Most individuals who have survived an acquired brain injury present consequences affecting the sensorimotor, cognitive, affective or behavioural components. These deficits affect the proper performance of daily living activities. The aim of this study is to identify functional differences between individuals with unilateral acquired brain injury using functional independence, capacity, and performance of daily activities.
MethodDescriptive cross-sectional design with a sample of 58 people, with right-sided injury (n=14 TBI; n=15 stroke) and left-sided injury (n=14 TBI, n=15 stroke), right handed, and with a mean age of 47 years and time since onset of 4±3.65 years. The functional assessment/functional independence measure (FIM/FAM) and the International Classification of Functioning (ICF) were used for the study.
ResultsThe data showed significant differences (P<.000), and a large size effect (dr=0.78) in the cross-sectional estimates, and point to fewer restrictions for patients with a lesion on their right side. The major differences were in the variables ‘speaking’ and ‘receiving spoken messages’ (ICF variables), and ‘Expression’, ‘Writing’ and ‘intelligible speech’ (FIM/FAM variables). In the linear regression analysis, the results showed that only 4 FIM/FAM variables, taken together, predict 44% of the ICF variance, which measures the ability of the individual, and up to 52% of the ICF, which measures the individual's performance. Gait alone predicts a 28% of the variance.
ConclusionsIt seems that individuals with acquired brain injury in the left hemisphere display important differences regarding functional and communication variables. The motor aspects are an important prognostic factor in functional rehabilitation.
La mayoría de las personas que han sobrevivido a un daño cerebral presentan secuelas que afectan a componentes sensoriomotores, cognitivos, emocionales o conductuales. Estos déficits repercuten en la correcta ejecución de actividades de la vida diaria. El objetivo de este estudio es identificar diferencias funcionales entre personas con daño cerebral adquirido (DCA) unilateral, mediante la independencia funcional, la capacidad y la realización de las actividades cotidianas.
MétodoDiseño transversal descriptivo con una muestra de 58 personas con lesiones derechas (n=14 TCE, n=15 ECV) e izquierdas (n=14 TCE, n=15 ECV), diestros, con una media de edad de 47 años y una media de 4±3.65 años de evolución. Las medidas utilizadas fueron la FIM FAM y la CIF.
ResultadosLos datos apuntan hacia la existencia de diferencias significativas (P<0.000) y un elevado tamaño del efecto (dr=0.78) en las estimaciones transversales, otorgando una menor restricción en la participación en las personas con lesión derecha. Las diferencias más destacadas se encuentran en las variables “recepción de mensajes hablados”, “escritura” y “habla inteligible”. Al hacer una regresión lineal, los resultados muestran que solo 4 variables de la FIM FAM predicen, en su conjunto, un 44% la variancia de la CIF que mide la capacidad del individuo y hasta un 52% de la CIF que mide la realización del sujeto. Tan solo la marcha predeciría un 28% de la variancia.
ConclusionesSe sugiere que las personas con DCA en el hemisferio izquierdo presentan importantes diferencias en variables funcionales y de la comunicación. Los aspectos motores representan un gran factor pronóstico para la rehabilitación funcional.
Acquired brain damage (ABD) is defined as an injury to the brain that occurs unexpectedly. The most recent data from Spain's National Statistics Institute1 show that there are 420,064 people with ABD living in Spain. A high percentage of cases (78%) were caused by stroke. Most patients who have survived an ABD present consequences affecting the sensorimotor, cognitive, affective, or behavioural components. These deficits directly affect occupational performance of activities of daily living (ADL).2 The patient's normal activities will not be carried out in the same way, and participation in these activities is reduced and may cease entirely. Therefore, one of the main targets of rehabilitation is achieving as much patient independence as possible, in both the home and community settings.
Some of the most frequently described impairments arising from unilateral brain injury are aphasia, apraxia, and right-sided motor limitations due to left hemisphere damage (LHD), and left-sided visuospatial impairments, hemineglect, and motor impairment due to right hemisphere damage (RHD).3–5 These deficits have unequal effects on a person's daily activities. Both lesions cause emotional and affective consequences. Titus et al.6 studied performance of ADL, although in a small selected sample (n=25) from one hospital including patients with cerebrovascular disease (CVD). Their performance was compared to results from patients with LHD and RHD. Both patient groups displayed deficits in the performance of ADL without any statistically significant differences between the two. However, results did show that deficits in patients with RHD mainly affected visuospatial perception, whereas patients with LHD had performance deficits. In 2011, a study conducted at the University of New Mexico7 included a control group of healthy adults (n=63) and a patient group with CVD in 2 subgroups by location: RHD (n=16) and LHD (n=30). Results for the LHD group showed aphasia as well as decreased independence; these patients needed more time to complete the task and their motor performance was poorer than the RHD group's. Both groups showed cognitive and motor impairments, but these were more pronounced in the LHD group. However, this study presents significant limitations, such as the context for performing functional tasks, which is far from real world. This factor might negatively impact performance by any of the groups.
As Bausela mentioned, Goldberg and Costa8 consider that the left hemisphere is more able to apply previously acquired codes, that is, completing tasks that are familiar or acquired, as in the case of ADL. From this, we extrapolate that a fully functioning left hemisphere can enable greater independence and participation in the daily activities that integrate a patient's lifestyle. According to Kielhofner, ‘we generate habits by consistently doing the same thing in the same context. What once required attention and concentration eventually becomes automatic’.9 Therefore, and assuming that everything to be learned requires increasingly complex cognitive processes, and therefore more effort, this task will become even more complicated if the hemisphere presumably responsible for it has suddenly been damaged.
The aim of this study is to identify functional differences between patients with unilateral ABD, CVDs, and right-sided vs left-sided head trauma. To this end, we have estimated functional independence, ability, and performance of daily activities.
Patients and methodsStudy designFor this cross-sectional study, we recruited 62 patients with unilateral brain damage who were classified into 2 subgroups, RHD and LHD. Patients were examined by a single clinician.
As required by current legislation, patients were asked to sign informed consent forms, and all of them consented and signed. Procedures conducted during the study complied with the ethical principles of the Declaration of Helsinki. They were reviewed by research committees at the comprehensive care centre for acquired brain damage (POLIBEA) and at the national centre of reference for acquired brain damage (CEADAC).
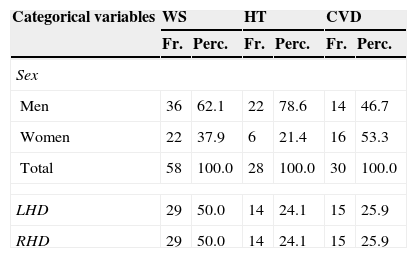
SampleThe study was conducted in the Madrid region. Fifty-two patients were from the POLIBEA centre and the remaining 10 were from the CEADAC centre. Inclusion criteria were as follows: being right-handed and older than 18 with a diagnosis of ischaemic CVD or head trauma and unilateral damage confirmed by a neurology department's report including an imaging study. The sample was selected by doctors and neuropsychologists working at the 2 centres named above. We excluded 4 patients based on the following exclusion criteria: progression time of less than one year, presence of other central nervous system conditions (tumours, anoxia, etc.), cardiorespiratory symptoms, severe brain damage, or scoring 6 or higher on Reisberg's Global Deterioration Scale (GDS),10 and/or a severely decreased level of consciousness or a score of 8 or less on the Glasgow Coma Scale (GCS).11 The final sample included 58 patients (36 men and 22 women) with a mean age of 47 years. All patients presented chronic impairment following a minimum period of one year since the event causing the lesion, and they had experienced no prior transient ischaemic lesions (Table 1).
Characteristics of patients with unilateral brain damage (n=58).
| Categorical variables | WS | HT | CVD | |||
|---|---|---|---|---|---|---|
| Fr. | Perc. | Fr. | Perc. | Fr. | Perc. | |
| Sex | ||||||
| Men | 36 | 62.1 | 22 | 78.6 | 14 | 46.7 |
| Women | 22 | 37.9 | 6 | 21.4 | 16 | 53.3 |
| Total | 58 | 100.0 | 28 | 100.0 | 30 | 100.0 |
| LHD | 29 | 50.0 | 14 | 24.1 | 15 | 25.9 |
| RHD | 29 | 50.0 | 14 | 24.1 | 15 | 25.9 |
| Continuous variables | M | SD | M | SD | M | SD |
|---|---|---|---|---|---|---|
| Age | 47 | 13.35 | 43 | 14.12 | 52 | 11.05 |
| Progression time, years | 4 | 3.65 | 5 | 4.15 | 4.20 | 3.05 |
| Age when damage occurred | 41 | 14.00 | 36 | 14.76 | 45.80 | 11.61 |
| GDS | 3 | 1.40 | 3.86 | 1.20 | 2.77 | 1.38 |
| GCS | 14.89 | 0.31 | 14.89 | 0.31 | – | – |
SD: standard deviation; Fr.: frequencies; WS: whole sample; Perc.: percentages; M: mean.
We selected the functional independence measure (FIM)12 and functional assessment measure (FAM)13 to measure the level of functional independence to perform ADL. Used together, these measures include 30 items that provide information mainly on motor and cognitive performance. Scores range from 1 to 7, with higher scores corresponding to better levels of functional independence. The assessment was performed based on observing task performance and on an interview.
The International Classification of Functioning, Disability and Health (ICF) is one of the classification systems of the World Health Organization, and it provides a wide range of information regarding health status.14 Our study analysed 23 domains that have been listed as some of the most relevant in the ADL context.15 Study methods were fundamentally based on interviews and direct observation. Scoring criteria followed the standards established by the ICF for the activities and participation component, since all items (or domains) belong to this component. For each item, we examined 2 of the 4 possible qualifiers: patient capacity and patient performance. Both qualifiers were assigned a numerical value, and the score ranges from 0 to 4 points: 0 (no problem), 1 (mild problem), 2 (moderate problem), 3 (severe problem), and 4 (complete problem). Minimum and maximum scores were 0 and 92, with higher scores indicating more impaired capacity and performance. The capacity qualifier describes the patient's actual ability to perform tasks without the help of any tools, technologies, or assistance by a third party. The performance qualifier describes the real daily experience of patients in their real settings with or without using support devices or products.
Cognitive impairment and level of consciousness were measured using the screening tests GDS10 and GCS,11 respectively. Imaging studies used to diagnose unilateral damage consisted of functional magnetic resonance images, computed tomography, and positron-emission tomography. These images were consulted for the sole purpose of selecting the sample.
The sole rater, an occupational therapist, administered the tests in the occupational therapy department ADL room at each of the institutions. This room is equipped with a kitchen, a bathroom, and objects used in normal domestic tasks, including an iron, a broom, and a washing machine. Rating performance was based on observations of the patient's activities in his/her normal setting, information provided in the interview, or the patient's description of his/her functional level.
Statistical analysisGiven the small size of the comparison subsamples, we used the non-parametric Mann–Whitney U test to study differences between independent subjects. Additionally, the effect size of the differences was estimated by transforming Cohen's d into a correlation coefficient (dr).16 Values of 0.20, 0.40, and 0.60 represent mild, moderate, and high effect sizes, respectively.
We first obtained descriptive data showing the frequency of the categorical variable, the mean, and the standard deviation (SD) of the remaining continuous variables. Reliability of the evaluation tools was estimated using the Cronbach α coefficient, which showed good reliability. Second, we analysed dependent variables as a cross-sectional performance study of all the patients included in comparison groups.
Data analysis was performed with SPSS v. 19 software by adding a syntax command for estimating effect size.
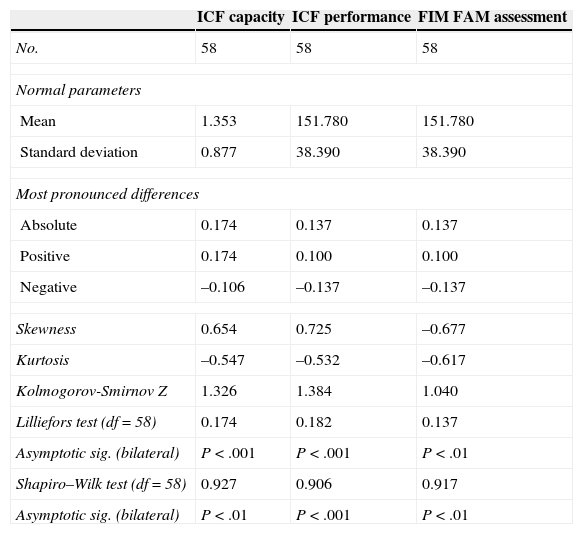
ResultsDescriptive data of the administered tests are listed in Table 2.
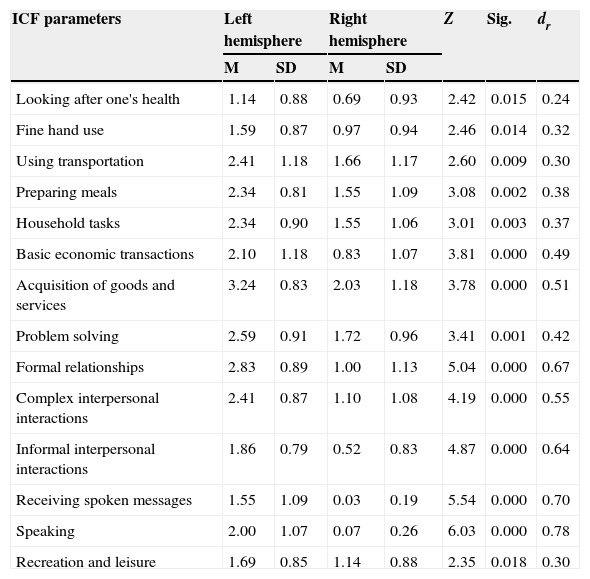
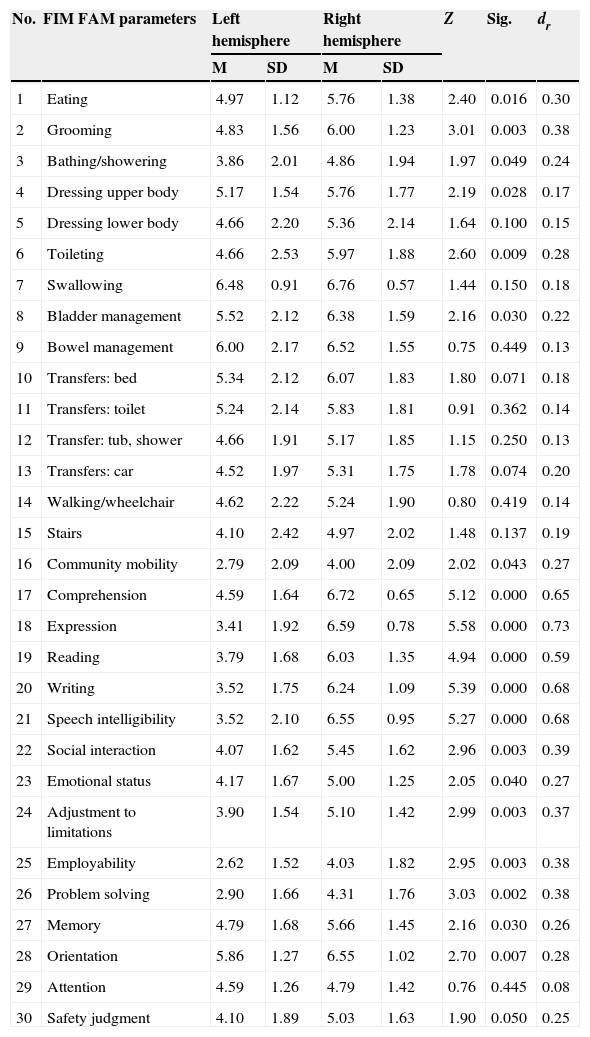
In the cross-sectional analysis of differences in variables between hemispheres, variables showing significant differences (P<.05) indicate less limited participation among patients with RHD, according to both the ICF assessment (Table 3) and the FIM FAM assessment (Table 4). The value of the effect size is moderate to high, which underscores the importance of these variables. The highest values were found for the variables ‘speech’, ‘receiving spoken messages’ (ICF variables), and ‘expression’, ‘writing’, and ‘speech intelligibility’ (FIM FAM variables).
Descriptive data of the administered tests and normality tests.
| ICF capacity | ICF performance | FIM FAM assessment | |
|---|---|---|---|
| No. | 58 | 58 | 58 |
| Normal parameters | |||
| Mean | 1.353 | 151.780 | 151.780 |
| Standard deviation | 0.877 | 38.390 | 38.390 |
| Most pronounced differences | |||
| Absolute | 0.174 | 0.137 | 0.137 |
| Positive | 0.174 | 0.100 | 0.100 |
| Negative | –0.106 | –0.137 | –0.137 |
| Skewness | 0.654 | 0.725 | –0.677 |
| Kurtosis | –0.547 | –0.532 | –0.617 |
| Kolmogorov-Smirnov Z | 1.326 | 1.384 | 1.040 |
| Lilliefors test (df=58) | 0.174 | 0.182 | 0.137 |
| Asymptotic sig. (bilateral) | P<.001 | P<.001 | P<.01 |
| Shapiro–Wilk test (df=58) | 0.927 | 0.906 | 0.917 |
| Asymptotic sig. (bilateral) | P<.01 | P<.001 | P<.01 |
ICF variables with significant differences between hemisphere groups.
| ICF parameters | Left hemisphere | Right hemisphere | Z | Sig. | dr | ||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | ||||
| Looking after one's health | 1.14 | 0.88 | 0.69 | 0.93 | 2.42 | 0.015 | 0.24 |
| Fine hand use | 1.59 | 0.87 | 0.97 | 0.94 | 2.46 | 0.014 | 0.32 |
| Using transportation | 2.41 | 1.18 | 1.66 | 1.17 | 2.60 | 0.009 | 0.30 |
| Preparing meals | 2.34 | 0.81 | 1.55 | 1.09 | 3.08 | 0.002 | 0.38 |
| Household tasks | 2.34 | 0.90 | 1.55 | 1.06 | 3.01 | 0.003 | 0.37 |
| Basic economic transactions | 2.10 | 1.18 | 0.83 | 1.07 | 3.81 | 0.000 | 0.49 |
| Acquisition of goods and services | 3.24 | 0.83 | 2.03 | 1.18 | 3.78 | 0.000 | 0.51 |
| Problem solving | 2.59 | 0.91 | 1.72 | 0.96 | 3.41 | 0.001 | 0.42 |
| Formal relationships | 2.83 | 0.89 | 1.00 | 1.13 | 5.04 | 0.000 | 0.67 |
| Complex interpersonal interactions | 2.41 | 0.87 | 1.10 | 1.08 | 4.19 | 0.000 | 0.55 |
| Informal interpersonal interactions | 1.86 | 0.79 | 0.52 | 0.83 | 4.87 | 0.000 | 0.64 |
| Receiving spoken messages | 1.55 | 1.09 | 0.03 | 0.19 | 5.54 | 0.000 | 0.70 |
| Speaking | 2.00 | 1.07 | 0.07 | 0.26 | 6.03 | 0.000 | 0.78 |
| Recreation and leisure | 1.69 | 0.85 | 1.14 | 0.88 | 2.35 | 0.018 | 0.30 |
FIM FAM variables showing significant differences between hemisphere groups.
| No. | FIM FAM parameters | Left hemisphere | Right hemisphere | Z | Sig. | dr | ||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | |||||
| 1 | Eating | 4.97 | 1.12 | 5.76 | 1.38 | 2.40 | 0.016 | 0.30 |
| 2 | Grooming | 4.83 | 1.56 | 6.00 | 1.23 | 3.01 | 0.003 | 0.38 |
| 3 | Bathing/showering | 3.86 | 2.01 | 4.86 | 1.94 | 1.97 | 0.049 | 0.24 |
| 4 | Dressing upper body | 5.17 | 1.54 | 5.76 | 1.77 | 2.19 | 0.028 | 0.17 |
| 5 | Dressing lower body | 4.66 | 2.20 | 5.36 | 2.14 | 1.64 | 0.100 | 0.15 |
| 6 | Toileting | 4.66 | 2.53 | 5.97 | 1.88 | 2.60 | 0.009 | 0.28 |
| 7 | Swallowing | 6.48 | 0.91 | 6.76 | 0.57 | 1.44 | 0.150 | 0.18 |
| 8 | Bladder management | 5.52 | 2.12 | 6.38 | 1.59 | 2.16 | 0.030 | 0.22 |
| 9 | Bowel management | 6.00 | 2.17 | 6.52 | 1.55 | 0.75 | 0.449 | 0.13 |
| 10 | Transfers: bed | 5.34 | 2.12 | 6.07 | 1.83 | 1.80 | 0.071 | 0.18 |
| 11 | Transfers: toilet | 5.24 | 2.14 | 5.83 | 1.81 | 0.91 | 0.362 | 0.14 |
| 12 | Transfer: tub, shower | 4.66 | 1.91 | 5.17 | 1.85 | 1.15 | 0.250 | 0.13 |
| 13 | Transfers: car | 4.52 | 1.97 | 5.31 | 1.75 | 1.78 | 0.074 | 0.20 |
| 14 | Walking/wheelchair | 4.62 | 2.22 | 5.24 | 1.90 | 0.80 | 0.419 | 0.14 |
| 15 | Stairs | 4.10 | 2.42 | 4.97 | 2.02 | 1.48 | 0.137 | 0.19 |
| 16 | Community mobility | 2.79 | 2.09 | 4.00 | 2.09 | 2.02 | 0.043 | 0.27 |
| 17 | Comprehension | 4.59 | 1.64 | 6.72 | 0.65 | 5.12 | 0.000 | 0.65 |
| 18 | Expression | 3.41 | 1.92 | 6.59 | 0.78 | 5.58 | 0.000 | 0.73 |
| 19 | Reading | 3.79 | 1.68 | 6.03 | 1.35 | 4.94 | 0.000 | 0.59 |
| 20 | Writing | 3.52 | 1.75 | 6.24 | 1.09 | 5.39 | 0.000 | 0.68 |
| 21 | Speech intelligibility | 3.52 | 2.10 | 6.55 | 0.95 | 5.27 | 0.000 | 0.68 |
| 22 | Social interaction | 4.07 | 1.62 | 5.45 | 1.62 | 2.96 | 0.003 | 0.39 |
| 23 | Emotional status | 4.17 | 1.67 | 5.00 | 1.25 | 2.05 | 0.040 | 0.27 |
| 24 | Adjustment to limitations | 3.90 | 1.54 | 5.10 | 1.42 | 2.99 | 0.003 | 0.37 |
| 25 | Employability | 2.62 | 1.52 | 4.03 | 1.82 | 2.95 | 0.003 | 0.38 |
| 26 | Problem solving | 2.90 | 1.66 | 4.31 | 1.76 | 3.03 | 0.002 | 0.38 |
| 27 | Memory | 4.79 | 1.68 | 5.66 | 1.45 | 2.16 | 0.030 | 0.26 |
| 28 | Orientation | 5.86 | 1.27 | 6.55 | 1.02 | 2.70 | 0.007 | 0.28 |
| 29 | Attention | 4.59 | 1.26 | 4.79 | 1.42 | 0.76 | 0.445 | 0.08 |
| 30 | Safety judgment | 4.10 | 1.89 | 5.03 | 1.63 | 1.90 | 0.050 | 0.25 |
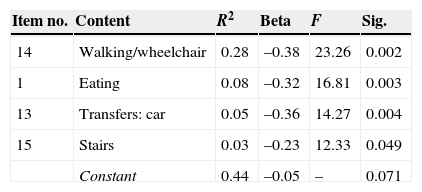
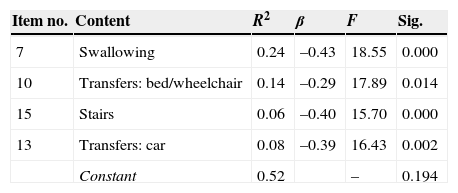
We analysed variance of the FIM FAM assessment as possible predictor of positive results on the ICF assessment to shed light on the significant relationship between variables and the sample. A linear regression analysis was performed using the following as predictor variables: FIM FAM items, time since lesion, age, and sex (transformed into dummy variables, values 1 and 2). Results show that only 4 FIM FAM variables, examined as a set, predict 44% of the variance on the ICF assessment of capacity (Table 5), and 52% of the variance on the ICF assessment of performance (Table 6).
Results from our study show a more pronounced deficit in the functional status of patients with LHD, as has been observed in previous studies. The main differences specifically involve variables related to communication (receiving spoken messages and speaking). These differences present a very high effect size for patients with LHD, which underscores their clinical relevance. This can probably be explained by the symptoms associated with LHD (language disorders).17–21 Functional rehabilitation programmes should take this into account, especially because neuroplasticity mechanisms can be stimulated to revert or compensate the lesion. These mechanisms are faster and more effective in both the damaged hemisphere and in homologous zones of the contralateral hemisphere when the rehabilitation programme intended to improve functionality is more intense and specific.20,22–27
Studies analysing the community integration of people with CVD and motor aphasia28,29 highlight that language disorders, together with other variables such as motivation, physical condition, decreased occupational performance, and advanced age, impact social participation in the long term. These findings suggest the importance of having intact speech abilities for proper integration in society. This concurs with findings by Sumathipala et al.,30 who stated that interactions with family and friends are key facilitators of functional improvement and integration for patients with brain damage. These findings correspond to differences in our study variables ‘formal relationships’ and ‘informal social relationships’, whose statistical significance and effect size are considerable.
In addition to the above, we have identified other variables belonging to the group of more complex activities related to instrumental skills: preparing meals, using transportation, completing household tasks and basic monetary transactions, and acquiring necessities (all variables from the ICF). If the analysis is performed with the FIM FAM assessment, the communication, social interaction, and community mobility parameters coincide, which highlights more basic parameters such as feeding, bathing, toileting, dressing, and grooming. Different studies31,32 suggest that mobility and social interaction are the areas showing the most impairment after an ABD. According to the results reviewed, mobility seems to be compromised in both groups with hemisphere damage, with the exception of fine hand use, considering that the differences on the ICF are statistically significant and show a large size effect for the LHD group. This may be due to the fact that the whole sample was right-handed: patients with RHD do not have to change dominant hands and/or retrain their motor skills, since the dominant hand retains a good functional level.
One of the most interesting findings in our study has to do with the predictor variables of the FIM FAM assessment. Results suggest that only 4 variables (walking, eating, car transfers, and stairs) predict 44% of the variance on the ICF assessment measuring capacity and up to 52% of the variance on the ICF assessment measuring performance. Furthermore, the variable ‘walking’ alone predicts 28% of variance, which indicates that if improvement is satisfactory, it will positively influence the remaining functional ADL. If the patient can move independently, eat, get into a car, and climb stairs unassisted, that level of independence will exert an influence on the patient's other activities (self-care, the more instrumental activities, and recreation and leisure activities). This position is coherent with the observation by DeJong et al.33 that more effective results obtained in gait training were correlated with positive outcomes for community mobility. These findings also highlight the importance of motor rehabilitation in achieving the best performance and occupational participation, which will promote independent performance of ADL.
Our study has identified significant differences in functionality between patients with RHD and those with LHD. However, other studies34,35 carried out in samples with similar characteristics did not find significant differences of any kind between the same groups (RHD and LHD). One explanation for the discrepancy could be that the above studies did not use specific scales to compare levels of autonomy. While the Bernspang and Fisher study34 did use a scale that measures functionality (AMPS), its sample included adults aged 60–92 years (mean age=73). This suggests that in addition to the lesion, deficits inherent to ageing may be present in both groups. As Fisher also observes in another article with similar characteristics,35 ageing is associated with a gradual decrease in ability to perform ADL. This reflection was also expressed by DeJong et al.33 Furthermore, according to Hernández-Muela et al.,36 neuroplasticity is greater during the earliest years of life and decreases gradually with age. Therefore, recovery at the age of 70 will not be as successful as at the age of 20.
This study has important limitations. The sample size limits our ability to draw general conclusions. Furthermore, we did not report the precise lesion localisation in every patient because the aim was to sort the sample by lesion hemisphere. Neuroimaging studies were used to determine lesion laterality; Miyai et al.37 found functional differences corresponding to lesion location in a sample of 46 subjects who had experienced stroke 3 months previously. However, as these patients were in the acute phase of recovery, changes can be subject to variability in neuroplasticity mechanisms.
Regarding the clinical implications of the data obtained, special emphasis must be placed on specific rehabilitation for patients since basic activities, such as walking or eating, predicts positive functional outcome in patients affected by ABD. Another important note is that patients with ABD probably need more intense interventions in the form of personalised treatment plans so as to offer patients optimum possibilities for recovery. Future studies should explore other variables related to the hemisphere affected by the lesion so as to implement other specific interventions that will foster improvements in the overall quality of treatments.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Huertas Hoyas E, Pedrero Pérez EJ, Águila Maturana AM, García López-Alberca S, González Alted C. Predictores de funcionalidad en el daño cerebral adquirido. Neurología. 2015;30:339–346.