The management of epilepsy during pregnancy requires optimal seizure control, avoiding the potential teratogenic effects of antiepileptic drugs.
ObjectivesThis study aims to describe the clinical characteristics and perinatal outcomes of pregnant patients with epilepsy; to analyse the factors associated with seizures during pregnancy; to describe the most commonly used antiseizure drugs in these patients; and to analyse changes in treatment regimens in 2 periods, 2000–2010 and 2011–2018.
MethodsWe conducted a prospective observational study of patients with epilepsy who reported their pregnancy between 2000 and 2018. Patients were evaluated in the first and second trimesters of pregnancy, after delivery, and at one year. Data were collected on demographic variables, epilepsy, and perinatal and obstetric variables.
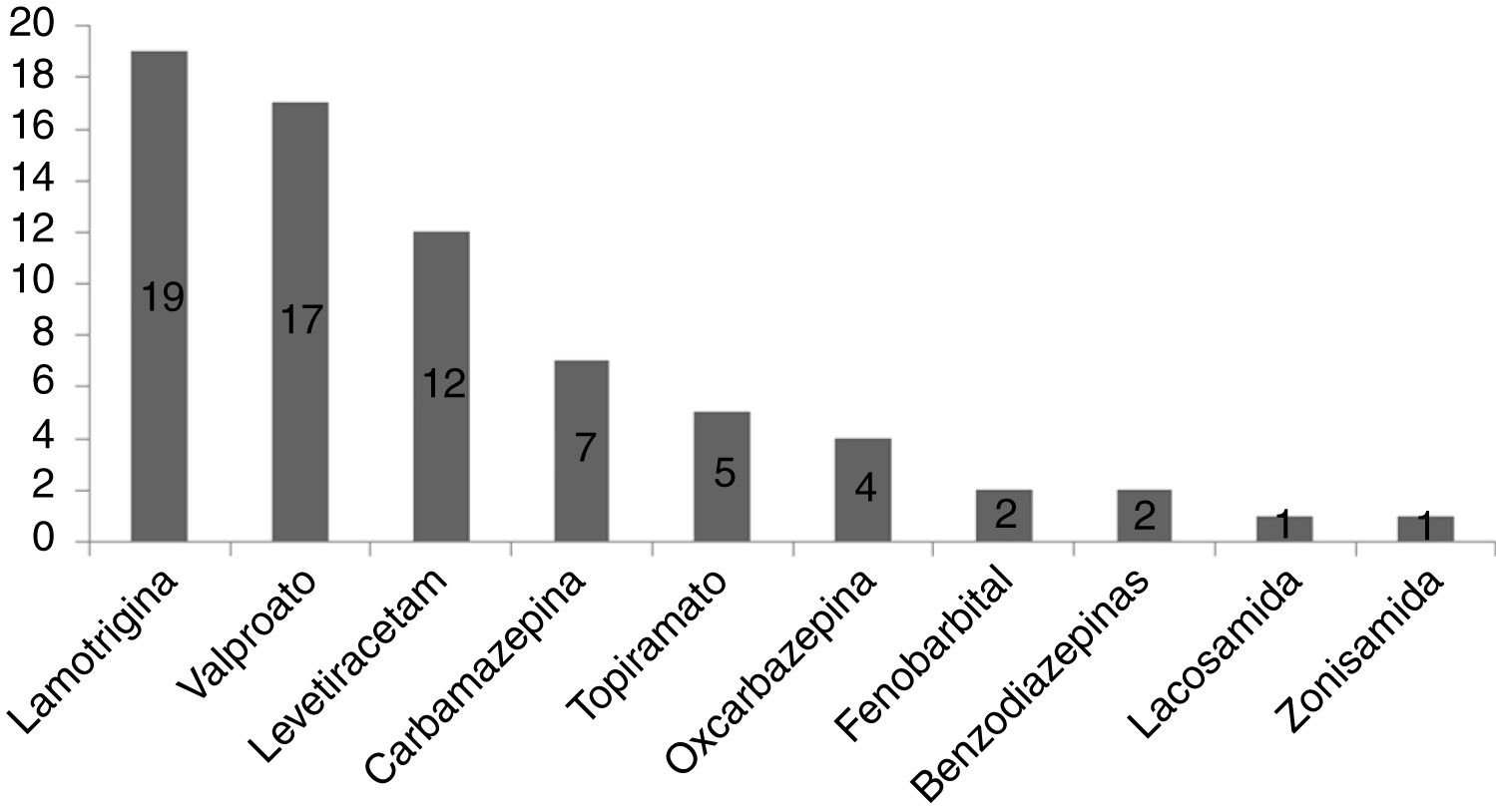
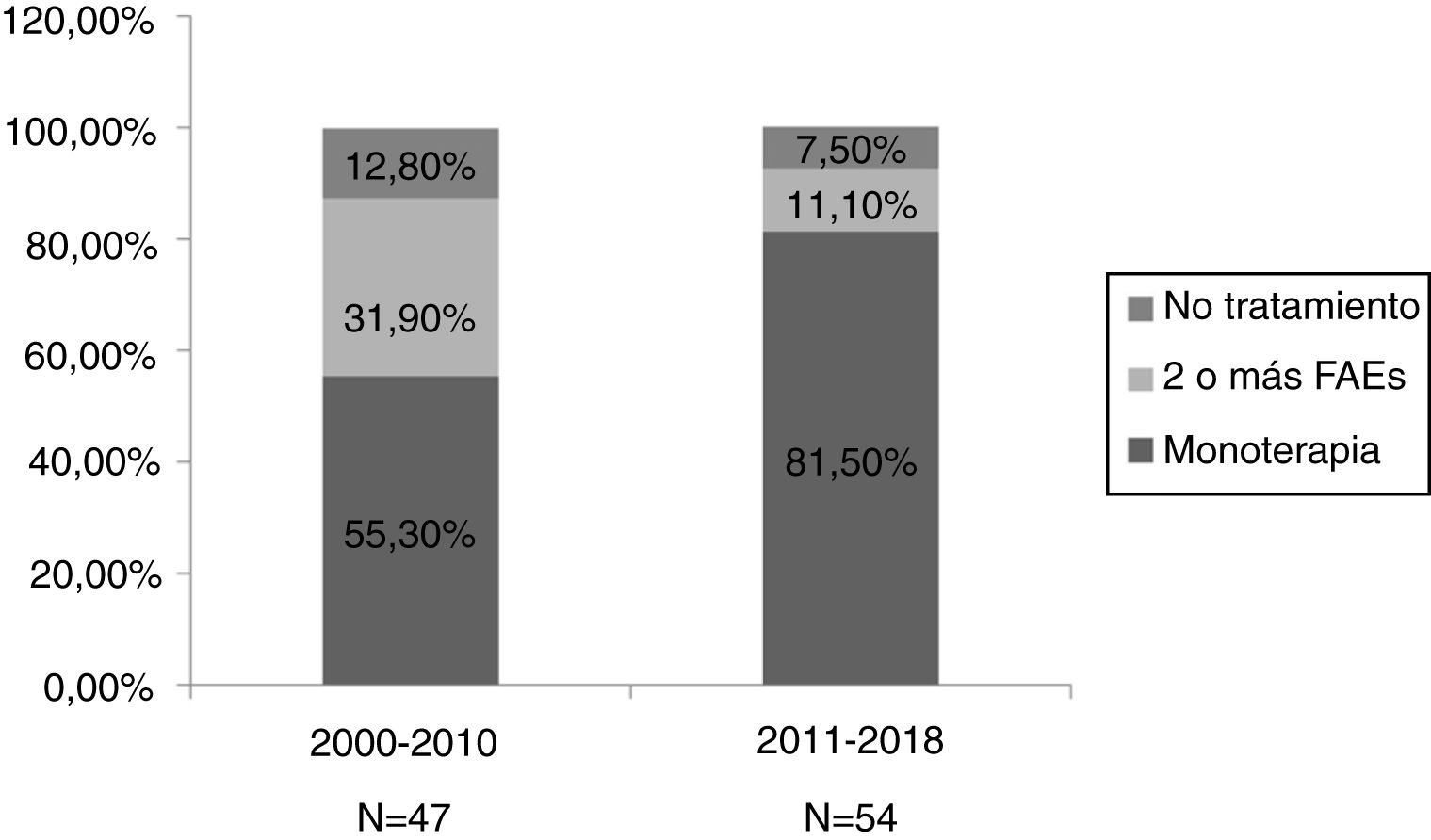
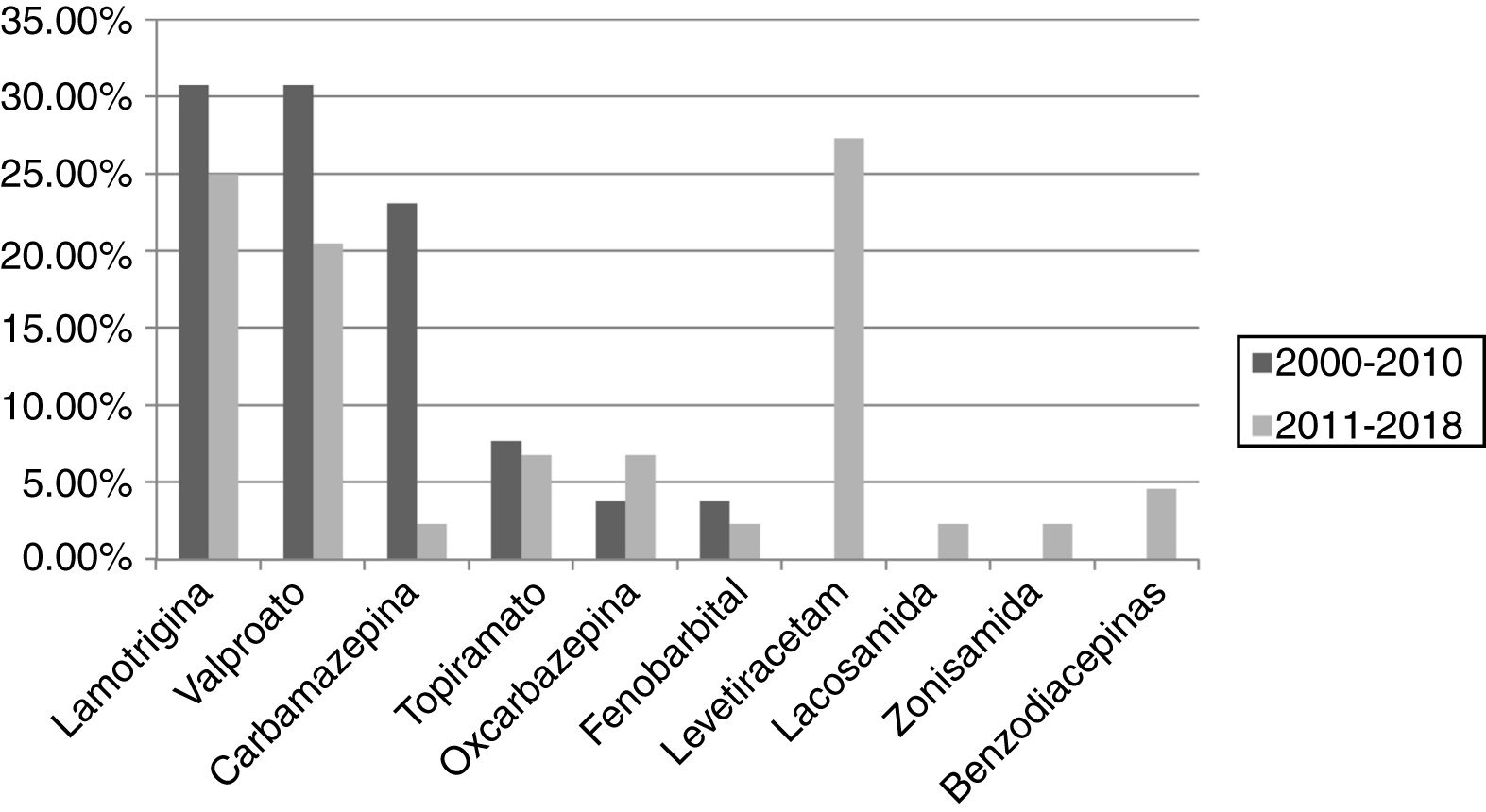
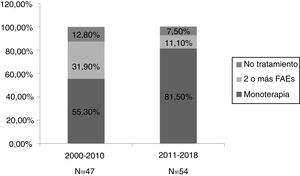
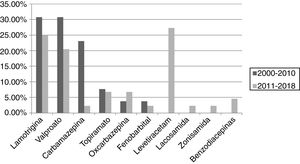
ResultsA total of 101 pregnancies were included. Patients’ mean age was 32.6 years; 55.4% had focal epilepsy, 38.6% had generalised epilepsy, and 5.9% had undetermined epilepsy. We recorded 90 live births, 9 miscarriages, and 5 cases of congenital malformations, 4 of which were born to women who received valproate monotherapy. Forty patients (39.6%) presented seizures, with 16 (40%) presenting generalised tonic-clonic seizures. The variables associated with seizures during pregnancy were poor seizure control in the year prior to pregnancy (66.7% vs 15.1%; P < .001), treatment with 2 or more antiseizure drugs (30% vs 14.8%; P < .001), and untreated epilepsy (25% vs 0%; P < .001). Antiseizure medications most widely used in monotherapy were lamotrigine (n = 19; 27.1%), valproate (n = 17; 24.2%), and levetiracetam (n = 12; 17.1%). In the most recent period (2011–2018), we observed a greater proportion of patients receiving monotherapy (81.5%, vs 55.3%), as well as a decrease in the use of carbamazepine (2.3%, vs 23.1%) and valproate (20.5%, vs 30.8%); and a marked increase in the use of levetiracetam (27.3%, vs 0%).
ConclusionsThe factors associated with the presence of seizures during pregnancy were previous poor seizure control, treatment with 2 or more antiseizure drugs, and lack of treatment during pregnancy. The most commonly used drugs were lamotrigine, valproate, and levetiracetam, with an increase in levetiracetam use and a decrease in valproate use being observed in the later period (2011–2018).
El manejo de la epilepsia durante la gestación requiere un control óptimo de las crisis, evitando los potenciales efectos teratogénicos del tratamiento antiepiléptico.
ObjetivosDescribir las características clínicas y los resultados perinatales de las pacientes con epilepsia gestantes. Analizar los factores que se asocian a la presencia de crisis durante la gestación. Describir los fármacos anticrisis más utilizados y analizar los cambios en el régimen terapeútico en dos periodos: de 2000–2010 y 2011–2018.
MétodosSe realizó un estudio prospectivo observacional de pacientes con epilepsia que notificaron su gestación en el periodo 2000−2018. Se evaluó a las pacientes en el primer y segundo trimestre de gestación, tras el parto y al año. Se recogieron variables demográficas, relacionadas con la epilepsia, perinatales y obstétricas.
ResultadosSe incluyeron 101 gestaciones. La edad media fue de 32,6 años, el 55,4% tenía una epilepsia focal, el 38,6% una epilepsia generalizada y el 5,9% indeterminada. Se registraron 90 nacidos vivos, 9 abortos espontáneos y 5 malformaciones congénitas, cuatro de ellas en monoterapia con valproato. En 40 gestaciones (39,6%) se registraron crisis, siendo tónico-clónicas generalizadas en 16 (40%). Las variables asociadas con la presencia de crisis durante el embarazo fueron el mal control el año previo a la gestación (66,7% vs 15,1%, p < 0,001), el tratamiento con dos o más fármacosanticrisis (30% vs 14,8% p < 0,001) y no recibir tratamiento (25% vs 0% p < 0,001). Los fármacos anticrisis más utilizados en monoterapia fueron lamotrigina (n = 19, 27,1%), valproato (n = 17, 24,2%) y levetiracetam (n = 12, 17,1%). En el periodo más reciente (2011–2018) se objetivó una mayor proporción de monoterapias (81,5% vs 55,3%), además de un descenso en el uso de carbamazepina (23,1% vs 2,3%) y valproato (30,8% vs 20,5%); y un aumento marcado de levetiracetam (0% vs 27,3%).
ConclusionesLos factores asociados con la presencia de crisis durante la gestación fueron el mal control previo, el tratamiento con dos o más fármacos anticrisis y la ausencia de tratamiento. Los fármacos más utilizados fueron lamotrigina, valproato y levetiracetam, con un incremento de éste último y un descenso de valproato en el periodo más reciente (2011–2018).
Epilepsy is the most frequent neurological disease requiring treatment during pregnancy, affecting 0.5% of all pregnant women. Most of these patients have a normal pregnancy and delivery, but an increased risk of complications during pregnancy has been reported.1 Seizures during pregnancy have been associated with increased risk of low birth weight, pre-term delivery, and small for gestational age (SGA), a paediatric term that describes neonates whose weight/length are 2 or more standard deviations (SD) below the mean established for the reference population and their sex and gestational age.2–8 Furthermore, antiseizure drugs (ASDs) may have teratogenic effects at both the morphological and the neurocognitive level.9–12 Valproate is the most teratogenic drug, with a risk of congenital malformations of 10%, which is dose-dependent. Polytherapy also represents a higher risk, especially if it includes valproate. This has led to a change in the prescription pattern of ASDs in recent years.13,14
Regarding the factors associated with seizures during pregnancy, the presence of at least one seizure in the year before conception, focal seizures, and combination therapy are described to act as predictors of seizures during pregnancy.15–17
Most information on the progression of pregnancy in women with epilepsy is from registries of patients treated with ASDs; there are few articles on patients in whom antiseizure treatment is withdrawn due to good seizure control.18 In this study, we describe the perinatal outcomes and the incidence of seizures during pregnancy in epileptic patients with and without antiseizure treatment; we analysed the factors associated with the presence of seizures and describe the most widely used AEDs, studying the changes in treatment in 2 periods: 2000–2010 and 2011–2018.
MethodsWe conducted an observational prospective registry of pregnant patients with epilepsy under follow-up by the epilepsy unit at a tertiary care hospital between 2000 and 2018, following the protocol of the EURAP registry established at the hospital. We conducted interviews in the first (notification of pregnancy) and second trimesters, after delivery, and at one year of follow-up. We also included untreated patients. We gathered data on demographic variables, epilepsy (type, aetiology, presence of generalised tonic-clonic seizures and other types of seizures during pregnancy, pharmacological treatment), obstetric variables (ultrasound findings, complications during pregnancy, treatment with folic acid, type of delivery), variables related to the newborn (sex, weight, head circumference, height, Apgar score, and presence of major congenital malformations until one year of life), and other congenital malformation risk variables (use of other drugs, smoking, alcohol consumption, family history of major congenital malformations). We also retrospectively reviewed previous seizure control in the year prior to pregnancy. We defined poorly-controlled patients as those presenting at least one seizure in the year prior to conception and well-controlled patients as those presenting no seizures in the year prior to conception.
In the calculation of the prevalence of congenital malformations, we excluded patients with miscarriages and abortions due to causes unrelated to major congenital malformations, as well as those who had not completed one year of follow-up after delivery.
Statistical analysisIn the study of variables associated with the presence of seizures during pregnancy, we used the chi-square test for categorical variables and the t-test/Wilcoxon test for continuous variables. Values of P < .05 were considered statistically significant. Statistical analysis was conducted using the SPSS software, version 21 (IBM; Chicago, IL).
ResultsSample characteristicsWe recorded 101 pregnancies in 76 different women, who notified their pregnancies in 2000−2018. Fifty-seven women reported a single pregnancy, 14 women reported 2 pregnancies, 3 reported 3 pregnancies, and one reported 4 pregnancies. Of all pregnancies, 96 were followed up for one year after delivery. Patients’ baseline characteristics are summarised in Table 1.
Baseline characteristics of the sample.
| Age at conception, mean (SD) | 32.6 (5.1) years |
|---|---|
| Type of epilepsy | Focal: 56 (55.4%) |
| Generalised: 39 (38.6%) | |
| Undetermined: 6 (5.9%) | |
| No seizures in the year prior to pregnancy | 58 (57.4%) |
| Smoking | 20 (19.8%) |
| Alcohol consumption | 1 (1%) |
| Adequate treatment with folic acid | 34 (33.7%) |
| Family history of congenital malformations | 4 (4%) |
Of the 101 pregnancies recorded, 88 were full-term, leading to 90 live newborns (2 pairs of twins and 86 single deliveries). Of the remaining 13 pregnancies, we recorded 9 miscarriages (9.3%) and 4 abortions (3.96%) (3 due to malformations detected prenatally and one due to severe intrauterine growth restriction). We recorded 38 normal vaginal deliveries (43.2%), 38 caesarean sections (43.2%), and 11 forceps deliveries (12.5%). Only 6 premature births were recorded (6.8%). Mean birth weight was 3 169.5 (486.5) grammes.
Of all pregnancies followed up for at least one year after delivery (n = 96), and having excluded miscarriages (n = 9) and one abortion not associated with malformation, we detected 5 cases of congenital malformations (of a total of 86; 5.81%), which are described in Table 2. In 4 cases, the mother was receiving monotherapy with valproate, at a mean dose of 1000 mg/day. The fifth patient received bitherapy with levetiracetam and carbamazepine. Prevalence of major congenital malformations amounted to 23.5% in patients treated with valproate (n = 17, after excluding miscarriages).4
Major congenital malformations in babies born to women with epilepsy from our sample.
| Patient age (years) | Type of malformation | Treatment | Dose (mg) | Family history of major congenital malformations | Time of detection | |
|---|---|---|---|---|---|---|
| Patient 1 | 34 | Thoracopagus conjoined twins | Valproate | 1000 | No | Prenatal (week 12) |
| Patient 2 | 29 | Spina bifida, hydrocephalus | Valproate | 900 | No | Prenatal (week 20) |
| Patient 3 | 40 | Interventricular communication, mild aortic stenosis | Valproate | 1300 | No | 0 months |
| Patient 4 | 29 | Craniosynostosis | Valproate | 800 | No | 6 months |
| Patient 5 | 30 | Anencephaly | Carbamazepine/levetiracetam | 400/1000 | No | Prenatal (week 13) |
Seizures were reported in 40 pregnancies, with generalised tonic-clonic seizures in 16 cases (40%). One patient presented peripartum absence status, with good response to pharmacological treatment, and no complications for the newborn.
Table 3 shows the factors associated with presence of seizures during pregnancy.
Factors associated with the presence of seizures during pregnancy.
| No seizures (n = 61) | Seizures (n = 40) | P | |
|---|---|---|---|
| Patient age, mean (SD) | 32.6 (5.1) | 32.5 (5.2) | .87 |
| Type of epilepsy | |||
| Focal | 29 (47.5%) | 27 (67.5%) | |
| Generalised | 28 (45.9%) | 11 (27.5%) | .14 |
| Undetermined | 4 (6.6%) | 2 (5%) | |
| Poor control (≥ 1 seizure in the previous year) | 8 (15.1%) | 24 (66.7%) | < .001 |
| Treatment | |||
| Monotherapy | 52 (85.2%) | 18 (45%) | |
| ≥ 2 antiseizure drugs | 9 (14.8%) | 12 (30%) | <.001 |
| No treatment | 0 (0%) | 10 (25%) | |
| Monotherapy with valproate | 12 (19.7%) | 5 (12.5%) | .42 |
| Monotherapy with lamotrigine | 15 (24.6%) | 4 (10%) | .07 |
| Monotherapy with levetiracetam | 10 (16.4%) | 2 (5%) | .12 |
| Miscarriage | 7 (11.5%) | 2 (5%) | .31 |
The presence of seizures during pregnancy was significantly associated with poor seizure control in the year prior to conception. Seventy-five percent of poorly controlled patients presented seizures during pregnancy, compared to only 25% of well-controlled patients (RR 11.25%, 95% CI: 4.05–31.28; P < .001).
Treatment with 2 or more ASDs and the absence of treatment were significantly associated with the presence of seizures during pregnancy. During pregnancy, 57.1% of the patients treated with 2 or more ASDs presented seizures, as compared to 27.5% of the patients receiving monotherapy (P < .001). All patients who were not under treatment with ASDs presented seizures during pregnancy. Of the 10 patients not receiving ASDs, 6 were well-controlled patients who had discontinued treatment due to good control, with seizure-free periods ranging from 3 to 9 years, and who had received no treatment for periods of 4–6 years. Four patients presented de novo seizures during pregnancy. The characteristics of patients not receiving antiepileptic treatment are listed in Table 4.
Characteristics of patients receiving no antiseizure treatment at conception.
| Patient age (years) | Epilepsy onset during pregnancy | Epilepsy type/aetiology | Time without seizure | Time without treatment | Major congenital malformations | Miscarriage | Obstetric complication | Management | |
|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | 31 | No | Focal cryptogenic | 4 years | 5 months | No | No | No | LMT onset in the third trimester |
| Patient 2 | 38 | Yes | Undetermined | No | No | No | CBZ onset in the second trimester | ||
| Patient 3 | 35 | Yes | Focal cryptogenic | No | No | No | LMT onset in the second trimester | ||
| Patient 4 | 38 | Yes | Focal symptomatic (cortical dysplasia) | No | No | No | LEV onset in the second trimester | ||
| Patient 5 | 29 | No | Focal cryptogenic | 3 years | 4 months | No | No | No | LMT onset in the second trimester |
| Patient 6 | 39 | No | Focal cryptogenic | 9 years | 6 years | No | No | No | CBZ onset in the third trimester |
| Patient 7 | 27 | No | Focal cryptogenic | 7 years | Unknown | No | No | No | VPA onset after delivery |
| Patient 8 | 31 | No | Focal cryptogenic | 4 years | 3 years | No | No | No | OXC onset |
| Patient 9 | 37 | No | Focal symptomatic (multiple sclerosis) | 4 years | 2 years | No | No | No | LEV onset in the third trimester |
| Patient 10 | 2 | Yes | Focal cryptogenic | No | No | PA | LEV onset in the second trimester |
CBZ: carbamazepine; LEV: levetiracetam; LMT: lamotrigine; OXC: oxcarbazepine; PA: placental abruption; VPA: valproate.
The type of epilepsy was not associated with the presence of seizures during pregnancy. No particular ASD in monotherapy was observed to be associated with poorer seizure control. We did not find an association between the presence of seizures and such perinatal variables as pre-term birth, birth weight, or type of delivery.
Pharmacological treatmentWe recorded 91 pregnancies in women receiving AEDs (90.1%), 70 (69.3%) in monotherapy and 21 (20.8%) with 2 or more ASDs. In the remaining 10 pregnancies, patients were not receiving treatment (9.9%) at the time of conception, with 9 starting antiepileptic treatment from the second trimester.
The most frequently used drug in monotherapy was lamotrigine, followed by valproate and levetiracetam, as shown in Fig. 1.
The mean daily dose of lamotrigine in monotherapy was 159.2 (65.2) mg, compared to 811.76 (323.81) mg for valproate and 1145.8 (405.34) mg for levetiracetam.
Of the patients receiving combination antiepileptic treatment (n = 21), 5 were receiving treatment with 3 AEDs, one of whom was also under treatment with vagus nerve stimulation. The remaining 16 were receiving bitherapy, with the most frequently used combinations being topiramate plus levetiracetam (n = 3) and valproate plus lamotrigine (n = 2).
We divided the sample into 2 periods, 2000–2010 (n = 47) and 2011–2018 (n = 54). We observed a higher percentage of monotherapy (81.5% vs 55.3%) and lower percentage of combination therapy (11.1% vs 31.9%) in the second period (Fig. 2). We did not observe significant differences between the 2 periods in such other variables as the presence of seizures during pregnancy, abortion, major congenital malformations, and pre-term birth.
The most widely used ASDs in monotherapy were lamotrigine and valproate in the first period (30.8% each), and levetiracetam (27.3%) and lamotrigine (25%) in the second. We observed a decrease in the use of carbamazepine (23.1% vs 2.3%) and valproate (30.8% vs 20.5%) between periods, as well as a considerable increase in the use of levetiracetam (0% vs 27.3%) and a less pronounced increase in the use of oxcarbazepine (3.8% vs 6.8%). The use of lamotrigine remained stable, with only a slight decrease (30.8% vs 25%). These results are shown in Fig. 3.
DiscussionMost pregnancies were uneventful. The rate of miscarriages amounted to 8.9%, a percentage similar to that observed in the general population, and a rate of major congenital malformations of 5.81%, which is higher than that observed in the general population (2%–3%). Four of the 5 cases of major congenital malformations occurred in the context of treatment with valproate in monotherapy, with a mean dose of 1000 mg/day, which represents 23.5% of the patients treated with valproate. This result is similar to, or even higher than, the rates published in the literature on the teratogenicity of valproate.10–12
Factors associated with seizures during pregnancy were the presence of at least one seizure in the year prior to conception, treatment with 2 or more ASDs, and receiving no treatment at the time of conception. The risk of seizures during pregnancy was 11 times greater in patients presenting at least one seizure in the year prior to conception. Treatment with 2 or more ASDs was also associated with a higher risk of seizures during pregnancy (62.5%, vs 30.5% of patients receiving monotherapy); this is probably due to the fact that the patients receiving this treatment regime had presented seizures in the year prior to pregnancy. These results are consistent with those reported in other articles in the literature. In a prospective observational study of 1297 pregnant patients with epilepsy, Thomas et al.15 observed that the presence of at least one seizure in the month prior to conception (OR: 15, 95% CI: 9–25), combination therapy (OR: 2.8, 95% CI: 2.3–3.9), and the presence of focal seizures (OR: 1.6, 95% CI: 2−2) were predictors of seizures during pregnancy.
Little information is available on pregnancy in patients with untreated epilepsy. In our sample, all patients who discontinued antiseizure treatment due to previous good control presented seizures during pregnancy. Similar findings were reported by Vadja et al.18 in a cohort of 148 untreated patients and 1532 treated patients from the Australian Register of Antiepileptic Drugs in Pregnancy. In that article, 56.1% of untreated patients and 46.9% of treated patients presented seizures during pregnancy. The probability of seizures during pregnancy in untreated patients was not dependent on the time without treatment, but was associated with the activity or inactivity of their epilepsy. In our case, all 6 patients were previously seizure-free but, despite this, presented seizures during pregnancy. For these reasons, in patients planning for pregnancy, it is advisable to maintain antiseizure treatment in monotherapy, avoiding drugs with higher teratogenic risk.
Several studies have reported that the presence of seizures during pregnancy, especially generalised tonic-clonic seizures, is associated with greater risk of pre-term birth, low birth weight, and small for gestational age.2–5 We did not observe this association, probably due to the small sample size.
In terms of pharmacological treatment, we observed an increase in the percentage of patients receiving monotherapy in recent years, which suggests better planning for pregnancy. Furthermore, as in other articles,13,14 we observed a pronounced increase in the use of newer drugs with lower teratogenic risk, such as levetiracetam and, to a lesser extent, oxcarbazepine, as well as a decrease in the use of carbamazepine and valproate. Although lamotrigine continues to be frequently used, alongside levetiracetam, its use has slightly decreased.
The limitations of our study include the small sample size and its single-centre design.
The conclusions of our study are as follows:
- •
Our findings confirmed the teratogenic effects of valproate, with an even higher prevalence of congenital malformations than that described in the literature.
- •
Seizures manifested in 39.6% of pregnancies. The factors significantly associated with the presence of seizures were poor previous seizure control, treatment with 2 or more ASDs, and the absence of treatment.
- •
The most widely used drugs were lamotrigine, valproate, and levetiracetam.
- •
We observed a higher proportion of monotherapy in the second period (2011–2018) than in the first, in addition to a decrease in the use of carbamazepine and valproate and increased use of levetiracetam and, to a lesser extent, oxcarbazepine.
The management of pregnant patients with epilepsy is complex; optimal seizure control is essential and potential teratogenic effects must be avoided. Current recommendations include planning for pregnancy and maintaining monotherapy at the minimum effective dose with an ASD presenting low teratogenic risk, such as lamotrigine or levetiracetam. In our experience, it is preferable not to suspend treatment in well-controlled patients due to the high probability of presenting seizures during pregnancy.
Conflicts of interestThe authors have no conflicts of interest to declare.