OnabotulinumtoxinA has been demonstrated to be effective as a preventive treatment in patients with chronic migraine (CM). Five years after the approval of onabotulinumtoxinA in Spain, the Headache Study Group of the Spanish Society of Neurology considered it worthwhile to gather a group of experts in treating patients with CM in order to draw up, based on current evidence and our own experience, a series of guidelines aimed at facilitating the use of the drug in daily clinical practice. For this purpose, we posed 12 questions that we ask ourselves as doctors, and which we are also asked by our patients. Each author responded to one question, and the document was then reviewed by everyone. We hope that this review will constitute a practical tool to help neurologists treating patients with CM.
OnabotulinumtoxinA ha demostrado ser eficaz como tratamiento preventivo en pacientes con migraña crónica (MC). El Grupo de Estudio de Cefalea de la Sociedad Española de Neurología ha considerado que sería de interés, a los 5 años de la aprobación en España de la onabotulinumtoxinA, reunir a un grupo de expertos en el tratamiento de pacientes con MC para elaborar con la evidencia actual y nuestra experiencia unas recomendaciones dirigidas a facilitar su uso en la práctica clínica diaria. Con este fin planteamos 12 preguntas que nos hacemos como médicos y que también nos realizan nuestros pacientes. Cada autor ha contestado una pregunta y luego el documento ha sido revisado por todos. Esperamos que esta revisión constituya una herramienta práctica para ayudar a los neurólogos que tratan a pacientes con MC.
One of the objectives of the Spanish Society of Neurology's Headache Study Group (GECSEN) is to issue consensus statements in order to establish good practice guidelines based on evidence and experience. The consensus statement on anaesthetic peripheral nerve block1 was the first of these; this document is intended to be a continuation of this work. The topics addressed in these documents were selected mainly due to the lack of clear consensus on how these techniques should be applied and assessed in clinical practice.
We also took into account the insight of neurologists working in reference headache units and who have published research on the subject; this expertise sheds light on the real-life effects of treatments administered after their approval in clinical trials.
This study aims to provide adequate answers to neurologists’ questions regarding the therapeutic management of chronic migraine (CM) with onabotulinumtoxinA (OnabotA), with a view to providing the best possible treatment and minimising the impact of migraine and the associated disability.
MethodsA group of neurologists specialising in the management and treatment of CM worked collaboratively to respond to the 12 questions considered most important regarding the use of OnabotA to treat CM, with answers drawing from their own expertise and the published evidence.
The issues addressed are grouped into 6 areas: (1) the action mechanism of OnabotA; (2) factors related to treatment response; (3) dosage and adjuvant treatments; (4) cost-effectiveness of the treatment; (5) safety of OnabotA; and (6) information for patients.
We performed a literature search on the MedLine database, including articles published up to April 2017. We also included bibliographical references cited in the articles identified, as well as databases pertaining to neurology organisations and societies, and clinical practice guidelines.
Each neurologist answered one question. Each response was critically reviewed by another expert blinded to the identity of the respondent; the final document was reviewed by all members of the panel.
1. What is the action mechanism of onabotulinumtoxinA?OnabotA (Botox®) is one form of botulinum toxin type A (BoNTA), which belongs to the large family of neurotoxins synthesised by the bacterium Clostridium botulinum. The protein is composed of one heavy- and one light-chain polypeptide, connected by a disulfide bond.2,3 Upon contact with presynaptic nerve terminals, the heavy chain binds to membrane receptors, and the toxin-receptor complex enters the neuron by endocytosis. The protein then undergoes a conformational change, with the disulfide bond breaking and the light chain being released into the neuronal cytoplasm.3,4 The light chain subsequently interacts with the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex, which is composed of a group of vesicular and membrane proteins and enables synaptic vesicle fusion with the synaptic membrane. Specifically, BoNTA cleaves synaptosomal associated protein-25 (SNAP-25), an essential SNARE complex protein which is anchored to the cytosolic face of the plasma membrane.5 By damaging the SNARE complex, the toxin prevents exocytosis of neurotransmitters and neuropeptides from nerve terminals into the synaptic cleft.3
The best understood effect of OnabotA is its capacity to inhibit acetylcholine release at the neuromuscular junction. This effect has been exploited extensively in the treatment of neurological conditions involving muscle hyperactivity. OnabotA also inhibits acetylcholine release from nerve terminals of the autonomic nervous system, hence its use in certain conditions presenting with autonomic dysfunction.4,6 Pain was recently included among the indications for OnabotA, given the drug's demonstrated efficacy for treating CM and other pain syndromes. Experimental studies have shown that BoNTA interferes with the transmission of painful stimuli.7–9 The exact mechanism of this antinociceptive effect is not fully understood. In vitro and animal studies have shown that BoNTA blocks the peripheral release of neuropeptides involved in neurogenic inflammation, such as substance P10,11 and calcitonin gene-related peptide (CGRP),11,12 and such excitatory neurotransmitters as glutamate.13,14 It can also block the translocation of membrane receptors to the surface of sensory neurons; examples are the transient receptor potential vanilloid 1 (TRPV1)15–17 receptor or the P2X3 purinergic receptor.16 These mechanisms enable BoNTA to reduce the sensitisation of peripheral nerve terminals, indirectly blocking central sensitisation.18 This appears to be the mechanism underlying the application of pericranial OnabotA infiltrations to treat CM. In fact, the Phase 3 Research Evaluating Migraine Prophylaxis Theory (PREEMPT) protocol establishes infiltration points near pericranial nerves with afferent fibres supplying the spinal trigeminal nucleus.19 Some experimental studies also suggest that BoNTA may directly modulate meningeal nociceptor signals through collateral branches crossing the cranial sutures.20,21 Regardless of the effects on peripheral nerves, experiments with high doses of BoNTA have found that it can reach the central nervous system via retrograde axonal transport and interneuronal transfer.22 However, experimental animals only display cleaved SNAP-25 fragments in the most peripheral neurons, making it unlikely that BoNTA infiltrations should have significant effects on the central nervous system.23
2. Which factors predict response to onabotulinumtoxinA?Since the first studies into the use of OnabotA as a possible treatment for CM, multiple study groups have searched for predictors of treatment response. We propose the following classification for analysing these factors:
Clinical/demographic factorsSeveral clinical variables have been proposed; these include markedly unilateral headache or allodynia,24 ocular or imploding migraine,25 and pericranial sensitivity during examination.26 These variables have not been confirmed in subsequent studies.27 Shorter progression time for migraine28 or CM29 is reported to be associated with better response.
Analytical factorsLevels of CGRP and to a lesser extent vasoactive intestinal peptide are reported to be predictive of response to OnabotA in patients with CM.30
Neuroradiological factorsA neurosonology study found a significantly higher ratio between flow velocity (measured by interictal transcranial Doppler ultrasound) in the middle cerebral artery and in the ipsilateral internal carotid artery in responders.31
A study published by Borsook's research group reports structural and functional differences between responders and non-responders in resting-state studies.32 This study included a small patient sample, and further study is needed to confirm the findings.
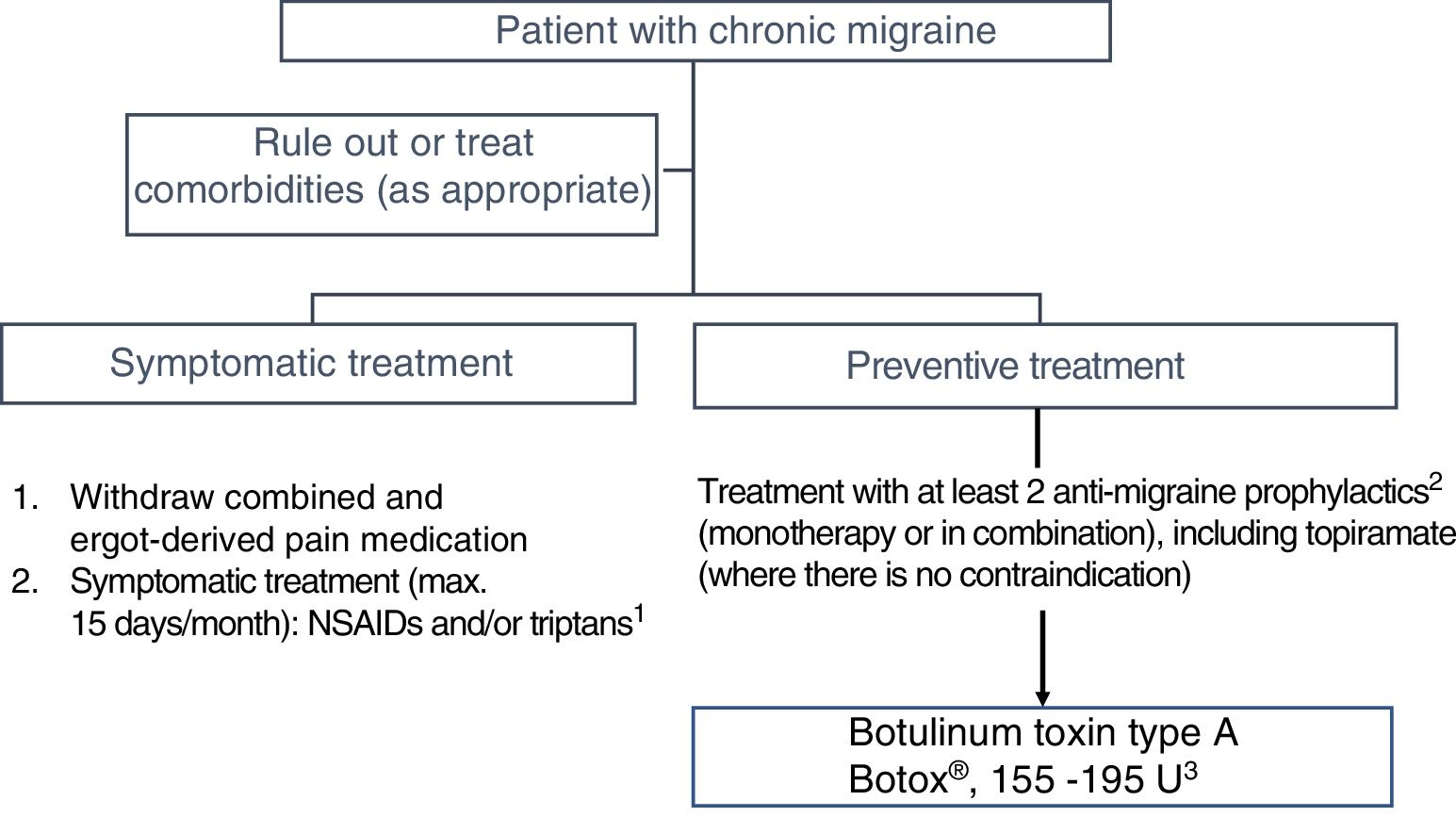
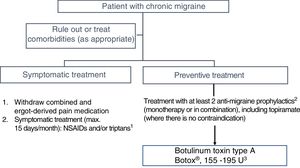
3. When should we ideally start treatment with onabotulinumtoxinA?In Spain, OnabotA was approved as a preventive treatment for CM in 2012 “in patients not responding satisfactorily or who are intolerant to preventive drug therapy for migraine.” GECSEN's 2015 Official Clinical Practice Guidelines for Headache,33 published after the PREEMPT studies,34,35 recommend starting treatment in patients with an intolerance, contraindication, or lack of response to at least 2 preventive drugs (topiramate and one beta-blocker) administered at the minimum recommended dose for at least 3 months (level of evidence IV, grade of recommendation: GECSEN) (Fig. 1).36
There is growing evidence that shorter progression time of migraine28,31 and particularly CM37 is associated with favourable progression and better response to OnabotA; early onset of preventive treatment is therefore recommended.38
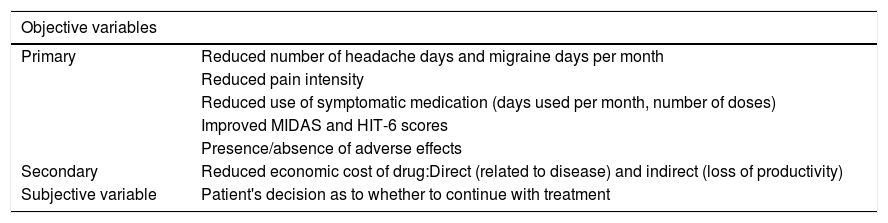
4. How should we assess treatment response?When assessing a patient's response to treatment with OnabotA, we should consider subjective variables, as well as objective, functional, and operative variables. Objective variables are quantified using a calendar on which patients mark days on which they experienced pain and disability, which assists the neurologist, together with the patient, in establishing treatment response.39
Subjective variables include headache intensity, tolerability, and the overall assessment of whether or not to continue treatment.40Table 1 summarises the main objective and subjective variables.
Response criteria for treatment of chronic migraine with onabotulinumtoxinA.
| Objective variables | |
|---|---|
| Primary | Reduced number of headache days and migraine days per month |
| Reduced pain intensity | |
| Reduced use of symptomatic medication (days used per month, number of doses) | |
| Improved MIDAS and HIT-6 scores | |
| Presence/absence of adverse effects | |
| Secondary | Reduced economic cost of drug:Direct (related to disease) and indirect (loss of productivity) |
| Subjective variable | Patient's decision as to whether to continue with treatment |
HIT-6: Headache Impact Test-6; MIDAS: Migraine Disability Assessment Test.
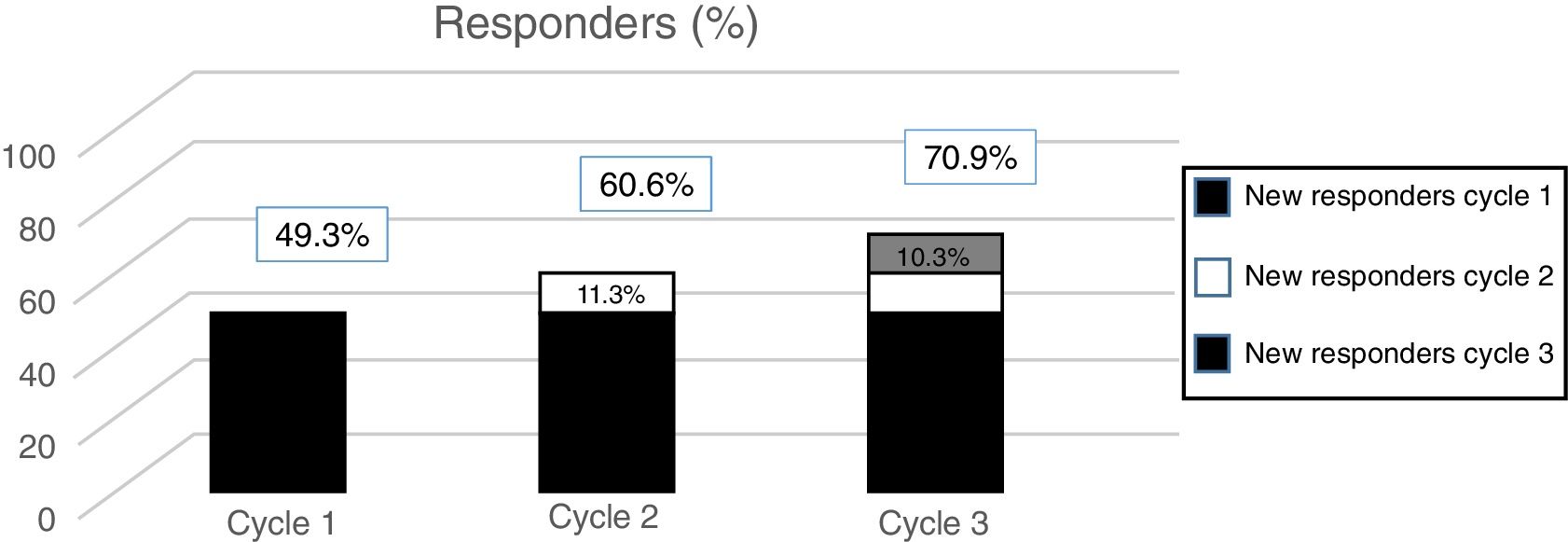
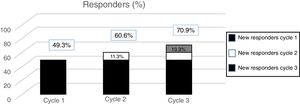
According to data from the PREEMPT programme,40 approximately 15% of patients (depending on the measurement of effectiveness used) begin to respond to the second cycle. In the light of this evidence, we should always administer a second cycle in patients apparently unresponsive to the first; increasing the dose to 195U should also be considered. On the one hand, these results should be expected, given the intrinsic variability of CM (which may mean that an apparent lack of response can be misleading); on the other, they suggest that the effect of OnabotA is cumulative, at least in the first treatment cycles.
The post hoc analysis of patients included in the PREEMPT study demonstrates that a third cycle of treatment can salvage as many as 10% of patients with apparently refractory migraine (Fig. 2).41 Therefore, the PREEMPT findings (which are consistent with observations from everyday practice) support administering at least 3 cycles of OnabotA, increasing dosage to 195U (even when treatment response is not satisfactory after 24 weeks),42 before establishing lack of response to this treatment (level of evidence IV, grade of recommendation: GECSEN).33
6. When should onabotulinumtoxinA be administered in doses above 155U?The current recommended dose of 155-195U is based on data obtained in various clinical trials on the use of OnabotA to treat migraine.43,44 Studies using higher doses (225-260U), which do not follow the PREEMPT injection paradigm, have demonstrated the safety of higher doses despite not achieving the primary objective of efficacy.45,46
PREEMPT studies34,35 use an initial dose of 155U, administered to fixed points. At the discretion of the researcher, and in accordance with pain location, an additional dose of 40U may be administered to the temporal, occipital, or trapezium regions, reaching a maximum dose of 195U. No clinical series have compared the different doses, although most authors recommend higher doses in patients not responsive to the initial low dose.47
A recent study demonstrated the sustained efficacy of a 195U dose over a period of 2 years in 143 patients with CM and medication overuse.42 Compared to a cohort of patients treated with 155U, patients receiving 195U showed greater treatment response from the first cycle, in terms of number of headache days, number of migraine days, and Headache Impact Test (HIT-6) scores. No significant difference was observed in the use of symptomatic medication until the fourth cycle.42
According to published data and clinical experience, there are 2 indications for increasing the dose after the first cycle: lack of response or insufficient response to the first infiltration, or insufficient duration of treatment response (worsening of symptoms 8-10 weeks after infiltration following initial good response).
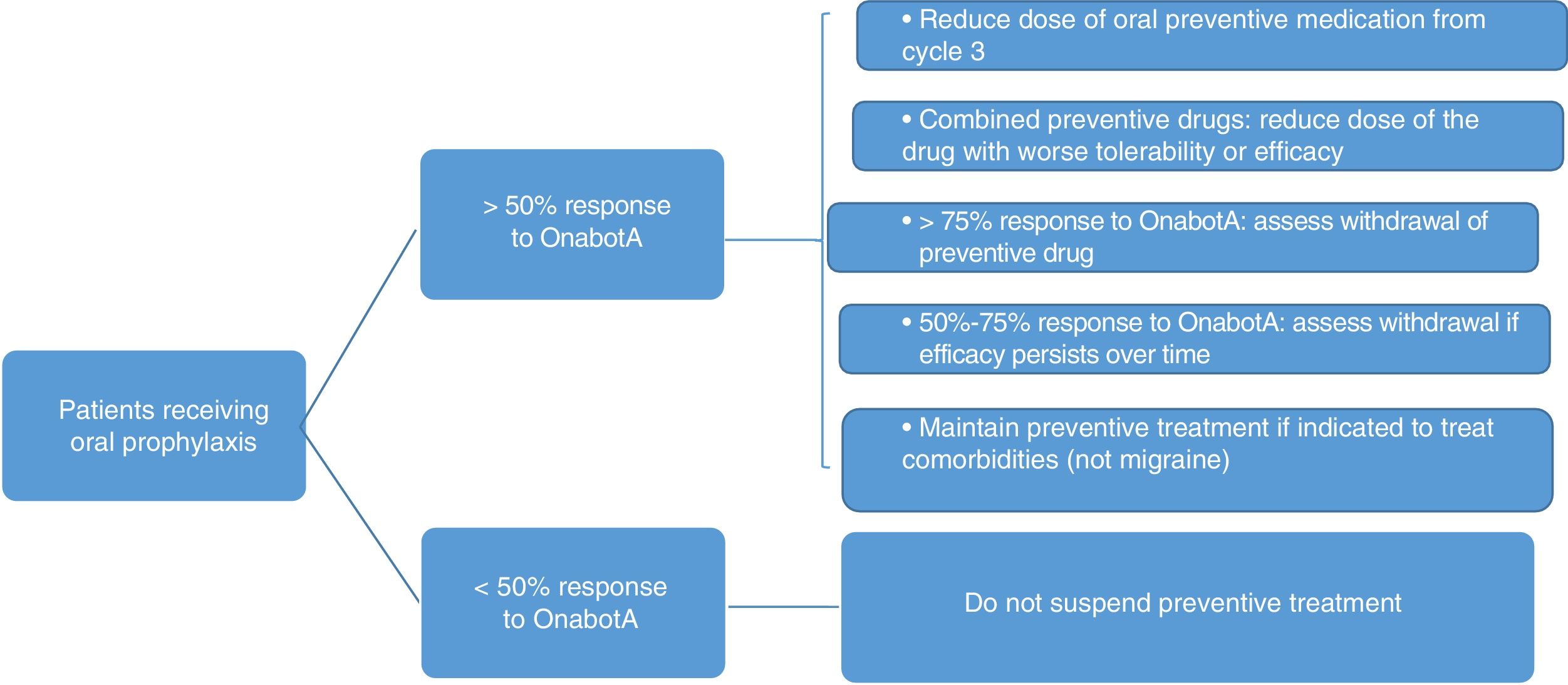
7. How should oral preventive treatment be managed in patients receiving onabotulinumtoxinA?It is relatively common in clinical practice to maintain oral treatment, despite suboptimal response, when starting treatment with OnabotA.48,49 Withdrawal or dose reduction may be justified by response to a first or second cycle of OnabotA. According to the literature, this is achieved in more than half of patients (complete withdrawal in 45.2% and dose reduction in 13.9%).49
Patients starting treatment with OnabotA will fall into one of the following 2 categories:
- •
Patients not receiving oral treatment due to intolerance, contraindication, or lack of response after at least 6 weeks of treatment.
- •
Patients receiving oral preventive medication in monotherapy or combined treatment, with some degree of response.
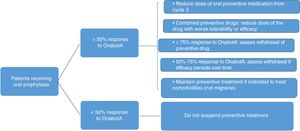
Fig. 3 summarises the protocol for withdrawing oral preventive treatment. The following must also be taken into account:
- •
The patient's opinion and consent.
- •
If symptoms worsen, the dose should be returned to the previous level.
- •
In patients receiving combined therapy, topiramate should be maintained if effectiveness and tolerability are equal.
- •
Preventive treatment may be indicated for concomitant diseases.
According to the published evidence, predicting when a patient will achieve maximum response to OnabotA treatment remains a challenge. The results of the PREEMPT study and patient series from clinical practice suggest that the number of headache days, pain intensity, and consumption of symptomatic medication may decrease progressively up to the fourth cycle of treatment.50–53 However, these studies are not directly comparable, as treatment response is measured in different ways. In the PREEMPT study,54 patients continued to improve from treatment onset, at least for the duration of the study's follow-up period. The prospective, observational, multi-centre REPOSE study52 included data from 783 patients treated with OnabotA for CM for one year, and found that effectiveness (reduction in the number of headache days and improvement in quality of life) persisted throughout the follow-up period. While these differences were calculated against baseline values, they do appear to increase progressively over the first 4 visits.
Guerzoni et al.50 published a prospective study in which 57 patients treated for CM with OnabotA were followed up for 18 months. The number of headache/migraine days per month significantly and progressively decreased with respect to baseline levels. At 6 months from treatment onset, pain days decreased by 22%, and consistently reduced by a further 18% after each cycle of treatment. Symptomatic medication use decreased by 26% at 6 months and 67% at 18 months.
The COMPEL study is a prospective, observational, multicentre, open-label study including 715 patients treated with OnabotA, with a 108-week follow-up period and 9 cycles of treatment.51 The authors of the study conclude that treatment efficacy increased sequentially, peaking after the ninth cycle.
Negro et al.53 performed a prospective, observational study, following up 132 patients with CM for a period of 2 years. Significant reductions from baseline values were observed for all the variables analysed (number of headache and migraine days per month and HIT-6 score), although increases in this benefit were less marked after the first year.
9. How should responders be managed in the long term?Given the long progression times characterising CM, we should consider how to manage these patients after the first year of treatment. There is no established consensus on this issue due to the lack of placebo-controlled studies of over one year's duration.55 However, recommendations can be made based on clinical data from patients receiving treatment for up to 5 years.
Several non-placebo-controlled studies report objective (>50% reduction in migraine days in 3 of 4 treatments) and subjective treatment response in around 70% of patients in the first year of treatment.47,56–59
Can the treatment be suspended at the end of the first year?The available data show that half of patients need to continue with quarterly OnabotA infiltrations, as they present systematic worsening of symptoms after 12 weeks. Of the remaining patients, injections can be postponed by one month (3 infiltrations per year) in 40%, and the treatment may be withdrawn in 10%.48
What happens to treatment response after one year?Clinical experience shows that only one in 10 patients who respond to treatment in the first year stop responding in the second year.48 Evidence from patients with 5 years’ follow-up suggests that loss of treatment response is highly unlikely after the second year. In the same series, no patient stopped responding to OnabotA between the third and fifth years of treatment. In addition to the reduced number of headache days, the long-term benefits include an over 50% decrease in the use of symptomatic medication and the number of emergency department visits for severe headache.48,60
OnabotA continues to show excellent tolerability after the first year. Adverse reactions are observed at a rate below 20%, accounting for very few cases of treatment withdrawal; the profile does not change with regard to those reported in short-term studies, with the exception of the potential for local muscle atrophy, which should lead us to reduce the dose administered to the affected muscle.48
What should be done if a patient does not respond to a given cycle of treatment?Treatment response may not be apparent. As CM can fluctuate and worsen over time, the proposed indicator of treatment response is an over 50% reduction in migraine days after at least 3 of 4 treatments.48 In the event of insufficient or absent response, OnabotA dose should be increased to 195U (see question 6).42
10. Is onabotulinumtoxinA a cost-effective treatment for chronic migraine?Recent studies show that the annual cost of CM ranges from €1500 to €3700, tripling that of episodic migraine.61 CM has higher direct costs, due to patients’ greater need for medical attention (at outpatient clinics, emergency departments, and inpatient wards) and complementary studies to confirm diagnosis, and significantly higher indirect costs due to absences from work and loss of productivity, which represents over 70% of the overall cost of migraine.62 Given the finite resources available to healthcare systems, controlling expenditure is one of the pillars of health policy.63 Therefore, treatments to be introduced into clinical practice must be both efficacious and cost-effective, particularly in relation to diseases that are prevalent, disabling, or associated with high levels of comorbidities and long duration, as is the case with CM.63,64
The first economic studies, based on estimates, demonstrate the cost-effectiveness of treatment with OnabotA, with reductions in both direct and indirect costs65,66; subsequent studies in the clinical setting have confirmed these findings.67,68 In a study comparing OnabotA to oral preventive treatments, only patients receiving the former showed a decrease in emergency department visits and hospital admissions.69 Various Spanish studies have shown that the treatment reduced the direct cost of CM, fundamentally due to the decreases in triptan use and emergency visits.48,69
Indirect cost is also considerably lower due to a marked reduction in rates of disability associated with migraine and patients’ improved quality of life.40 Therefore, it seems reasonable to conclude that the treatment has an impact beyond merely reducing direct costs; in parallel with the reduction in disability, there is a decrease in indirect costs associated with absence from work and loss of productivity.
11. Is treatment with onabotulinumtoxinA safe?According to the safety and tolerability analysis performed as part of the 5 trials conducted prior to the drug's indication for CM,70 the rate of treatment withdrawal due to adverse reactions was 3.4%. The most frequent issues were neck pain (12.6%), muscle weakness (8%), muscle stiffness (6.1%), and ptosis (4.6%). Although 72.9% of patients receiving OnabotA reported at least one adverse effect, only 5.4% considered it serious (vs 3% in the placebo group).
In the PREEMPT programme, the treatment-related adverse event rate for patients undergoing 5 injection cycles was only 34.8%. The percentage of patients with adverse reactions decreased in successive cycles, from 48.3% in the first to 19.1% in the fifth.54
Neck stiffness and ptosis were somewhat more prevalent in the prospective study with the largest patient series published to date than in clinical trials, affecting 14.5% and 11% of patients, respectively. The authors also report occasional migraine exacerbations in the first days after injection (4.3%), and dysphagia (1.96%).59
Pascual et al.48 described frontotemporal muscle atrophy in 2 patients treated with OnabotA for longer than 5 years. Both cases were merely observations, with no functional or aesthetic impact, and did not require treatment discontinuation. Finally, Negro et al.42 observed similar rates of adverse reactions in patients receiving 155- and 195-U doses.
12. How should we manage patients’ expectations? How should patients be informed?Regarding the management of expectations in patients treated with OnabotA, the questions listed below should be answered with the following information:
How much better will I get, and how long will it take?Both clinical trials and everyday practice have found OnabotA to achieve a 50% reduction in the number of headache and migraine days per month, and to decrease pain intensity (number of days using symptomatic medication and doses).28,42,48,58,59,71–74 Approximately 80% of patients respond to OnabotA infiltration during and after the first year of treatment.38,42,48,58,71–75 In some cases, this enables progressive withdrawal of preventive medication after the third cycle of treatment; these drugs can be fully suspended in almost half of patients.42 This improvement is also observed on scales measuring quality of life and headache impact, and persists over time.49,71
Therapeutic compromiseRecent studies report that shorter progression time of migraine in general28,76 and CM in particular, as well as earlier onset of treatment, are associated with better treatment response.38 After an initial cycle with 155U of OnabotA, we should consider increasing the dose to 195U in patients with no response, suboptimal response, or if response does not persist until the next cycle (worsening at 8-10 weeks following a good initial response).42,47
Is this treatment safe?Yes. Data from clinical trials34,35 and everyday practice52,59 suggest that adverse reactions are mild and transient: neck pain, muscle weakness, muscle stiffness, and ptosis. These effects do not change for long-term treatment48 or at higher doses,42 which shows that OnabotA is a safe treatment for CM; treatment discontinuation due to adverse reactions is anecdotal.
How often and for how long do I need the treatment?There is no consensus as to when OnabotA should be withdrawn in responders. It is apparent that the interval between cycles can be extended to 4 or 5 months in approximately 20% of cases.75 Therefore, the effect of the drug appears to be cumulative over successive cycles, although it continues to be difficult to establish when the maximum benefit is reached. Loss of response is rare after the first year of treatment; where it does occur, we should consider increasing the dose to 195U. Once the response returns, the dose may be readjusted according to the patient's needs. Five-year follow-up data suggests that the excellent tolerability of the drug persists over time.48
ConclusionsTable 2 shows the conclusions of this consensus statement, which are derived from the questions initially presented and are based on published evidence and clinical experience.
Conclusions.
| Mechanism of action in chronic migraine |
| BoNTA blocks the exocytosis of neuropeptides and neurotransmitters involved in the generation and propagation of pain impulses (substance P, CGRP, glutamate) and the translocation of membrane receptors to the surface of sensory neurons (TRPV1, P2X3). |
| Predictors of treatment response |
| Predictors of response to the drug are related to its mechanism of action (plasma CGRP levels) and parameters potentially related to reduced possibility of “dechronification” of migraine (age, progression time, and structural or functional neuroimaging changes). |
| When to start OnabotA |
| Early treatment is recommended for all patients with CM with no response, intolerance, or contraindications for 2 oral preventive treatments. |
| Variables related to response |
| In addition to objective and quantitative variables (reduced number of headache/migraine days per month and number of pain-free days), it is important to consider subjective variables (patient's decision to continue with the treatment). |
| How many cycles should be tried if there is no initial response? |
| Even in the absence of a satisfactory response at 24 weeks, it is reasonable to complete 3 cycles of treatment with OnabotA, increasing the dose to 195U before concluding that it is ineffective. We should also assess the presence of factors related to chronic or refractory pain, comorbidities, or the co-presence of another cause of pain or primary headache. |
| When to administer over 155U of OnabotA |
| A dose of 195U is recommended if there is no response to the first infiltration, or if the response is suboptimal or does not persist (pain worsens 8-10 weeks after infiltration following an initial good response). It is important to assess the correct development of the neck muscles, given the increased risk of adverse reactions. |
| How should oral preventive treatment be managed in patients receiving OnabotA? |
| In patients with persisting improvement (>75%), it is recommended to completely withdraw the oral preventive drug. In patients showing improvement of 50%-75%, we should aim to reduce doses in patients receiving monotherapy or to remove one of the 2 drugs in patients receiving combined therapy. In the latter case, topiramate should be maintained if effectiveness and tolerability are equal. Oral prophylaxis should be continued in patients with <50% improvement. |
| Is response to OnabotA cumulative? |
| The effect of OnabotA in preventing CM appears to be cumulative over successive cycles, although it continues to be difficult to establish at which point the maximum benefit is reached. |
| How should responders be managed in the long term? |
| After the first year of treatment, OnabotA is withdrawn in 10% of patients due to good response; in 40% of patients the infiltration can be administered one month later per cycle; around half of patients continue with quarterly cycles. Loss of response after the first year is rare; where it does occur, we should consider increasing the dose to 195U. |
| Is OnabotA a cost-effective treatment for chronic migraine? |
| Yes. Data from clinical practice have confirmed that the treatment reduces both the direct and the indirect costs of CM. Given its excellent tolerability, it is not associated with increased resource use due to adverse reactions, as these are infrequent and mild. |
| Is OnabotA safe? |
| Yes. The treatment is rarely withdrawn due to adverse effects in patients with CM, although no data is available for follow-up periods of over 5 years. |
CGRP: calcitonin gene-related peptide; CM: chronic migraine; OnabotA: onabotulinumtoxinA; TRPV1: transient receptor potential vanilloid 1.
The authors have no conflicts of interest to declare.
The authors are grateful to Allergan S.A. for their assistance in the editorial process and the logistics of meetings.
Allergan was not involved in drafting the document and had no influence over the conclusions reached.
Dr Antonio Martínez of Ciencia y Deporte S.L. provided assistance in the preparation of the manuscript, with funding from Allergan.
Please cite this article as: Gago-Veiga AB, Santos-Lasaosa S, Cuadrado ML, Guerrero ÁL, Irimia P, Láinez JM, et al. Evidencia y experiencia de bótox en migraña crónica: Recomendaciones para la práctica clínica diaria. Neurología. 2019;34:408–417.