Simultaneous facial diplegia, or acute bilateral facial nerve palsy, is an unusual condition.1,2 Unlike the unilateral form, facial diplegia is idiopathic in only 20% of cases.2 Intracranial dermoid cysts are infrequent, and their rupture even rarer; it may occur spontaneously, after cranial trauma, or as a complication of tumour resection.3,4
Our case involved a 25-year-old woman with a medical history of gestational diabetes. In December 2009 she experienced skin abscesses in the scapular and perineal regions and was treated with amoxicillin-clavulanic acid. Two months later the patient presented at the hospital with stupor, hypotension, hyperglycaemia (479mg/dL), metabolic acidosis (venous blood gas analysis: pH 6.87, pCO2 17mm Hg, pO2 33mm Hg, bicarbonate 1.6mmol/L), Kussmaul breathing, and severe dehydration associated with diabetic ketoacidosis, and was therefore admitted to the ICU. Meticillin-sensitive Staphylococcus aureus was isolated in pure culture from purulent exudate from the skin abscesses (the name of the antibiotic was changed from methicillin to meticillin in 2005). After the patient recovered consciousness she exhibited grade V bilateral facial paralysis according to the House-Brackmann Facial Nerve Grading System; all other results from the neurological examination were normal. The results of angiotensin-converting enzyme, serology, autoantibody, and tumour marker tests were all within normal limits. The patient showed positive anti-glutamate decarboxylase and anti-tyrosine phosphatase titres, 13% glycated haemoglobin, and microalbuminuria. A chest radiography, echocardiography, and abdominal ultrasound showed no noteworthy findings. A lumbar puncture disclosed clear cerebrospinal fluid (CSF) with an opening pressure of 12cm of water, 1 leucocyte/μL, 2 red blood cells/μL, elevated protein levels (115mg/dL), and hypoglycorrhachia (135mg/dL in CSF; glycaemia: 406mg/dL). Gram stain, CSF culture, cryptococcal antigen test, VDRL test, Ziehl-Neelsen stain, and mycobacteria culture yielded negative results.
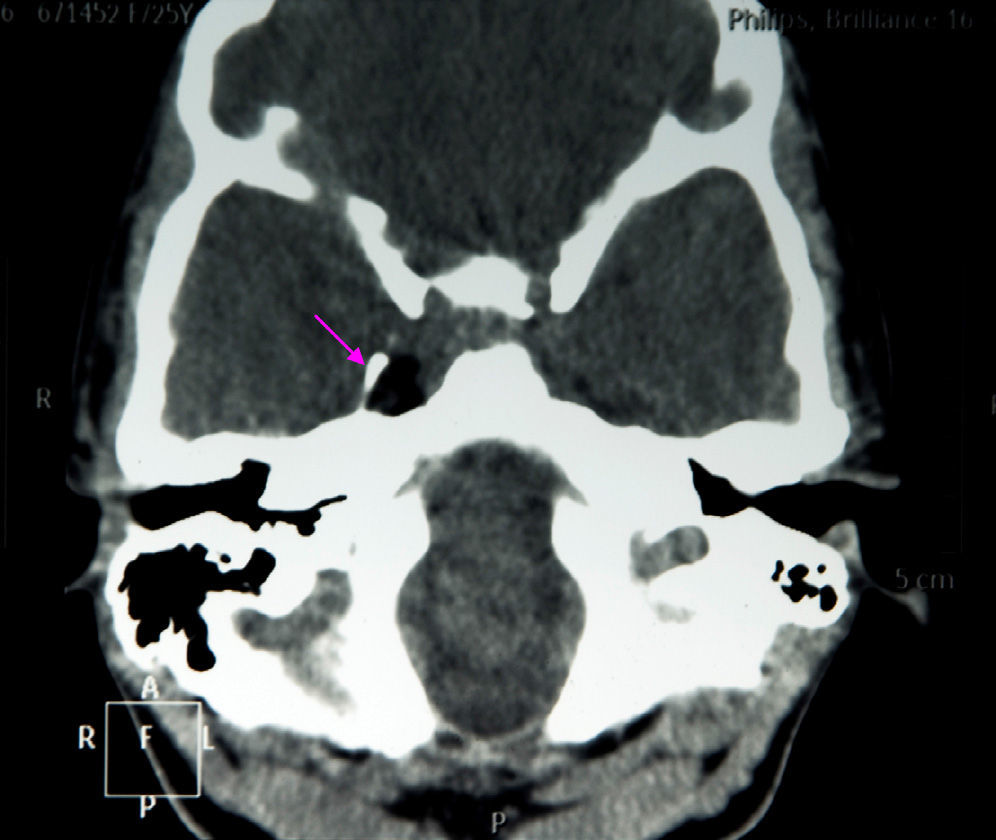
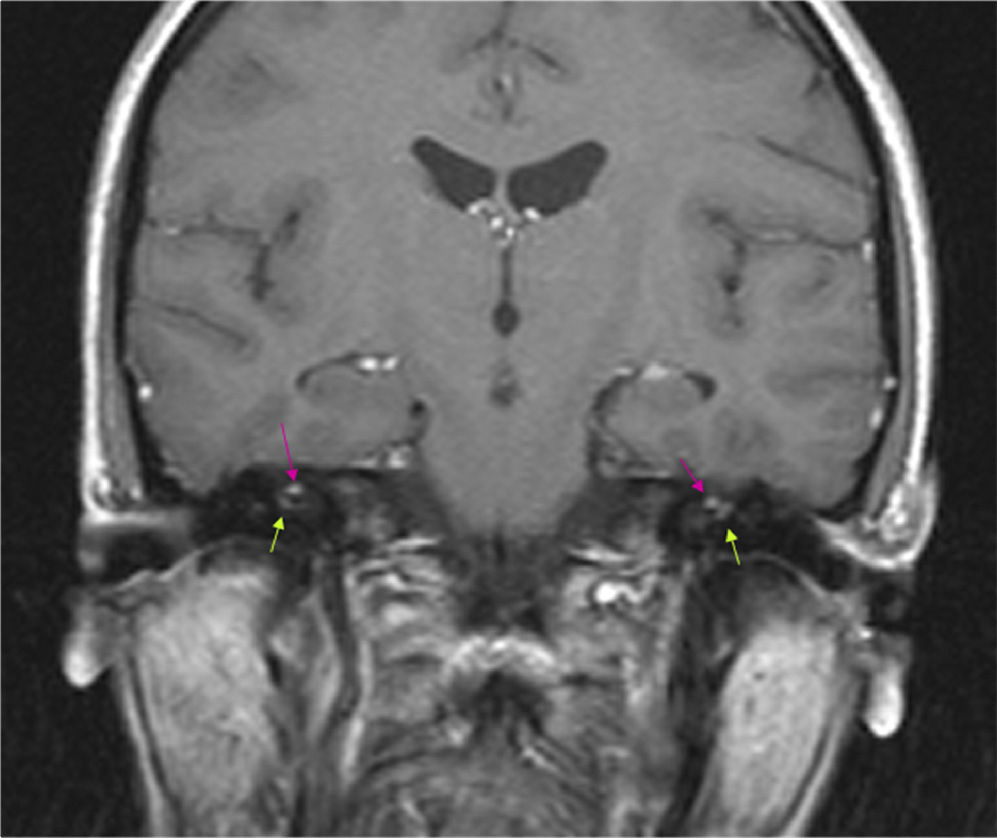
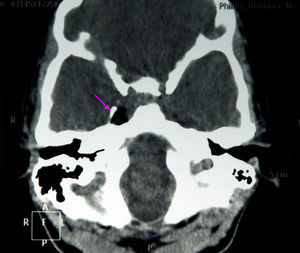
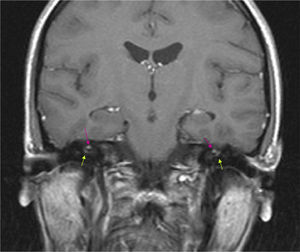
The results of a neurophysiological study (electroneurography, electromyography, and blink reflex test) were compatible with moderate-to-intense axonal damage in the form of decreased amplitude in both facial nerves. Motor and sensory nerve conduction studies of the median, ulnar, and peroneal nerves showed normal conduction velocity and amplitude. A cranial CT scan revealed a heterogeneous well-defined lesion 14mm in diameter in the right parasellar region, surrounded by a ‘bone shell’ bordering the lateral side of the clivus, which was compatible with a dermoid cyst (Fig. 1). Brain MRI scans both with and without gadolinium disclosed a lesion of 15mm anteroposterior diameter and 10mm transverse diameter, located in the right cavernous sinus (hyperintense on T1, T2, and STIR images), as well as micronodular lesions in cisternal spaces and the intracanalicular segment of both facial nerves and diffuse uptake in the infratentorial meninges after gadolinium administration, compatible with possible dermoid cyst rupture with leptomeningeal and cisternal seeding (Fig. 2).
Brain MRI (coronal T1-weighted image) with gadolinium. Hyperintense micronodules associated with chemical shift artefacts (fat) in both internal auditory canals (upper arrows). Increased uptake in the first segment of the right facial nerve (intracanalicular) and the second segment of the left facial nerve (labyrinthine) (lower arrows).
After treatment with dexamethasone IV, the patient improved ad integrum, although another MRI scan disclosed that the cystic lesion remained in the cavernous sinus, without meningeal enhancement or any other alterations. The patient was still asymptomatic 2 and a half years later. No additional follow-up neuroimaging scans were performed.
Until now no cases of acute facial diplegia secondary to a ruptured dermoid cyst have been reported in the literature. Clinical manifestations of ruptured intracranial dermoid cysts include headache, seizures, aseptic meningitis, hydrocephalus, acute ischaemic stroke, neuropsychiatric symptoms, olfactory hallucinations, vision loss, facial paresthaesia, and diplopia.5,6 Headache and seizures are the most common symptoms. The compression or mass effect that the cyst or fragments of it exert on nearby nervous or vascular tissue explains these symptoms. The literature reports several cases of mononeuropathies which affect the optic nerve,7 the facial nerve,4,5,8 the oculomotor nerve,9 and the trochlear nerve,10 but no reports have thus far described a case involving the bilateral facial nerve. Several cases of decompensated diabetes associated with acute bilateral facial paralysis have been reported; oxidative stress and ischaemia have been suggested as the aetiopathogenic mechanism.11
In our case, the neuroimaging findings may explain the aetiopathogenesis of facial diplegia. The occupation of both internal auditory canals with tissue having the same characteristics as the right cavernous sinus lesion supports the hypothesis that both facial nerve lesions are a result of extrinsic compression. We hypothesise that, in the context of diabetic ketoacidosis, cerebral oedema secondary to changes in plasma osmolarity may lead to the rupture of the dermoid cyst, resulting in leptomeningeal seeding and occupation of the intracanalicular portion of both facial nerves. This condition may be a variant of Guillain-Barré syndrome.12 Complementary tests ruled out other possible causes, especially Guillain-Barré syndrome, given the presence of neurophysiological alterations compatible with axonal damage to both facial nerves, supporting the hypothesis that the lesion was due to compression, and the CSF biochemical profile (low glucose and high protein levels). Some studies have found enhancement of the intracanalicular portion of the facial nerve in post-contrast T1-weighted images in the context of other disorders.13 However, copresence of T1-weighted hyperintense micronodules in both internal auditory canals, near both facial nerves, on the one hand, and other micronodules with the same characteristics dispersed throughout the leptomeningeal space and displaying the same signal as the parasellar lesion suggestive of a dermoid cyst on the other, supports the hypothesis that the facial nerve lesion was caused by the compressive effect of these micronodules.
In conclusion, we describe and illustrate the first reported case of acute bilateral facial nerve palsy as a neurological complication of the rupture of an intracranial dermoid cyst.
To Dr Francisco García Fuentes of the internal medicine clinical management unit at Hospital Punta de Europa (healthcare area of Campo de Gibraltar).
Please cite this article as: Gil de Castro R, de la L. Peinado Cantero M, Ruiz Padilla FJ, Cánovas Delgado SI. Diplejía facial como forma de presentación clínica de la rotura de un quiste dermoide intracraneal: a propósito de un caso. Neurología. 2016;31:424–425.