strategy in 6 patients (6.5%) who displayed normal physical examination and ECG findings. At one year of follow-up, 3 patients had experienced stroke (3.2%) and 1 patient experienced MI (1%); no vascular deaths were identified.
ConclusionsIn our TIA patients, early vascular study and detecting patients with silent cardiomyopathy may have contributed to the low rate of vascular disease recurrence.
El riesgo de infarto cerebral dentro de los primeros 90 días tras un ataque isquémico transitorio (AIT) se estima entre un 8-20%. Existe escaso consenso sobre cuál es la estrategia diagnóstica más eficaz. Nuestro objetivo fue evaluar del beneficio del estudio precoz con ecocardiografía transtorácica (ETT) y ultrasonografía carotídea y transcraneal (DTSA/TC) en los pacientes con AIT.
MétodosEvaluamos de forma prospectiva todos los pacientes con AIT atendidos en urgencias durante 24 meses. Recogimos variables demográficas, factores de riesgo vascular y escala ABCD2. La etiología del AIT fue clasificada según criterios TOAST. En todos los pacientes se realizó el estudio vascular precoz (<72h) con ETT y DTSA/TC. Los objetivos primarios fueron la recurrencia vascular cerebral, infarto de miocardio (IAM) o muerte vascular durante el primer año.
ResultadosEvaluamos 92 pacientes con una edad media de 68,3±13 años y el 61% fueron hombres. La media de la escala ABCD2 fue de 3 puntos (≥5 en un 30%). La distribución etiológica fue la siguiente: aterotrombótico de gran vaso 12%; cardioembólico 30%; pequeño vaso 10%; indeterminado 40% e inhabitual 8%. Los hallazgos de la ETT cambiaron el tratamiento en 6 pacientes (6,5%) con exploración física y ECG normal. Al año de seguimiento 3 pacientes (3,2%) sufrieron un infarto cerebral, uno (1%) un IAM y no detectamos ninguna muerte vascular.
ConclusionesEl estudio etiológico precoz en los pacientes con AIT y la detección de pacientes con cardiopatía silente puede haber contribuido a la baja tasa de recurrencia vascular.
The risk of stroke after a transient ischaemic attack (TIA) is estimated at 4% to 10% during the first week and 8% to 20% during the first 90 days.1–5 TIA is considered a medical emergency due to its high morbidity and mortality; mortality rate during the first year ranges from 1.9% to 7.9%, while the risk of myocardial infarction is estimated at 1.7% to 2.7%.6,7 However, there is no consensus as to which diagnostic strategy is the most effective for reducing the risk of vascular recurrence.
Previous studies have shown that the risk of suffering a stroke after TIA is associated with many different clinical factors (older age, diabetes mellitus, symptom duration >10minutes, presence of motor symptoms or language impairment), the aetiological subtype (large-artery atherosclerosis or cardioembolism),5,8 and presence of acute lesions in brain MRI.9,10
International guidelines support urgent assessment of the supra-aortic trunks in the first 48hours using ultrasound or other diagnostic techniques, whereas an echocardiography study should be conducted in patients with clinical and/or electrocardiographic findings suggestive of heart disease, or in patients with TIA of undetermined cause (UND).11
Risk of vascular recurrence is very high in patients with TIA, and few studies have demonstrated that early diagnosis and treatment can change this.12,13 Our purpose is to evaluate the benefits of early transthoracic echocardiography (TTE) in patients with TIA who were followed for one year, and compare our results with those reported in previous series.
Patients and methodsWe conducted a prospective study of all consecutive patients with TIA seen in our emergency department during a 24-month period. All patients underwent early assessment by a neurologist specialising in cerebrovascular disease. TIA was suspected in all patients displaying a sudden-onset focal cerebral or retinal neurological deficit of possible vascular aetiology and lasting less than 24hours. We included all patients offering sufficient clinical suspicion to have undergone a vascular study.
In our normal clinical practice, all patients with TIA are admitted to hospital to undergo aetiological studies and receive treatment.
The expert neurologist gathered the following data from each patient in the emergency department: clinical history, complete physical examination, blood test including a serum electrolyte study, complete blood count, biochemical analysis including kidney and liver function, coagulation test, electrocardiogram, chest radiography, brain CT, and ABCD score2 (≥60 years old=1 point; systolic blood pressure≥140mmHg or diastolic blood pressure≥90mmHg=1 point; hemiparesis=2 points; speech alterations without weakness=1 point; symptom duration≥60minutes=2 points; 10 to 59minutes=1 point; <10minutes=0 points; diabetes mellitus=1 point).
When patients presented no neurological symptoms indicating a specific affected arterial territory (for example, pure motor syndrome), the arterial territory was considered to be undetermined.
All patients underwent a complete study with TTE and carotid and transcranial Doppler ultrasound in the first 72hours from onset of TIA. Patients were considered to have intracranial stenosis when they presented a mean flow velocity>80cm/s with a side-to-side asymmetry of >30cm/s and some degree of turbulent flow, and when this diagnosis was confirmed by brain CT angiography or MRI angiography studies. Carotid stenosis was classified according to the NASCET criteria as mild (<50%), moderate (50% to 69%), and severe (≥70%). Complementary studies, such as Holter ECG, CT angiography, brain MRI, or coagulation studies, were performed in specific cases according to clinical criteria.
Definition of vascular risk factorsOnly patients with an active tobacco habit were regarded as smokers. Hypertension was defined as systolic blood pressure≥140mmHg or diastolic blood pressure≥90mmHg; patients under hypertensive treatment were also considered to have hypertension. Diabetes mellitus was defined as fasting baseline glycaemia≥126mg/dL or use of antidiabetic treatment, and hypercholesterolaemia was defined as total cholesterol levels≥220mg/dL or use of hypolipidaemic drugs.
Aetiological classification of TIA and clinical objectivesTIAs were classified by aetiology as large-artery atherosclerosis (LAA), small-vessel occlusion (SVO), cardioembolism (CE), stroke of other determined cause (OC), or stroke of UND, following TOAST criteria.14
We gathered vascular recurrences during hospitalisation, at 90 days, and at one-year follow-up in face-to-face consultations with the expert neurologist. Stroke, new TIA, myocardial infarction, and vascular death at any time after admission to the emergency department were considered vascular recurrences.
Stroke was defined as a sudden focal cerebral or retinal neurological deficit of apparent vascular aetiology and a duration >24hours.
All patients signed informed consent forms before being included in the study. The study protocol was approved by our hospital's Clinical Research Ethics Committee.
Statistical analysisResults from normally distributed variables are presented as means±SD, and non-normally distributed variables as medians and interquartile ranges.
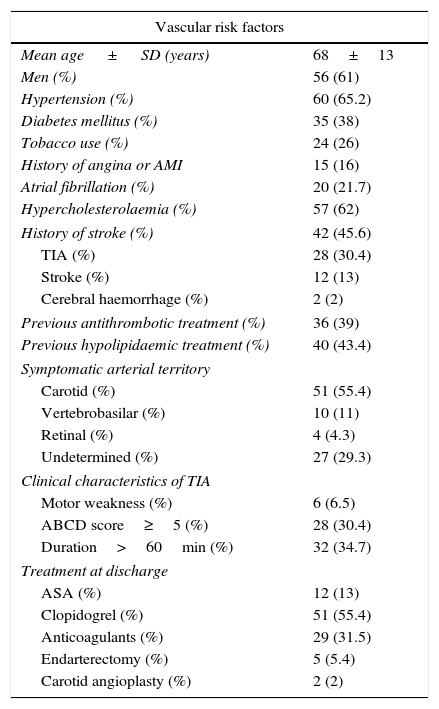
ResultsWe assessed a total of 101 patients in 24 months. Nine of these patients (9%) presented no cerebrovascular disease: they included 4 cases of hypertensive encephalopathy and one case each of epilepsy, hypoglycaemia, myelopathy, migraine, and hypercapnic encephalopathy. The final sample included 92 patients with a mean age of 68.3±13 years; 61% were men. Table 1 summarises vascular risk factors in our sample.
Risk factors and clinical manifestations of TIA.
| Vascular risk factors | |
|---|---|
| Mean age ± SD (years) | 68±13 |
| Men (%) | 56 (61) |
| Hypertension (%) | 60 (65.2) |
| Diabetes mellitus (%) | 35 (38) |
| Tobacco use (%) | 24 (26) |
| History of angina or AMI | 15 (16) |
| Atrial fibrillation (%) | 20 (21.7) |
| Hypercholesterolaemia (%) | 57 (62) |
| History of stroke (%) | 42 (45.6) |
| TIA (%) | 28 (30.4) |
| Stroke (%) | 12 (13) |
| Cerebral haemorrhage (%) | 2 (2) |
| Previous antithrombotic treatment (%) | 36 (39) |
| Previous hypolipidaemic treatment (%) | 40 (43.4) |
| Symptomatic arterial territory | |
| Carotid (%) | 51 (55.4) |
| Vertebrobasilar (%) | 10 (11) |
| Retinal (%) | 4 (4.3) |
| Undetermined (%) | 27 (29.3) |
| Clinical characteristics of TIA | |
| Motor weakness (%) | 6 (6.5) |
| ABCD score≥5 (%) | 28 (30.4) |
| Duration>60min (%) | 32 (34.7) |
| Treatment at discharge | |
| ASA (%) | 12 (13) |
| Clopidogrel (%) | 51 (55.4) |
| Anticoagulants (%) | 29 (31.5) |
| Endarterectomy (%) | 5 (5.4) |
| Carotid angioplasty (%) | 2 (2) |
ASA: acetylsalicylic acid; TIA: transient ischaemic attack.
Thirty percent of our patients had a history of TIA, and in 45% of these patients, the previous TIA had occurred within 15 days of the TIA that motivated the visit to the emergency department.
The mean ABCD score2 was 3 points (range, 0-7), and 30% of patients scored>5 points.
Mean time elapsed from symptom onset to arrival at the emergency department was 2hours; 59% of the patients visited the emergency department in the first 3hours after symptom onset. Mean duration of symptoms was 30minutes (range, 1-1440minutes); symptoms lasted for more than 60minutes in 34% of the patients. Mean hospitalisation time was 3.2±2.8 days.
The affected arterial territories were the carotid (55.4%), vertebrobasilar (11%), and retinal (4.3%); territory was undetermined in 29.3%.
The distribution of TIAs by aetiology was as follows: LAA, 12%; CE, 30.4%; SVO, 9.8%; UND, 40.2%; OC, 7.6% (3.2% due to haemodynamic causes, 2.2% due to paradoxical embolism, and 2.2% due to haematological disorders). Two or more causes of TIA were identified in 1.1% of the cases.
We detected significant extracranial stenosis in 9 patients (9.7%); in 83 patients, the study yielded normal results or indicated mild stenosis (<50%), one patient presented moderate stenosis (50%-69%), 7 patients had severe stenosis (≥70%), and one patient presented complete occlusion of the internal carotid artery. Symptomatic intracranial stenoses were confirmed in 2% of the patients.
Arterial revascularisation was performed in 7 patients (7.6%; endarterectomy in 5 and stent-supported angioplasty in 2). Mean time elapsed from symptom onset to arterial revascularisation was 22 days (range, 13-90) for endarterectomy and 71 days (range, 52-90) for angioplasty.
In 28 patients (30%), TIA was due to cardioembolic causes: 20 (21.5%) had atrial fibrillation; 8 (8.6%) had other cardioembolic causes detected with TTE during the first 72hours (Table 2). In 6 of the 8 patients with other cardioembolic causes, results from the physical examination and baseline ECG were normal; the remaining 2 presented mitral valve rumble and inferior Q wave, respectively. Therefore, TTE results in 6.5% of patients were responsible for a change in diagnosis of TIA (from stroke of UND to CE) and in the initial treatment during the first 72hours (antiplatelet treatment started in the first 24hours is discontinued and anticoagulant treatment is initiated).
Patients in whom TTE changed the therapeutic strategy.
| Patients (n) | Findings in the ECG and physical examination |
|---|---|
| Severe mitral stenosis (1) | Normal ECG; mitral valve rumble in physical examination |
| Akinesia of the LV and EF<35% (4) | Normal ECG in 3 and inferior Q wave in 1 |
| Ventricular thrombus (1) | Normal ECG and physical examination |
| AF not detected in the ECG (1) | Normal ECG and physical examination |
| Dilated cardiomyopathy with EF<35% (1) | Normal ECG and physical examination |
ECG: electrocardiogram; AF: atrial fibrillation; EF: ejection fraction; LV: left ventricle.
MRI was performed in 72% of the patients with a mean delay time of 10 days from symptom onset. We found acute lesions in DWI sequences in 26% of cases; the diagnosis in these patients was not TIA but rather stroke (lacunar infarct, 7.6%; non-lacunar infarct in the carotid territory, 15%; vertebrobasilar stroke, 2%; bilateral acute lesions, 1%). According to the aetiology of TIA, acute lesions are distributed as follows: LAA, 4.3%; CE, 34.7%; UND, 39%; SVO, 8.7%; and OC, 13%.
Objectives at 90 days and at one year of follow-upThree patients (3.2%) experienced a stroke a mean time of 29 days after the TIA that caused them to seek emergency care. Of this subgroup, 2 patients presented significant carotid stenosis which was not accessible to endarterectomy and experienced strokes while waiting to undergo carotid angioplasty in another centre (our hospital does not perform endovascular procedures). The remaining patient had atrial fibrillation and an absolute contraindication to anticoagulants, and he experienced a stroke at 90 days from the TIA.
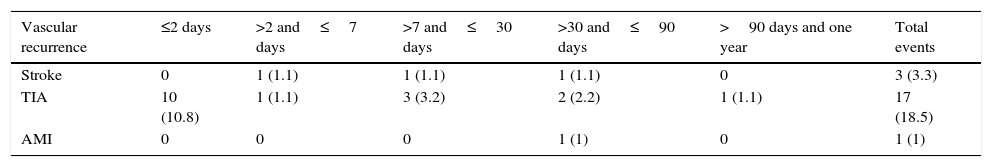
A total of 17 patients (18.5%) experienced TIA recurrence a mean of 3 days after the initial TIA. The distribution of these new TIAs by aetiology was as follows: CE, 7; LAA, 1; SVO, 2; OC, 2; and UND, 5 (Table 3).
Timeline for cerebral vascular recurrence or myocardial infarction after initial TIA.
| Vascular recurrence | ≤2 days | >2 and≤7 days | >7 and≤30 days | >30 and≤90 days | >90 days and one year | Total events |
|---|---|---|---|---|---|---|
| Stroke | 0 | 1 (1.1) | 1 (1.1) | 1 (1.1) | 0 | 3 (3.3) |
| TIA | 10 (10.8) | 1 (1.1) | 3 (3.2) | 2 (2.2) | 1 (1.1) | 17 (18.5) |
| AMI | 0 | 0 | 0 | 1 (1) | 0 | 1 (1) |
Data are expressed as numbers (percentages).
TIA: transient ischaemic attack; AMI: acute myocardial infarction.
One patient experienced a myocardial infarction at 90 days; we did not detect any cerebral haemorrhages or vascular deaths during the one-year follow-up period.
DiscussionAt one year of follow-up, only 3.2% of patients experienced stroke and 1% experienced myocardial infarction. In addition, we found no cases of cerebral haemorrhage or vascular death. These numbers are significantly lower than those reported by previous series (stroke rates of 8% to 20% at 90 days in patients with a history of TIA). This pronounced decrease in stroke rates may be linked to early assessment with TTE and Doppler ultrasound.
Risk of TIA recurrence was higher in the first 2 days after the initial TIA, and CE was the leading cause of recurrence. However, only one patient with CE (and an absolute contraindication to anticoagulants) experienced a stroke during the one-year follow-up period.
In our study, CE was the second most common aetiological subtype of TIA (30.4%), following UNDs (40.2%). At discharge, 29 patients (31%) were treated with anticoagulant agents for the following reasons: CE, 26 (28.2%); OC, 2 (2.1%). One patient (1%) was classified as UND for presenting 2 possible reasons. Previous studies of hospitalised TIA patients who were given clinical interviews by a neurologist specialising in vascular disease15–19 have reported percentages of anticoagulant use after discharge ranging from 5% to 30%; the risk of stroke is higher in the studies reporting lower percentages of anticoagulant use. These differences may be explained by the fact that TTE was not systematically performed in all patients; this may lead to underestimating CE, which would in turn increase the risk of vascular recurrence.
Current guidelines do not support systematic use of TTE in all patients with TIA. These recommendations are based on the lack of sufficient scientific evidence supporting the benefits of performing TTE in all cases of TIA.11 However, this lack of conclusive scientific evidence does not demonstrate that TTE provides no benefits for patients with TIA. TTE can detect low- and medium-risk cardioembolic causes and provides complementary information on cardiac function or a possible underlying cardiomyopathy. It is also helpful for adjusting treatment for hypertension, arrhythmia, and heart failure.
According to previous studies of TIA, the risk of myocardial infarction after one year of follow-up is 1.7% to 2.7% and the mortality rate due to vascular causes ranges from 1.9% to 7.9%.6,20 In our study, 1% of patients experienced myocardial infarction and there were no cases of vascular death at one year of follow-up; these findings are in line with those reported by other studies performing TTE systematically.19,21 Based on the fact that studies with higher percentages of anticoagulant use after discharge show lower stroke rates,17,19 and that the studies systematically using TTE show lower rates of stroke, myocardial infarction, and vascular death, we may conclude that systematic TTE helps detect more cases of CE and consequently contributes to decreasing vascular recurrence.
In our study, TTE was performed on all patients even if results from the physical examination and the ECG were normal. Cardioembolic causes were detected in 6 patients (6.5%) whose results from these studies had initially been normal. Patients who were found to have silent ischaemic cardiomyopathy were referred to the cardiology department for further assessment and treatment of ischaemic heart disease. Identifying these patients and providing early treatment for cardiopathy may have contributed to the low rate of vascular recurrence and the lack of vascular deaths. Randomised controlled studies are necessary to prove the benefits of early TTE for reducing vascular events.
The risk of stroke after a TIA is associated with various clinical factors (older age, diabetes mellitus, symptom duration>10minutes, presence of motor symptoms or language impairment); aetiological subtype (LAA or CE)5,8; and presence of acute lesions in brain MRI.9,10 At 90 days, the risk of stroke for patients with TIA is estimated at 9.8% for those scoring 4 to 5 on the ABCD scale and 17.8% for those scoring≥6.20,22 Presence of acute lesions in MR images, combined with a symptom duration>60minutes, is an independent predictor of future cerebral ischaemic events (OR=5.02).9 In our study, 30% of patients scored≥5 points on the ABCD scale, and 28% of patients showed acute lesions on MRI. In 12% of all patients showing acute lesions, symptom duration was≥60minutes. Our low rates of recurrence are therefore not very likely to be due to sample characteristics.
Our study has several limitations: (1) A greater sample size may have provided a better evaluation of the risk of vascular recurrence in patients with TIA. (2) Transoesophageal echocardiography (TOE) may improve the aetiological classification and better assess ventricular function in patients with a poor acoustic window. TOE is more sensitive than TTE for detecting other potential cardioembolic sources. (3) We cannot quantify the relative impact of secondary prevention treatments (for example, antiplatelet drugs, statins, and antihypertensive drugs) on the decrease in vascular recurrence. In our study, treatments for secondary prevention were initiated immediately and all patients were assessed by a neurologist specialising in vascular disease, both in the emergency department and in outpatient clinics at 90 days and at one year. This follow-up regimen resulted in excellent adherence to treatment. (4) Our results represent patients from a single hospital, which may limit the extent to which these results can be generalised to other populations. (5) We did not use ABCD scores to stratify the prognosis due to the small sample size. (6) Since our study was not a randomised controlled study, we cannot prove the benefits of early TTE for reducing vascular events and can only compare our results with those reported in other series. (7) Delays for undergoing carotid angioplasty were excessive, which may have been responsible for some cases of vascular recurrence. Our hospital is currently implementing a new protocol intended to reduce delays. (8) In our centre, TIAs are not monitored during the first 48hours. Early monitoring of these patients may have provided more information about cardiac rhythm disorders. However, of all patients in whom TTE resulted in a change in diagnosis, only one had atrial fibrillation that was not detected by the initial ECG, while the rest had CE.
Previous studies have shown that assessment of TIAs by specialists results in lower risk of vascular recurrence. According to the EXPRESS13 and SOS-TIA12 studies, early assessment and treatment of patients with TIA is associated with lower stroke rates at 90 days (2.1% and 1.2%, respectively). In our study, assessment by an expert neurologist plus early vascular and cardiology studies yielded low recurrence rates for stroke, myocardial infarction, and vascular death. These rates remained low during the one-year follow-up period.
ConclusionOur results suggest that early assessment of TIA patients in the emergency department is useful for determining the pathophysiology of cerebral ischaemia. In our study, performing an early vascular assessment and identifying patients with silent ischaemic cardiomyopathy may have contributed to the low one-year mortality rates from stroke, myocardial infarction, and vascular death. The main contribution of the present study is the fact that we achieved a significant decrease in stroke risk at one year compared to other series. This decrease may have been influenced by a number of factors, including secondary prevention, assessment by a neurologist specialising in cerebrovascular disease, and probably the cardiology study, since CE was the second most frequent cause of TIA in our series. It is therefore logical to believe that detecting and treating the most frequent cause of TIA is beneficial for reducing recurrence. Based on our results, we recommend that patients with TIA be evaluated by a neurologist specialising in cerebrovascular disease and undergo early vascular and cardiology studies even if results from the ECG and the physical examination are normal.
Conflicts of interestThe authors have no conflicts of interest to declare.
We would like to thank the emergency department for their help with this study.
Please cite this article as: Cocho D, Monell J, Planells G, Ricciardi AC, Pons J, Boltes A, et al. Baja tasa de infarto cerebral, infarto de miocardio y muerte vascular con el diagnóstico y tratamiento etiológico rápido del ataque isquémico transitorio. Neurología. 2016;31:18–23.
This study was presented orally at the 63rd Annual Meeting of the Spanish Society of Neurology. Neurología. 2011;26:33.