The neuropathic pain is the most habitual problem in the neuropathy induced by chemotherapy (NIQ) and the one that more interferes in the quality of life of the patients. His precocious detection turns out to be fundamental to reduce or to eliminate the problems that from this one stem. The aims of this study were: 1) determine the incident and NIQ's characteristics and neuropathic pain in patients with mieloma multiple (MM) treated with bortezomib, and 2) to evaluate the impact of the neuropathic pain in the activities of the daily life (AVD).
MethodAll the patients diagnosed of MM candidates for treatment with bortezomib attended in the Hospital Joan XXIII during 2013, took part. The participants were interviewed individually and were reporting on the presence, the characteristics and the impact of the pain, as well as of the adverse effects of the bortezomib.
ResultsThere took part 22 persons, of which NIQ presented the half, being the degree 2 the predominant one. The most habitual location of the neuropathic pain was hands and feet; it was appearing in a spontaneous and progressive way deteriorating in rest and during the night, with predominance of positive symptoms. The impact of the pain was reflected in all the AVD. The principal limitation was the disability to enjoy the life. The peripheral neuropathy occupied the first place in order of subjective importance for the patient followed by the fatigue and the constipation.
ConclusionsA proper assessment and early detection of neuropathic pain is critical to minimising its impact on the quality of life of patients.
El dolor neuropático es el problema más habitual en la neuropatía inducida por quimioterapia (NIQ) y el que más interfiere en la calidad de vida de los pacientes. Su detección precoz resulta fundamental para reducir o eliminar los problemas que de este se derivan. Los objetivos de este estudio eran: 1) determinar la incidencia y las características de NIQ y dolor neuropático en pacientes con mieloma múltiple (MM) tratados con bortezomib, y 2) evaluar el impacto del dolor neuropático en las actividades de la vida diaria (AVD).
MétodoTodos los pacientes diagnosticados de MM candidatos a tratamiento con bortezomib atendidos en el Hospital Joan XXIII durante 2013, participaron. Los participantes eran entrevistados individualmente e informaban sobre la presencia, las características y el impacto del dolor, así como de los efectos adversos del bortezomib.
ResultadosParticiparon 22 personas, de las cuales la mitad presentaron NIQ, siendo el grado 2 el predominante. La localización más habitual del dolor neuropático era manos y pies; aparecía de manera espontánea y progresiva empeorando en reposo y durante la noche, con predominio de síntomas positivos. El impacto del dolor se reflejó en todas las AVD. La limitación principal fue la incapacidad para disfrutar de la vida. La neuropatía periférica ocupó el primer lugar en orden de importancia subjetiva para el paciente seguido de la fatiga y el estreñimiento.
ConclusionesUna adecuada evaluación y detección precoz del dolor neuropático es fundamental para minimizar su impacto en la calidad de vida del paciente.
Multiple myeloma (MM) is the second most frequent haematologic neoplasia, after lymphoma, accounting for approximately 1% of all cases of cancer in Spain1 and the rest of Western Europe.2 It is estimated that 10% to 13% of all patients with haematologic neoplasia have MM, with an incidence of 3 to 5 cases per 100000 person-years in Spain,1 a rate somewhat lower than that reported for Europe (4-6 cases per 100000 person-years).
The introduction of bortezomib as a treatment for MM has significantly improved the life expectancy of these patients. However, this drug is associated with a significant risk of peripheral neuropathy.3-5 The neurotoxic effect of this drug is caused by a number of mechanisms that affect axonal transport and cause inflammatory damage to sensory neurons.6 In addition to other pathophysiological mechanisms, such as increased expression of inflammatory mediators, primarily cytokines7 (tumour necrosis factor8 or interleukin-18,9), we should also mention such other mechanisms as genetic predisposition to the disease and changes in ion channels and intracellular signalling.7 As a result, neuropathic pain is the main clinical expression of chemotherapy-induced peripheral neuropathy (CIPN) associated with bortezomib.10 In CIPN, small nerve fibres conveying temperature and pain information (unmyelinated C fibres and thinly myelinated Aδ fibres) undergo early, selective degeneration (small fibre neuropathy [SFN]).5,11 At more advanced stages, the disease affects the entire peripheral nerve, including thicker myelinated fibres (Aß fibres),11,12 leading to severe sensory deficits (vibration and tactile hypoaesthesia, mixed fibre neuropathy [MFN]), and can even involve motor fibres, causing impaired mobility.3,12,13 This pain is sometimes difficult to describe; it is normally intense, stabbing, or burning, and may be accompanied by a tingling sensation or anodynia. This type of pain interferes with sleep and the activities of daily living (ADL) and can be highly incapacitating.
Neuropathic pain is therefore of great importance, both as an early marker of CIPN associated with bortezomib, and because of its great impact on quality of life.14 It affects multiple functional and emotional aspects of a patient's main daily living areas, decreasing sleep quality15,16 and negatively affecting mood,17-19 mobility,3,18,20 and social interaction.19 However, neuropathic pain is difficult to characterise, since on occasion it is non-specific or disproportionate to the objective data on the patient's neuropathy. This difficulty is compounded by the fact that neuropathic pain is similar to or adds to other types of pain, such as bone pain due to pathological fractures. Therefore, specialised care and staff training are required. Furthermore, no easy-to-administer scales assessing both neuropathic pain and CIPN are available to date.
The purpose of our study is twofold: to identify and describe neuropathic pain in a group of patients diagnosed with MM and treated with bortezomib, and to evaluate the impact of pain on ADLs. In line with previous studies, we hypothesised that a significant percentage of patients would report neuropathic pain and that this would be associated with the presence of significant limitations in all ADLs.
MethodsParticipantsAll adult patients diagnosed with MM, receiving intravenous bortezomib (Velcade®), and treated at Hospital Universitario Joan XXIII, in Tarragona, in 2013, were considered for inclusion in our study. The inclusion and exclusion criteria were as follows:
Inclusion criteria: being 18 or older, having a diagnosis of MM, and being eligible for treatment with bortezomib.
Exclusion criteria: presenting cognitive or psychiatric alterations that may interfere with proper comprehension of interview questions, experiencing severe complications of the disease, having been admitted to the intensive care unit, or having a history of polyneuropathy or diabetes mellitus.
All participants were included in the study after undergoing haematological diagnosis in their first treatment visit (at the haematology day centre at Hospital Universitario Joan XXIII), agreeing to participate in the study, and signing informed consent forms. The study was approved by our centre's Ethics Committee.
Variables and measurement instruments- -
Descriptive and sociodemographic data: we recorded each patient's age and sex. Medical histories were reviewed to gather data about the type of chemotherapy used for each patient.
- -
Degree of neuropathy: we used the World Health Organization Common Toxicity Criteria for Peripheral Neuropathy, which establishes 5 different grades: grade 0, no symptoms of neuropathy; grade 1, paraesthesia or weakness but no pain or function loss; grade 2, pain affecting function but not interfering with ADLs; grade 3, pain interfering with ADLs; and grade 4, motor impairment and/or disabling pain.21
- -
Presence of neuropathic pain: we used the Spanish-language version of the Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) pain scale,22 which identifies neuropathic pain by assessing symptoms in 6 dimensions. Scores higher than 12 indicate a high likelihood of neuropathic pain.
- -
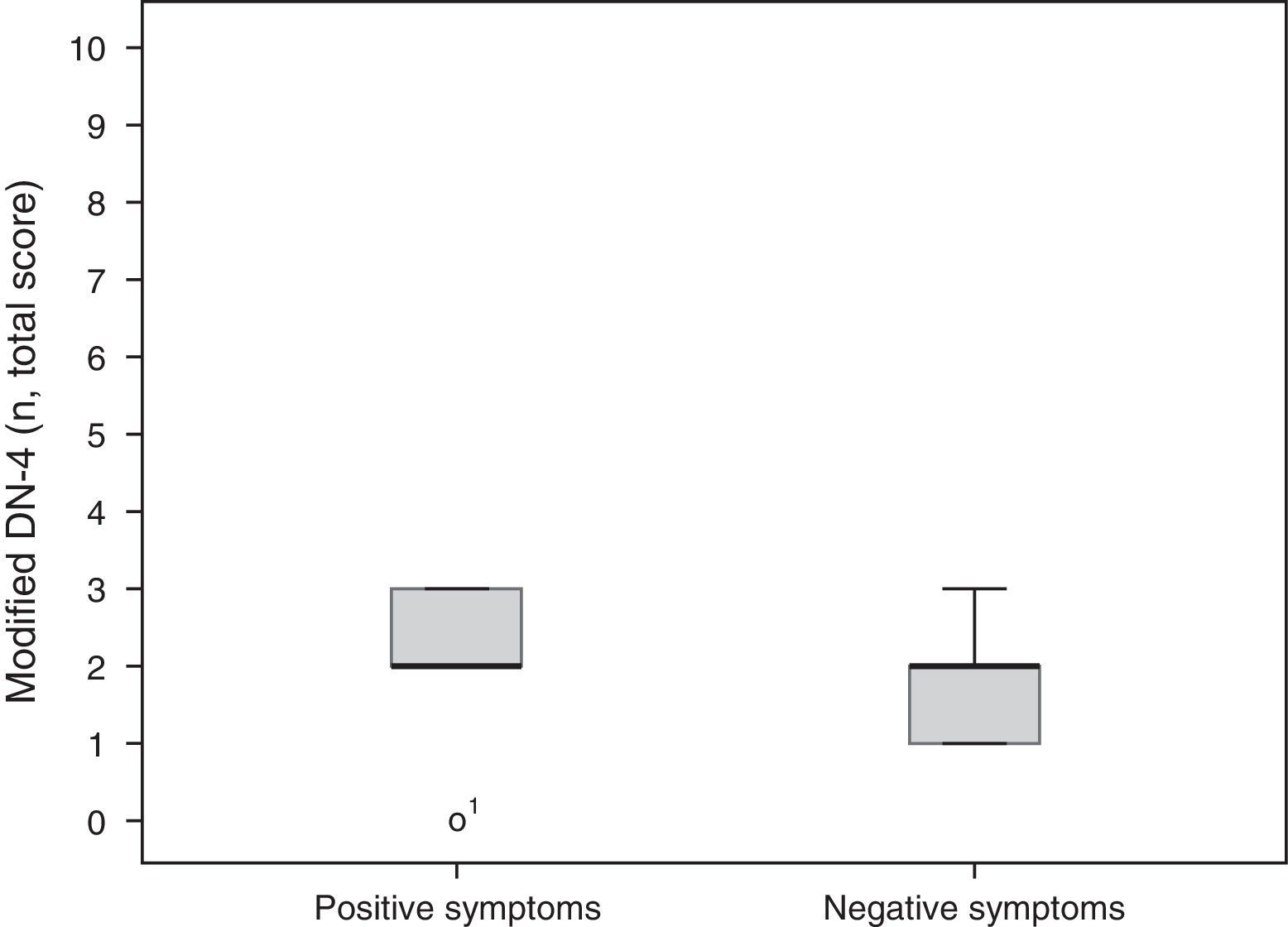
Symptom characterisation and clinical examination of neuropathic pain: we used the Spanish-language version of the Neuropathic Pain Diagnostic Questionnaire (DN-4),23 which evaluates symptoms of neuropathic pain and the main signs of sensory neuropathy with 4 items: 1) pain characteristics: 1.1) burning, 1.2) painful cold, 1.3) electric shocks; 2) sensory symptoms: 2.1) tingling, 2.2) pins and needles, 2.3) numbness, 2.4) itching; 3) sensitivity: 3.1) hypoaesthesia to touch, 3.2) hypoaesthesia to prick; and 4) presence of dynamic mechanical allodynia. The neuropathy examination also assessed the presence or absence of Achilles reflexes. Therefore, assessment of the signs and symptoms included in the DN-4, combined with evaluation of presence or absence of Achilles reflexes, was regarded as the modified DN-4. We classified as “positive” symptoms those assessed by items 1.1, 1.2, 3.2, and 4 of the DN-4, which were regarded as the most representative symptoms of SFN. “Negative” symptoms, compatible with mixed or global involvement of sensory nerve fibres (mixed peripheral neuropathy [MPN]), were assessed with items 2.3 and 3.1; an additional point was scored for each absent Achilles reflex (right or left). We thus determined whether patients met at least 3 of the 4 “positive” (SFN) or “negative” (MPN) symptoms. This classification did not include items 1.3, 2.1, 2.2, or 2.4, since these assess non-specific symptoms shared by both clinical entities.
- -
Impact of pain on ADLs: we administered the Spanish-language version of the Brief Pain Inventory.24 This questionnaire includes 11 items evaluating the impact of pain on different ADLs on a numeric scale from 0 (no pain/no impact on ADLs) to 10 (worst pain possible/major impact on ADLs). Pain intensity is classified as mild or no pain (0-3), moderate (4-6), or severe (≥7).
- -
Pain-related disability: the Spanish-language version of the Oswestry Disability Index was used.25 This index is obtained by dividing the total score of the questionnaire by the number of sections answered and multiplying by 100. Values of 0% to 20% indicate minimal disability, 21% to 40% moderate disability, 41% to 60% severe disability, and 61% to 80% indicates that the patient is unable to work or perform ADLs, and that an intervention may be required. Values over 80% should be interpreted with caution as they may be indicative of symptom exaggeration.26
- -
Symptoms regarded by the patient as adverse effects were recorded at the end of the treatment. Evaluating quality of life based on subjective perceptions is common practice.27,28 We enquired about the symptoms described in the literature: nausea and/or vomiting, diarrhoea, constipation, fatigue, anorexia, and pain. Furthermore, the last question was open-ended so that patients could report any additional symptoms that were not listed on the scale. Participants were asked to assess the impact of these symptoms on quality of life on a scale from 0 (no impact) to 10 (greatest possible impact).
We conducted a descriptive analysis of data (mean age±SD, age range, sex distribution, and diagnosis). The Kruskal-Wallis test was then used to compare the different groups of neuropathy. The association between disability and the degree of neuropathy was assessed with the Spearman correlation coefficient. Statistical analysis was performed using SPSS, version 15.
ResultsA total of 22 adults who had been diagnosed with MM and were undergoing treatment with bortezomib participated in the study. Half of the patients developed a significant degree of neuropathy; 9 of these (41% of the total sample) completed the study and the remaining 2 died before study completion. Four patients were administered thalidomide during the study period. In one of these, Velcade® was replaced by thalidomide due to onset of peripheral neuropathy and severe neuropathic pain. In the remaining 3 patients, both drugs were combined from the time chemotherapy was started, although at low doses. All patients with CIPN (n=14) showed a similar MM stage and degree of bone damage (presence or absence of pathological fractures). Eleven of these (79%) had bone pain. Additional patient characteristics are listed in Table 1.
Participants’ characteristics.
| Participants (N) | 22 |
|---|---|
| Mean age, years±SD | 63.8±11.7 |
| Sex, n (%) | |
| Men | 11 (50%) |
| Women | 11 (50%) |
| Neuropathy | |
| Yes | 11 |
| No | 11 |
| Symptom onset | 100% (2nd cycle) |
| Neuropathy grade (0-4) | |
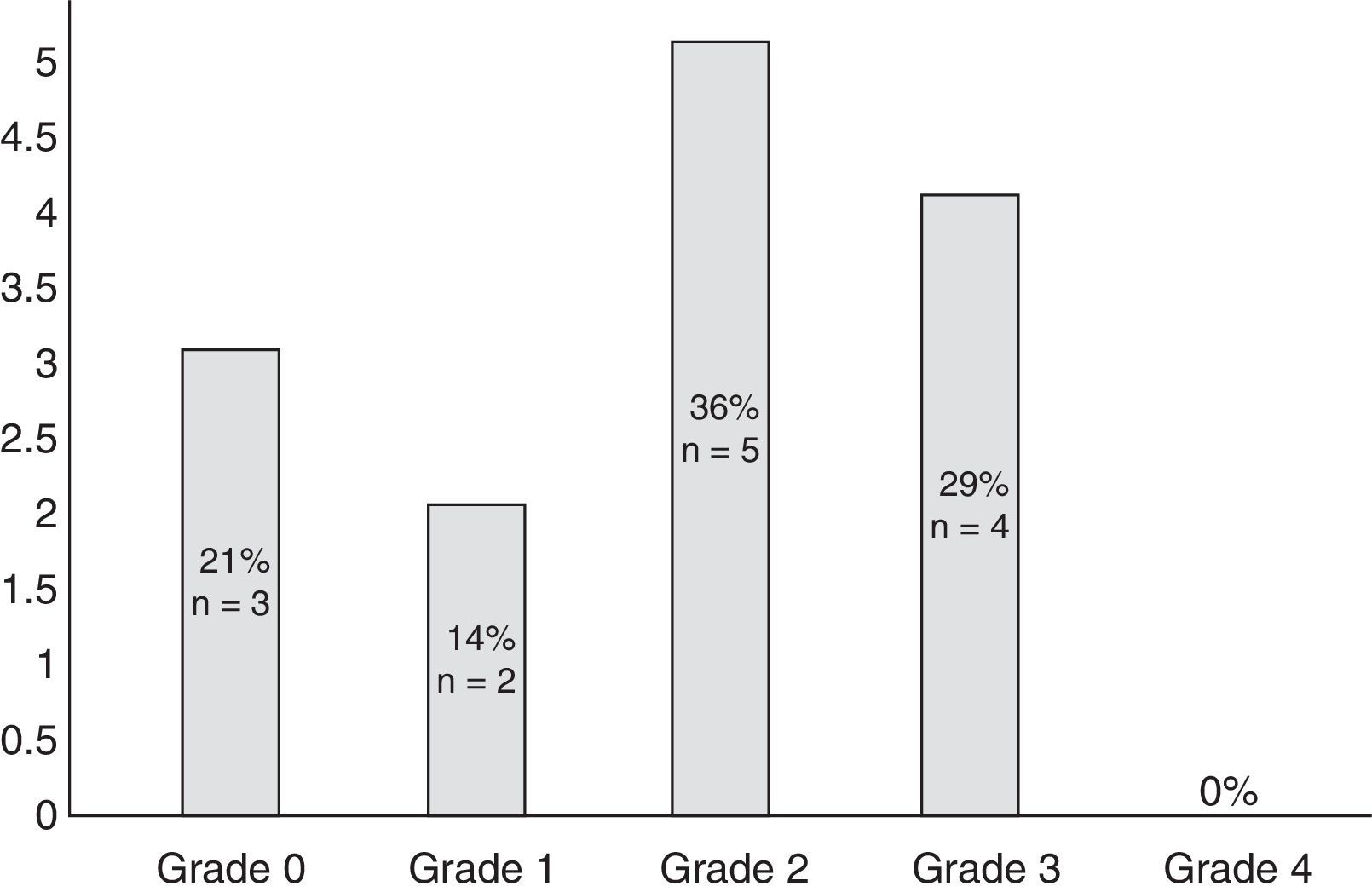
| 1 | 2 (14%) |
| 2 | 5 (36%) |
| 3 | 4 (29%) |
| 4 | 0 (0%) |
| Disability (0%-100%) | |
| Total no. of participants: 9 | 50.88±25.22 |
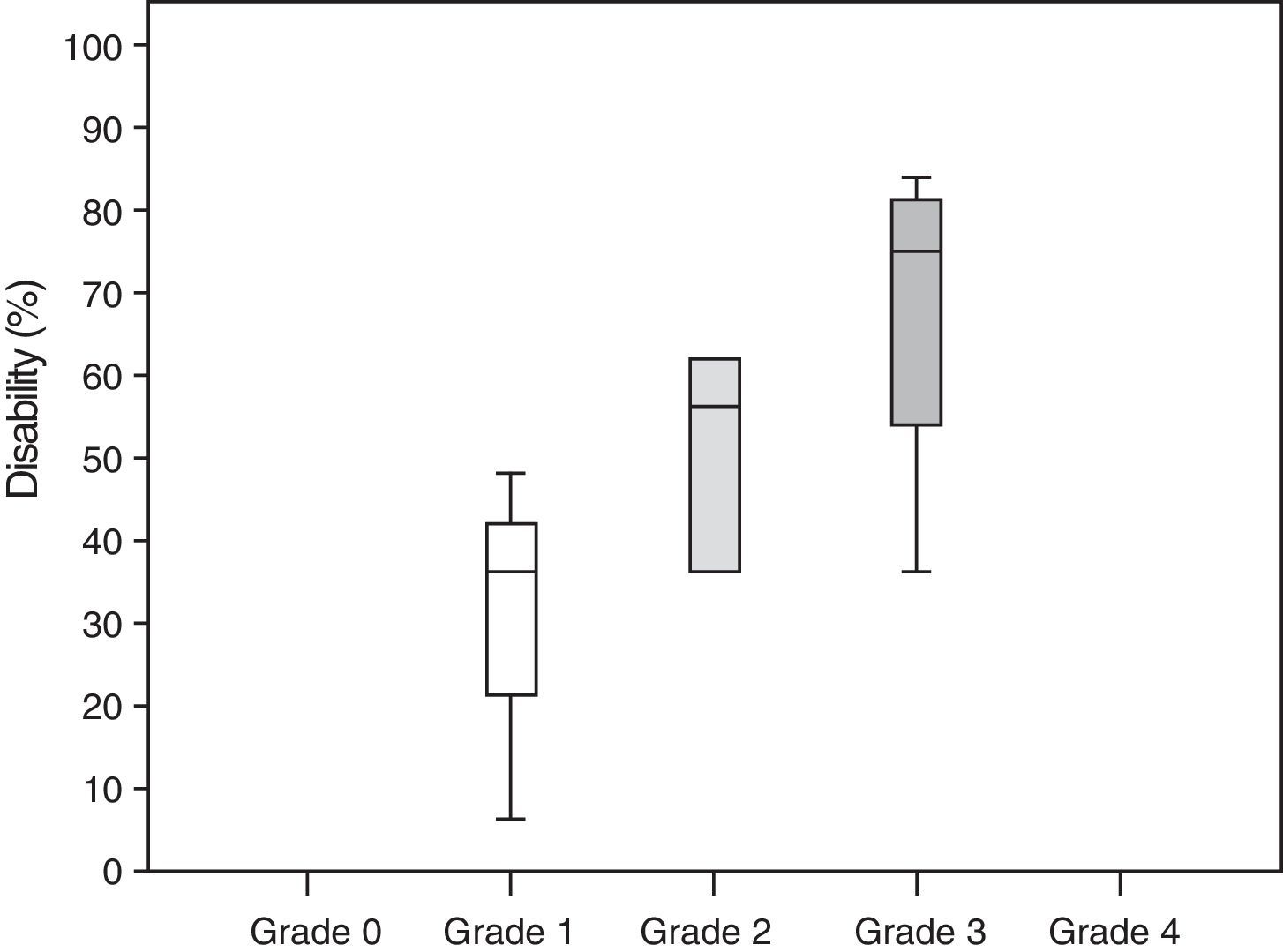
| Grade 1 neuropathy | 30±21.6 |
| Grade 2 neuropathy | 49±18.4 |
| Grade 3 neuropathy | 67±21.6 |
| Grade 4 neuropathy | 0% |
| DN-4 (nerve fibre involvement) | |
| SFN | 5 (56%) |
| MPN | 2 (22%) |
| SFN=MPN | 2 (22%) |
| Pain intensity (0-10) | |
| Mean maximum pain intensity | 7.8±2.5 |
| Mean minimum pain intensity | 2.4±1.6 |
| Mean pain intensity | 4.5±2.3 |
| Location | |
| Lower limbs (toes and soles) | 9 (100%) |
| Upper limbs (hands) | 5 (56%) |
| LANSS (0-24) | |
| Total no. of participants: 9 | 10.11±4.22 |
| Grade 1 neuropathy | 8.33±3.51 |
| Grade 2 neuropathy | 14.5±6.36 |
| Grade 3 neuropathy | 9.25±2.98 |
| Grade 4 neuropathy | 0% |
SD: standard deviation; N: number of patients; SFN: small fibre neuropathy; MPN: mixed peripheral neuropathy.
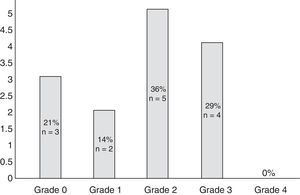
Eleven participants (50%) met the WHO criteria for peripheral neuropathy; in all of these patients, symptoms appeared after the second cycle of bortezomib. Fig. 1 classifies patients by degree of neuropathy. Grade 2 was the most common (n=5; 36%). Pain usually affected the toes and the soles of the feet (100% of the patients reported pain in these locations) and, to a lesser extent, the upper limbs, especially the hands (56%). No significant neuropathy was identified in 3 patients (14%) after treatment was completed (grade 0 of the WHO scale). In the remaining participants (n=8; 36%), neuropathy and/or its association with the drug could not reliably be determined.
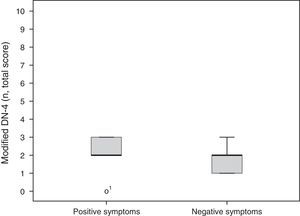
A total of 5 participants (56%) mainly had positive symptoms (modified DN-4 2.2±0.97), whereas 2 patients (22%) predominantly had negative symptoms (modified DN-4 1.7±0.66). These results are shown in Fig. 2. Two patients had similar scores for both SFN and MPN items. No statistically significant differences were found between groups for degree of neuropathy or level of disability according to the Oswestry Disability Index (Kruskal-Wallis H [2]=0.17, P=.92 and H [6]=3.2, P=.78, respectively).
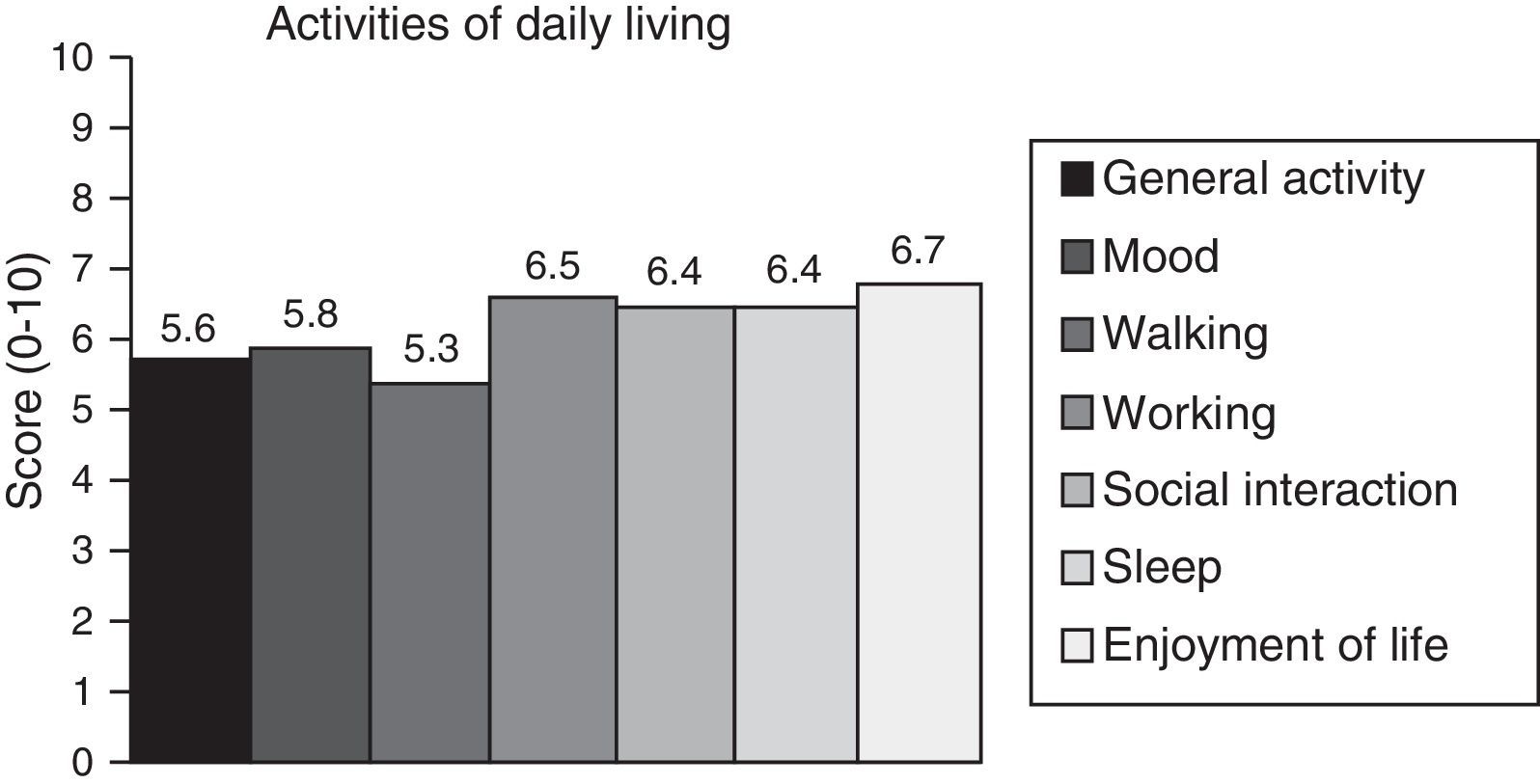
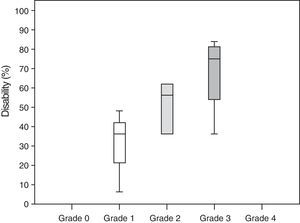
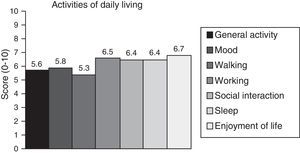
Neuropathic pain: impact on activities of daily livingPain had a significant or very significant impact on ADLs. Of all ADLs included in the study, neuropathic pain had the greatest impact on ability to enjoy life and the least pronounced impact on walking ability. Regarding the degree of neuropathy, our results showed a trend towards statistical significance (Spearman rho=0.65, P=.06); the higher the degree of neuropathy, the more severe the disability (Fig. 3). Furthermore, neuropathic pain was observed to have an impact on ADLs even when pain intensity and the degree of neuropathy were low (pain intensity <4, grade 1 of the WHO scale). Fig. 4 shows the impact of pain on ADLs.
Other adverse effects of bortezomibPeripheral neuropathy and fatigue were the main adverse effects of bortezomib in our sample (78% and 67%, respectively). Other adverse effects were reported to a lesser extent: constipation (55%), diarrhoea (44%), anorexia (33%), nausea and vomiting (22%), and sweating (11%).
DiscussionThis study has evaluated the presence of neuropathic pain, its characteristics, and its impact on ADLs in a sample of adult patients with MM undergoing treatment with bortezomib. Our results show that neuropathic pain is a frequent complication in these patients and has a negative impact on ADLs in patients treated with bortezomib.
The percentage of patients with CIPN associated with bortezomib in our sample (49%) is similar to those reported in the literature (30%-60%).10,29,30 This type of neuropathy is mainly sensory; neuropathic pain and other “positive” symptoms are the symptoms most frequently detected during examination. Neuropathic pain may therefore be considered an indicator of the toxicity of this drug.11 Various neurophysiological and histological techniques have been developed to precisely determine the onset of axonal damage.31 However, these techniques can be complex. The use of such clinical scales as those used in this study may therefore be of assistance in this process and provide a valid approach using the descriptors of the DN-4 scale and other factors for evaluating neuropathy, such as Achilles reflexes. Although our results are satisfactory, further studies with larger sample sizes are necessary to confirm their validity and reliability.
According to our study, neuropathic pain has a significant impact on ADLs. In fact, our results confirm a strong association between neuropathic pain and disability, with disability rates of 30% in patients with grade 1 neuropathy and up to 67% in those with grade 3 neuropathy.
The parameter most severely affected by neuropathic pain was ability to enjoy life, although such other areas as functionality, night-time sleep, and mood have also been found to be significantly altered in these patients. However, although these areas may be affected by other disease-related factors, properly evaluating different areas such as sleep, physical activity, or mood may help establish more suitable treatments to minimise the impact on patients’ quality of life. As shown in our study, even low degrees of neuropathy (≤WHO grade 2) may be associated with significant disability.
Among all the reported adverse effects of bortezomib, peripheral neuropathy is the most disabling.32,33 In fact, in our study this adverse effect was considered the most disabling by the participants themselves. Long-term disability associated with CIPN, including bortezomib-induced disability, has a negative impact on quality of life, which explains the insistence on the development of neuroprotective agents and early diagnosis of neurotoxicity. In line with this idea, for example, physicians may develop an assessment protocol to detect neuropathy at all stages of treatment, follow up neuropathic pain symptoms, and adapt treatment to each patient's needs. Further research should aim to identify other risk factors that have not yet been defined and which may be helpful in designing more personalised treatments. This information may also be useful in designing specific support programmes to improve the quality of life of these patients once treatment is completed.
Our study is not exempt from certain limitations. First, the occurrence of acute complications and even patient drop-outs during the study period due to disease progression or death may prevent us from generalising our results. However, we hypothesise that these factors may have had little to no effect, given that our results are consistent with those reported in the literature. Second, the cross-sectional nature of this study prevents us from determining whether residual neuropathy is permanent or resolves at a later time. Future research should focus on determining the degree of long-term impairment and disability affecting these patients. This information will be helpful for designing treatment plans or guidelines to minimise the impact of neuropathy on patients’ ADLs and quality of life. Lastly, the lack of data from other neurophysiological (nerve conduction studies or thermal threshold testing) or histological studies (study of cutaneous innervation) conducted before or after treatment makes it impossible to determine the precise percentage of asymptomatic patients who developed subclinical peripheral neuropathy.
Despite these limitations, our study further emphasises the importance of assessing neuropathic pain in cancer patients undergoing chemotherapy, especially with bortezomib. We recommend conducting a baseline assessment using easy, quick-to-administer tools for early detection of the initial symptoms of neuropathy. Likewise, analysing ADLs in these patients helps clearly identify the most affected areas with a view towards designing specific preventive treatments or strategies. The limitations perceived by patients themselves with regard to the main adverse effects underscore the need to train patients to identify and report initial symptoms as early as possible. Our results may help the healthcare professionals managing these patients to adapt drug administration protocols to their individual characteristics and their objective and perceived needs.
FundingThis study has received no funding of any kind.
Conflicts of interestThe authors have no conflicts of interest to declare.
We would like to thank the patients from the haematology department at Hospital Universitario Joan XXIII in Tarragona for participating in this study.
Please cite this article as: Vizcaíno SE, Casanova-Mollà J, Escoda L, Galán S, Miró J. Dolor neuropático en pacientes oncológicos en tratamiento con bortezomib. Neurología. 2018;33:28–34.