Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease that causes severe dysphagia and weight loss. Percutaneous endoscopic gastrostomy (PEG) is currently the technique of choice for the enteral nutrition of these patients.
ObjectivesTo analyse mortality and complications in a series of patients diagnosed with ALS who underwent PEG, and to evaluate factors related to patient survival after the procedure.
Materials and methodsWe performed a prospective, observational study including all patients diagnosed with ALS and treated by our hospital's Gastroenterology Department in the period 1997-2013. We studied mortality, complications, and clinical and biochemical parameters, and correlated these with the survival rate.
ResultsThe study included a total of 57 patients, of whom 49 were ultimately treated with PEG. ALS onset was bulbar in 30 patients and spinal in 19. Mortality during the procedure and at 30 days was 2% (n=1). Six patients (12.2%) experienced major complications; 17 (34.7%) experienced less serious complications which were easily resolved with conservative treatment. No significant differences were observed in forced vital capacity, albumin level, or age between patients with (n=6) and without (n=43) major complications.
ConclusionsPEG is an effective, relatively safe procedure for the enteral nutrition of patients with ALS, although not without morbidity and mortality. Neither forced vital capacity nor the form of presentation of ALS were associated with morbidity in PEG.
La esclerosis lateral amiotrófica (ELA) es una enfermedad neurodegenerativa que produce disfagia grave y pérdida de peso. La gastrostomía endoscópica percutánea (GEP) es en la actualidad la técnica de elección para la nutrición enteral de estos pacientes.
ObjetivosAnalizar la mortalidad y las complicaciones en una serie de pacientes diagnosticados de ELA a los que se realizó la GEP y evaluar los factores relacionados con la supervivencia después del procedimiento.
Material y métodosEstudio observacional prospectivo en el que se incluyeron los pacientes diagnosticados de ELA atendidos en el Servicio de Gastroenterología (años 1997-2013) a los que se realizó GEP. Se estudiaron la mortalidad, las complicaciones y los parámetros clínicos y analíticos, correlacionándolos con la tasa de supervivencia.
ResultadosSe incluyeron 57 pacientes, de los que finalmente se pudo realizar la GEP en 49. La ELA fue de inicio bulbar en 30 y espinal en 19. La mortalidad durante el procedimiento y a los 30 días fue del 2% (n=1). Se registraron complicaciones mayores en 6 pacientes (12,2%) y complicaciones de menor gravedad, que se resolvieron fácilmente con tratamiento conservador, en 17 (34,7%). No se observaron diferencias en la capacidad vital forzada, la cifra de albúmina o la edad entre los pacientes con (n=6) o sin (n=43) complicaciones mayores.
ConclusionesLa GEP en los pacientes con ELA es un procedimiento eficaz y relativamente seguro para la nutrición enteral de estos pacientes, aunque no exento de morbimortalidad. Ni la capacidad vital forzada ni la forma de inicio de la enfermedad fueron factores asociados a morbilidad en la GEP.
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease which affects the upper and lower motor neurons, causing loss of strength and muscle atrophy.1 Cognitive function and sphincter control are generally preserved until the more advanced stages of the disease.
ALS mainly affects adults aged between 40 and 70 years. There is currently no curative treatment; prognosis is poor, with 5-year survival rates of approximately 20%.2 For this reason, management of patients with ALS requires a multidisciplinary approach focused on reducing the complications that arise during the progression of the disease. The most frequent complications include respiratory failure and dysphagia, which is associated with weight loss and aspiration pneumonia.3
Dysphagia, representing one of the most severe complications of ALS, is normally associated with bulbar involvement, and manifests in approximately 60% of cases.4 Several methods are available for identifying dysphagia; these include videofluoroscopy and guided clinical history enquiring specifically about difficulty swallowing. Adverse effects secondary to dysphagia are also assessed, as are changes to body mass index or weight.
Treatment of dysphagia depends on the stage of ALS progression. Thus, in the initial stages, we can try to optimise food intake capacity through swallowing rehabilitation. In more advanced stages, food consistency should be adapted to the patient's capacity through the introduction of thickeners, food fragmentation, and the use of blended and semisolid foods. Lastly, patients with a highly impaired food intake capacity must undergo invasive procedures, including percutaneous endoscopic gastrostomy (PEG).
This technique was introduced in 1980 by Ponsky and Gauderer, and is currently the method of choice for enteral nutrition of patients who are unable to eat but have an intact digestive tract. Current clinical guidelines for ALS recommend performing PEG early, when the forced vital capacity (FVC) is still higher than 50%.5
There is controversy regarding the association between improved survival rates and the use of PEG in patients with ALS. PEG is associated with a 30-day mortality rate ranging from 10% to 25%, according to the results reported by different clinical trials.6,7 Survival after gastrostomy is usually associated with age, respiratory function, and C-reactive protein and serum albumin levels.8,9
The aim of this study is to analyse the mortality and complications of PEG in a series of patients diagnosed with ALS at our hospital, and to study the factors associated with post-intervention survival rates.
Materials and methodsPopulation and designWe conducted a prospective observational study including all patients diagnosed with ALS and treated at our hospital's gastroenterology department between 1997 and 2013 who underwent PEG. Of a total of 665 patients with ALS who attended our department, 57 were eligible for PEG. Surgery could be performed in 49 patients; ALS onset was bulbar in 30 patients and spinal in 19.
The main indications for PEG were severe dysphagia, choking, and/or weight loss> 10%. All patients underwent a series of clinical studies before the procedure, including a complete blood count, complete biochemical analysis, coagulation study, chest radiography, electrocardiogram, arterial blood gas analysis, and spirometry. The minimum FVC required has been 1L since late 1997. PEG was performed using the Gauderer-Ponsky technique,7 with the same team of endoscopists and healthcare staff participating in all cases. Once the intervention was completed, the inflated air was aspirated to avoid deterioration of respiratory function. No sedation was used in the first 34 patients included in the study. Conscious sedation (propofol, midazolam, and/or fentanyl), introduced in 2009, was administered to 15 patients, as decided by the intensive care specialist participating in the procedure.
All patients were administered antibiotic prophylaxis with cefazolin (before PEG and every 8h after the procedure until discharge). Patients with allergy to beta-lactam antibiotics received vancomycin.
In order to estimate the progression of the patients included in the study, we recorded the time elapsed between ALS diagnosis and PEG performance, and the time elapsed between gastrostomy and death. We analysed mortality and complications occurring during the procedure and in the 30 days thereafter, especially those complications related to respiratory failure, pneumonia, and cardiorespiratory arrest. Complications associated with mortality, significant prolongation of hospital stay, surgical intervention, or an additional endoscopic procedure was regarded as major complications. The remaining complications were considered to be minor. These parameters were correlated with age, sex, FVC, and serum albumin level.
Statistical analysisThese parameters, expressed as absolute values or percentages, were analysed using the Spearman rank correlation test, Wilcoxon matched pairs test, and the Mann–Whitney U-test. Means were compared using the t test. All statistical tests were performed using version 13 of the SPSS software package (SPSS Inc., North Chicago, IL, USA).
ResultsPEG was indicated in 57 of the 665 patients diagnosed with ALS and treated during the study period. Gastrostomy was performed in most of these patients (n=49, 86%); the procedure could not be performed in 8 (14%; 5 men and 3 women; mean age, 61 years; range, 40-75). Conditions preventing performance of the procedure were intolerance to endoscopy in 3 patients (37%), inability to perform transillumination in 3 (37%), and laryngospasm in the 2 remaining patients (25%).
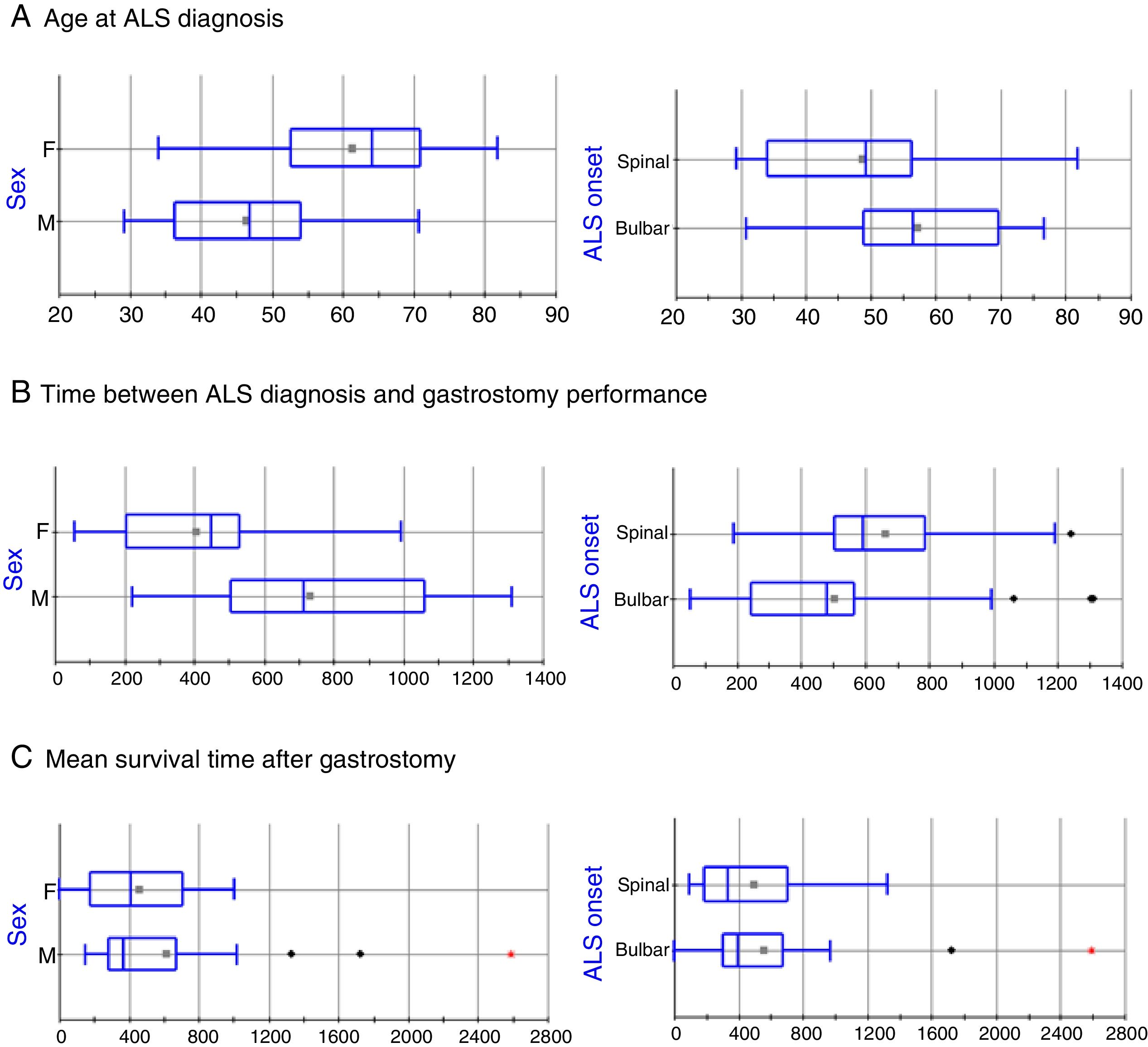
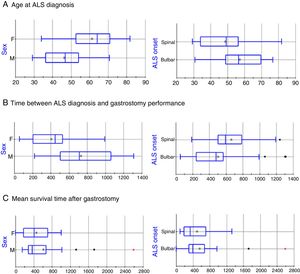
The clinical and sociodemographic characteristics of the 49 patients who underwent surgery are listed in Table 1. The series includes 24 men and 25 women, with statistically significant differences in age at ALS diagnosis (mean±SD: 46.36±11.7 vs 61.3±11.82 years, respectively; P=.0005, Fig. 1A). ALS onset was bulbar in 30 patients (61.2%) and spinal in 19 (38.8%); again, there were statistically significant differences between these groups in the age at ALS diagnosis (57.37±12.44 vs 48.63±14.65 years, respectively; P=.0302; Fig. 1A). The mean time elapsed between ALS diagnosis and gastrostomy was 46.91±27.25 months. The factor with the strongest influence on this parameter was patient sex (61.06±28.4 vs 33.90±18.61 months in men and women, respectively, P=.0003; Fig. 1B). Furthermore, patients diagnosed with bulbar-onset ALS displayed a tendency to shorter delays before PEG performance, in comparison with patients with spinal-onset ALS, although these differences were not statistically significant (41.86±28.07 vs 55.33±24.25 months, respectively; P=.0978; Fig. 1B).
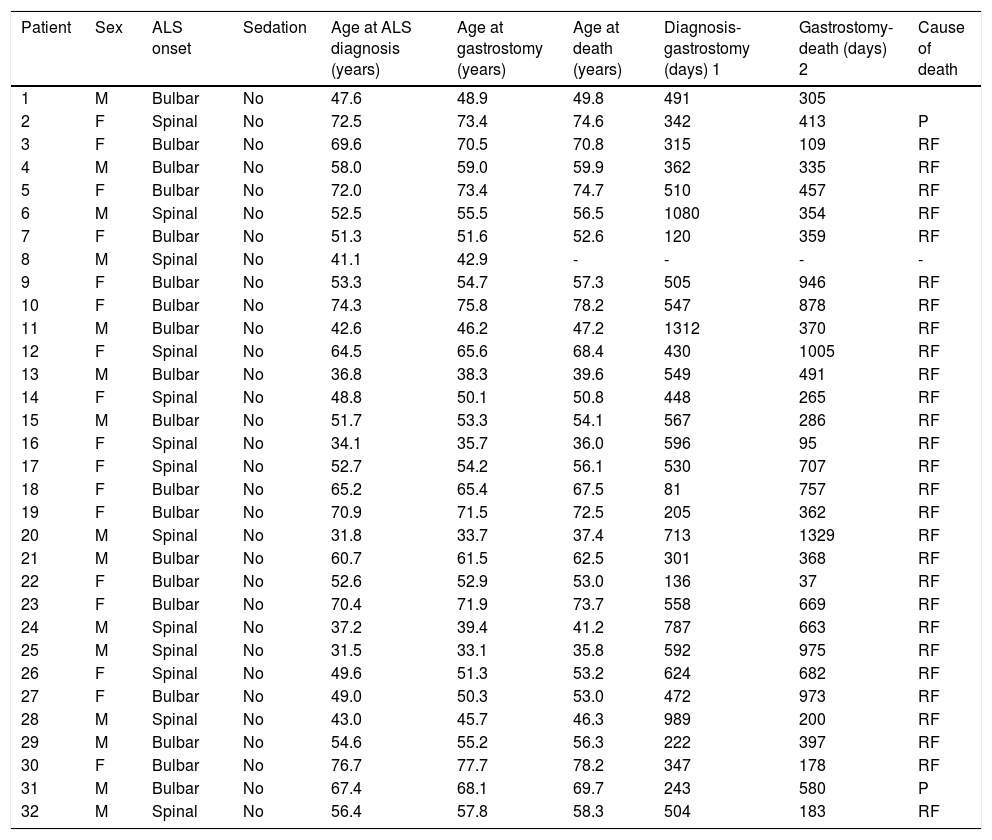
Clinical characteristics of patients who underwent percutaneous endoscopic gastrostomy.
| Patient | Sex | ALS onset | Sedation | Age at ALS diagnosis (years) | Age at gastrostomy (years) | Age at death (years) | Diagnosis-gastrostomy (days) 1 | Gastrostomy-death (days) 2 | Cause of death |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | Bulbar | No | 47.6 | 48.9 | 49.8 | 491 | 305 | |
| 2 | F | Spinal | No | 72.5 | 73.4 | 74.6 | 342 | 413 | P |
| 3 | F | Bulbar | No | 69.6 | 70.5 | 70.8 | 315 | 109 | RF |
| 4 | M | Bulbar | No | 58.0 | 59.0 | 59.9 | 362 | 335 | RF |
| 5 | F | Bulbar | No | 72.0 | 73.4 | 74.7 | 510 | 457 | RF |
| 6 | M | Spinal | No | 52.5 | 55.5 | 56.5 | 1080 | 354 | RF |
| 7 | F | Bulbar | No | 51.3 | 51.6 | 52.6 | 120 | 359 | RF |
| 8 | M | Spinal | No | 41.1 | 42.9 | - | - | - | - |
| 9 | F | Bulbar | No | 53.3 | 54.7 | 57.3 | 505 | 946 | RF |
| 10 | F | Bulbar | No | 74.3 | 75.8 | 78.2 | 547 | 878 | RF |
| 11 | M | Bulbar | No | 42.6 | 46.2 | 47.2 | 1312 | 370 | RF |
| 12 | F | Spinal | No | 64.5 | 65.6 | 68.4 | 430 | 1005 | RF |
| 13 | M | Bulbar | No | 36.8 | 38.3 | 39.6 | 549 | 491 | RF |
| 14 | F | Spinal | No | 48.8 | 50.1 | 50.8 | 448 | 265 | RF |
| 15 | M | Bulbar | No | 51.7 | 53.3 | 54.1 | 567 | 286 | RF |
| 16 | F | Spinal | No | 34.1 | 35.7 | 36.0 | 596 | 95 | RF |
| 17 | F | Spinal | No | 52.7 | 54.2 | 56.1 | 530 | 707 | RF |
| 18 | F | Bulbar | No | 65.2 | 65.4 | 67.5 | 81 | 757 | RF |
| 19 | F | Bulbar | No | 70.9 | 71.5 | 72.5 | 205 | 362 | RF |
| 20 | M | Spinal | No | 31.8 | 33.7 | 37.4 | 713 | 1329 | RF |
| 21 | M | Bulbar | No | 60.7 | 61.5 | 62.5 | 301 | 368 | RF |
| 22 | F | Bulbar | No | 52.6 | 52.9 | 53.0 | 136 | 37 | RF |
| 23 | F | Bulbar | No | 70.4 | 71.9 | 73.7 | 558 | 669 | RF |
| 24 | M | Spinal | No | 37.2 | 39.4 | 41.2 | 787 | 663 | RF |
| 25 | M | Spinal | No | 31.5 | 33.1 | 35.8 | 592 | 975 | RF |
| 26 | F | Spinal | No | 49.6 | 51.3 | 53.2 | 624 | 682 | RF |
| 27 | F | Bulbar | No | 49.0 | 50.3 | 53.0 | 472 | 973 | RF |
| 28 | M | Spinal | No | 43.0 | 45.7 | 46.3 | 989 | 200 | RF |
| 29 | M | Bulbar | No | 54.6 | 55.2 | 56.3 | 222 | 397 | RF |
| 30 | F | Bulbar | No | 76.7 | 77.7 | 78.2 | 347 | 178 | RF |
| 31 | M | Bulbar | No | 67.4 | 68.1 | 69.7 | 243 | 580 | P |
| 32 | M | Spinal | No | 56.4 | 57.8 | 58.3 | 504 | 183 | RF |
| Patient | Sex | ALS onset | Sedation | Age at ALS diagnosis (years) | Age at gastrostomy (years) | Age at death (years) | Diagnosis-gastrostomy (days) 1 | Gastrostomy-death (days) 2 | Cause of death |
|---|---|---|---|---|---|---|---|---|---|
| 33 | F | Spinal | No | 81.8 | 82.4 | 83.2 | 191 | 313 | RF |
| 34 | F | Bulbar | No | 55.1 | 56.6 | 58.1 | 516 | 561 | RF |
| 35 | F | Bulbar | Yes | 62.3 | 62.5 | 63.6 | 74 | 388 | RF |
| 36 | M | Bulbar | Yes | 30.8 | 32.8 | 37.5 | 738 | 1722 | RF |
| 37 | M | Bulbar | Yes | 36.0 | 38.3 | 40.1 | 825 | 674 | RF |
| 38 | F | Bulbar | Yes | 59.8 | 60.0 | 61.3 | 56 | 499 | P |
| 39 | M | Bulbar | Yes | 53.5 | 55.6 | 56.6 | 765 | 359 | RF |
| 40 | M | Spinal | Yes | 29.3 | 30.7 | 31.5 | 510 | 287 | RF |
| 41 | M | Spinal | Yes | 50.0 | 53.2 | 53.6 | 1190 | 147 | RF |
| 42 | M | Spinal | Yes | 33.8 | 37.2 | 37.7 | 1242 | 185 | P |
| 43 | M | Bulbar | Yes | 70.8 | 73.7 | 80.8 | 1061 | 2588 | RF |
| 44 | F | Bulbar | Yes | 43.1 | 45.9 | 45.9 | 992 | 0 | CRA |
| 45 | F | Bulbar | Yes | 71.6 | 72.9 | 73.0 | 445 | 48 | RF+BA |
| 46 | M | Bulbar | Yes | 46.3 | 49.9 | 50.6 | 1304 | 271 | RF |
| 47 | F | Bulbar | Yes | 67.0 | 68.3 | 70.2 | 452 | 725 | RF |
| 48 | F | Spinal | Yes | 64.2 | 66.0 | 66.3 | 677 | 108 | RF |
| 49 | M | Spinal | Yes | 49.3 | 50.6 | 53.4 | 506 | 1020 | RF |
ALS: amyotrophic lateral sclerosis; BA: bronchoaspiration; CRA: cardiorespiratory arrest; F: female; M: male; P: pneumonia; RF: respiratory failure; 1: time period between the date of diagnosis of amyotrophic lateral sclerosis and the date of gastrostomy performance; 2: time period between the date of gastrostomy performance and the date of death.
Box-plot of the effects of sex and ALS onset on disease progression time. Statistically significant differences were observed for age at diagnosis and for time elapsed between diagnosis and gastrostomy; however, no differences were identified in mean survival time after the intervention in any patient group. The horizontal axis represents time in years (A) or days (B and C).
During the intervention, we recorded easily resolved minor complications in 17 patients (34.7%): loss of a tooth (n=1), allergic reaction to vancomycin (n=1), mild stomal haemorrhage (n=2), laryngospasm resolved during the procedure (n=6), hypertensive crisis and supraventricular tachycardia (n=1), gastric wall haematoma (n=1), benign pneumoperitoneum (n=1), wound infection (n=1), mild gastrointestinal haemorrhage (n=1), stoma leakage (n=1), and desaturation (n=1). Furthermore, major complications were detected in 6 patients (12.2%): pneumonia (n=2); severe gastrointestinal haemorrhage requiring transfusion, resolved with endoscopic treatment (n=1); perforation of a Riedel lobe by the gastrostomy tube, making it necessary to perform a second PEG (n=1); dislocation of the jaw (n=1); and death due to cardiorespiratory arrest (n=1). The 2 cases of pneumonia occurred in the group of 15 patients who were not administered sedation during the procedure. There were no significant differences between patients with major complications (n=6) and the remaining patients (n=43) in FVC, albumin levels, or age. In the few patients whose C-reactive protein levels were determined before the PEG, we did not observe significant differences between those presenting major complications and the remaining patients.
Mortality rate during the procedure and at 30 days was 2.04% (1 of 49 patients). Mean hospitalisation time was 3.1 days (range, 2-15). Significant differences were observed in age at death between men and women (50.28±11.94 vs 63.68±11.71 years, respectively; P=.0003) and between patients with bulbar- and with spinal-onset ALS (60.27±12.18 vs 52.23±14.45 years, respectively; P=.0446). These differences seem to be due not to the endoscopic procedure, but to differences in the time elapsed between ALS diagnosis and surgery in both groups. No differences were observed in mean survival time after the PEG in any patient group (Fig. 1C).
DiscussionPEG is considered the method of choice for enteral nutrition of patients with ALS presenting severe dysphagia, choking, or significant weight loss. However, this is an invasive procedure which patients and families usually oppose in early stages of the disease. Therefore, it is usually performed in advanced stages of ALS, and is subsequently associated with high mortality rates (up to 25%) in the 30 days following the intervention.7 Only a limited number of our patients underwent PEG, either due to patients’ or family members’ resistance to the procedure, or due to excessively decreased FVC values.
The mortality rate observed in our series is lower than those reported in previous studies.3,10 Mortality at 30 days after the intervention was only recorded in 1 patient (2%). The only recorded death occurred during PEG performance and corresponded to a patient who was at a very advanced stage of the disease, with a FVC below 25%, which probably contributed to this outcome. Furthermore, 6-month survival rates were also considerably higher than those reported in previous studies: 7 (14%) patients died during the first 6 months of follow-up. This percentage is lower than that reported in a series of Spanish patients with similar characteristics to those included in our study (mortality rate of approximately 50%).11 However, we should highlight the close follow-up of the patients by our multidisciplinary team; this, together with the exclusion criteria (FVC of less than 1L), may explain the low mortality rate at 30 days. On the contrary, while the mortality rate in our series was lower, the number of patients presenting major complications was higher than that reported by other studies.6,10,12
The 2 reported cases of pneumonia occurred in patients who received conscious sedation for the PEG. Sedation may have favoured aspiration of oropharyngeal secretions due to the decreased cough reflex. The other 2 complications, gastrointestinal haemorrhage and perforation of the liver by the gastrostomy tube, are infrequent, unpredictable, and difficult to avoid. Jaw dislocation resolved easily. Minor complications were very frequent (36.7% of patients) but resolved quickly and easily. In all likelihood, the high prevalence of this type of complication is due to the nonspecificity of the criteria used to define minor complications during data collection.
Survival time showed no statistically significant differences associated with age, FVC, albumin level, or type of ALS onset; we are therefore unable to demonstrate that any of these parameters may represent an independent factor associated with the development of complications after the intervention. However, the number of major complications was surprisingly low, preventing analysis with sufficient statistical power. Other authors have shown the importance of age, FVC, and previous hospital stays9,13 as independent factors influencing mortality and morbidity rates associated with PEG. On the contrary, as mentioned earlier, sedation during the procedure seems to play a role in the development of complications. These results support those previously obtained by Mathus-Vliegen et al.10 in their series of 55 patients with ALS, who presented a mortality rate of 1.8% during the procedure, 3.6% during the first 24hours, and 11.5% at 30 days. In that study, death during the first 24hours was attributed to CO2 retention secondary to sedation and gastric hyperinflation. We should mention that Mathus-Vliegen et al.10 used midazolam, which has a longer half life and probably a higher risk of complications than propofol. Chio et al.3 obtained similar results in a comparative study between radiological and endoscopic gastrostomy, reporting a mortality rate of 4.3%, associated in this case with respiratory failure.
Our study has several main limitations. Firstly, a very long time elapsed between the first and the last gastrostomy of the series (16 years: 1997-2013). Also, this is a single-centre study in which PEG was always performed by the same physicians. Lastly, this is a prospective study, the first performed in Spain. To our knowledge, ours is the largest series of patients with ALS undergoing PEG during advanced stages of the disease.
In short, we can conclude that PEG is an efficient and relatively safe procedure for enteral nutrition of patients with ALS. In our series, respiratory failure and type of ALS onset were not found to be associated with post-intervention mortality and morbidity.
Conflicts of interestThe authors have no conflicts of interest to declare.
We would like to thank the team members of the Intensive Care Unit and the Endoscopy Unit at Hospital Universitario La Paz/Carlos III.
Please cite this article as: Perseguer JC, Seiz AM, Portales MR, Hernández JM, Mora Pardina JS, García-Samaniego J. La gastrostomía endoscópica percutánea en pacientes diagnosticados de esclerosis lateral amiotrófica: mortalidad y complicaciones. Neurología. 2019;34:582–588.