The main inhibitory neurotransmitter in the central nervous system is γ-aminobutyric acid (GABA). Autoimmunity against glutamic acid decarboxylase (GAD), a key element in the synthesis of GABA from glutamic acid, selectively inhibits GABAergic neurotransmission, causing such neurological conditions as stiff person syndrome, progressive encephalomyelitis with rigidity and myoclonus, epilepsy, and cerebellar ataxia. It may be associated with neoplasms, autoimmune polyglandular syndrome, type 1 diabetes mellitus, and autoimmune thyroiditis.1–3 We present a case of myoclonus-dystonia and cerebellar ataxia in association with anti-GAD autoimmunity. The study was approved by our local healthcare research ethics committee, and the patient gave written informed consent.
Our patient was a 41-year-old man who 14 years earlier presented acute extension contracture of the right upper limb, which lasted several weeks. Sequelae consisted of involuntary movements of the hand and flexion of the wrist, which subsequently also appeared in the left upper limb. Symptoms remained stable until 2 years ago, when the patient’s condition progressively worsened despite treatment with baclofen, tizanidine, and oxcarbazepine; the patient was left unable to write or use utensils.
The physical examination revealed dystonic upper limb posture, with arms in adduction and elbows in extension, and forced flexion of the wrists, and sudden, asynchronous jerks predominantly affecting distal muscles, both spontaneous and provoked by voluntary movement; the left lower limb showed abnormal posture in external rotation. Finger-to-nose and heel-to-knee tests showed dysmetria, stance and gait appeared broad-based, and the patient was unable to walk in tandem. During admission, the patient was clinically stable.
A blood analysis revealed anti-GAD65 antibodies at a titre of >1:30 000. The analysis of the cerebrospinal fluid (CSF) detected a positive band for anti-GAD65 antibodies; no other antineuronal antibodies were detected in the serum or CSF.
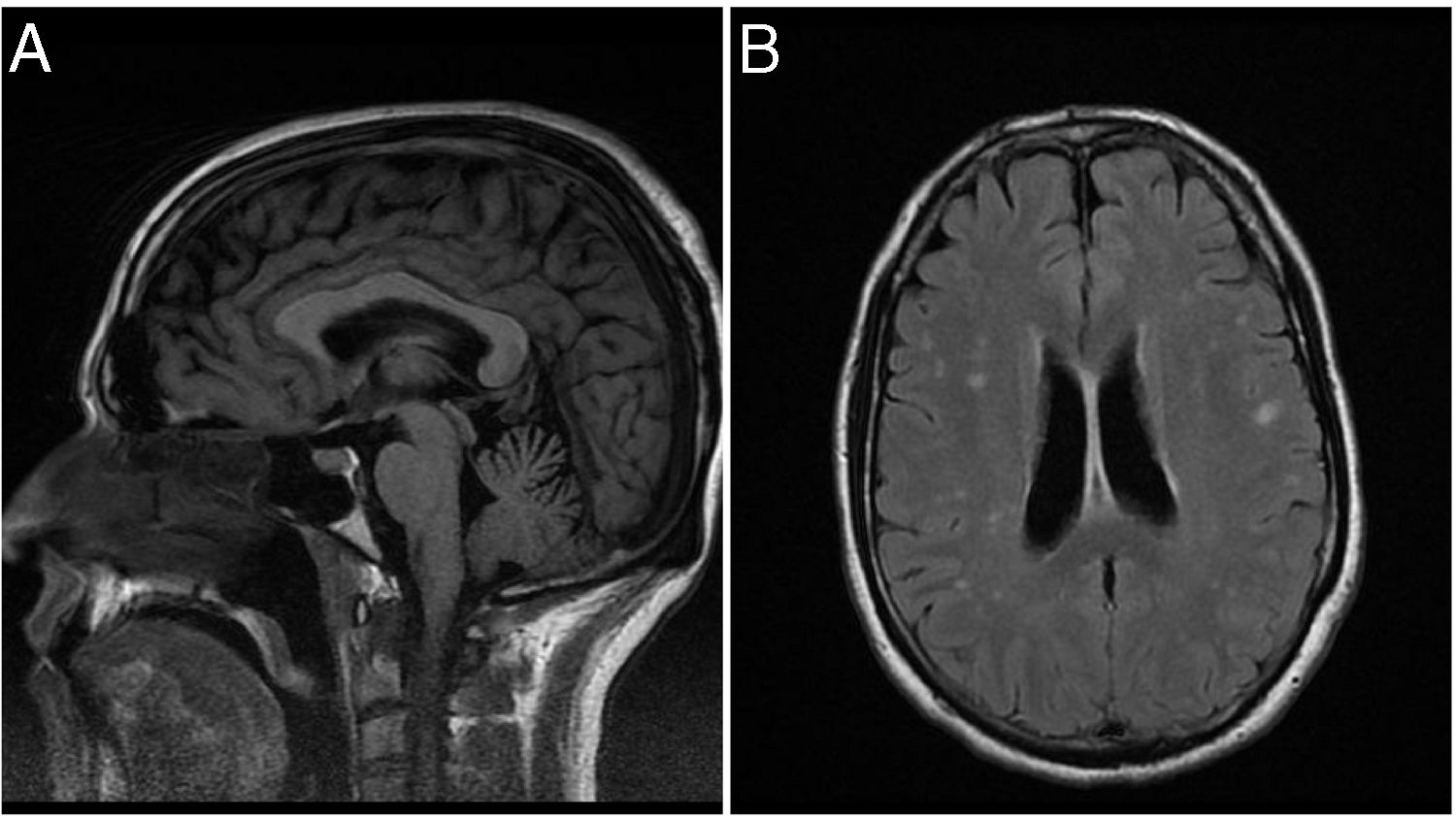
T1-weighted brain MRI sequences showed superior cerebellar vermis atrophy; T2-weighted FLAIR sequences displayed enlarged cerebellar fissures, reduced size of gyri, and hyperintensities in both cerebral hemispheres (Fig. 1). Chest and abdomen CT scan findings were normal. An electromyography study revealed involuntary muscle spasms in the right triceps, which were compatible with myoclonus; an electroencephalography study did not reveal epileptiform activity.
We administered 2 cycles of intravenous methylprednisolone at 1 g/day for 5 days, and one cycle of intravenous immunoglobulins at 0.4 g/kg/day for 5 days; no improvement was observed. After treatment with 2400 mg/day gabapentin, the patient was able to walk in tandem, the posture of the limbs improved, and the amplitude and frequency of myoclonus decreased. We then added tiagabine at 15 mg/day and the patient regained the ability to perform such manual tasks as writing or using cutlery.
Myoclonus-dystonia, also known as DYT11 dystonia, is a syndrome characterised by dystonic limb posture accompanied by muscular jerks; the syndrome follows an autosomal dominant inheritance pattern. It manifests in the first or second decade of life; location in the body varies, intensity fluctuates, and symptoms improve with alcohol consumption. Our patient’s symptoms resemble those of hereditary myoclonus-dystonia. Treatment consists of anticholinergics, pimozide, and tetrabenazine.4–6 The improvement observed with gabapentin, which increases GABA concentration in nervous tissue in healthy volunteers,7 and tiagabine, a synaptic GABA reuptake inhibitor,8 may suggest a GABA deficiency, as this protein is abundant in the basal ganglia.9
Ataxia secondary to anti-GAD autoimmunity is explained by the inhibition of GABAergic neurotransmission due to the loss of Purkinje cells,1,2,10 which use GABA as a neurotransmitter.11 Gabapentin has been shown to improve ataxia in late cortical cerebellar atrophy,12 in which the loss of Purkinje cells causes selective reduction of GABA in the dentate nuclei and CSF.13,14 Also, in a case of adult-onset GM2 gangliosidosis, cerebellar ataxia improved with administration of tiagabine12; the drug also improved ataxia associated with anti-GAD autoimmunity in our case.
Initial treatment for anti-GAD autoimmunity consists of corticosteroids or immunoglobulins, followed by immunosuppressants for maintenance of remission.3,15 This immunosuppressive treatment was probably ineffective because the disease was not at the active inflammation phase, as demonstrated by the absence of pleocytosis or high CSF protein levels and/or abnormal signal intensity on MRI, and the prolonged, gradual progression of symptoms. Nevertheless, ataxia, dystonia, and myoclonus considerably improved with gabapentin and tiagabine.
The association between anti-GAD autoimmunity and myoclonus-dystonia has not previously been reported in the literature, and it should be considered in sporadic cases of the disease. The improvement achieved with gabapentin and tiagabine in our case is noteworthy given the resistance to immunomodulatory treatment. Further studies with larger samples are therefore needed to confirm the efficacy of GABAergic agents in treating the different syndromes caused by anti-GAD autoimmunity.
Please cite this article as: Isern de Val Í, Gazulla J. Distonía mioclónica y ataxia cerebelosa en la autoinmunidad antiglutámico-descarboxilasa. Neurología. 2020;35:423–425.