To review the available scientific evidence about the effectiveness of auditory cues during gait initiation and turning in patients with Parkinson's disease.
MethodsWe conducted a literature search in the following databases: Brain, PubMed, Medline, CINAHL, Scopus, Science Direct, Web of Science, Cochrane Database of Systematic Reviews, Cochrane Library Plus, CENTRAL, Trip Database, PEDro, DARE, OTseeker, and Google Scholar. We included all studies published between 2007 and 2016 and evaluating the influence of auditory cues on independent gait initiation and turning in patients with Parkinson's disease. The methodological quality of the studies was assessed with the Jadad scale.
ResultsWe included 13 studies, all of which had a low methodological quality (Jadad scale score ≤2). In these studies, high-intensity, high-frequency auditory cues had a positive impact on gait initiation and turning. More specifically, they (1) improved spatiotemporal and kinematic parameters; (2) decreased freezing, turning duration, and falls; and (3) increased gait initiation speed, muscle activation, and gait speed and cadence in patients with Parkinson's disease.
ConclusionsWe need studies of better methodological quality to establish the Parkinson's disease stage in which auditory cues are most beneficial, as well as to determine the most effective type and frequency of the auditory cue during gait initiation and turning in patients with Parkinson's disease.
Revisar la evidencia científica sobre la efectividad de la aplicación de estímulos auditivos en la fase de iniciación de la marcha y giro en pacientes con enfermedad de Parkinson.
MétodosSe realizó la búsqueda en las bases de datos Brain, PubMed, Medline, Cinahl, Scopus, Science Direct, Web of Science, Cochrane Database of Systematic Reviews, Biblioteca Cochrane Plus, CENTRAL, Trip Database, PEDro, DARE, OTSeeker y Google Académico. Se incluyeron estudios que analizasen la influencia de estímulos auditivos sobre el inicio y el giro de la marcha independiente en pacientes con enfermedad de Parkinson, publicados entre 2007 y 2016. Su calidad metodológica fue valorada mediante la escala Jadad.
ResultadosSe seleccionaron 13 artículos, todos ellos de baja calidad (Jadad≤2) que mostraron resultados positivos en relación con el uso de estímulos auditivos a alta frecuencia e intensidad sobre el inicio de la marcha y la ejecución de giros. En concreto, 1) mejoraron los parámetros espaciotemporales y cinemáticos, 2) disminuyeron la congelación, el tiempo de giro y las caídas y 3) aumentaron la velocidad de iniciación de la marcha, la activación muscular y la velocidad y cadencia de la marcha en los pacientes con EP.
ConclusionesSe requiere un mayor número de estudios y de mayor calidad metodológica para justificar y comprender en qué estadio los pacientes se beneficiarían más de esta señal sensorial, así como el tipo de guía auditiva y la frecuencia de estimulación más eficaz en la fase de iniciación de la marcha y de giro en pacientes con Parkinson.
Parkinson's disease (PD) is an idiopathic, insidiously progressive, neurodegenerative process. From a histological viewpoint, it is characterised by death of dopaminergic neurons in the striatum and substantia nigra pars compacta, and presence of Lewy bodies in various areas of the brain.1–3 PD is the most prevalent neurodegenerative disease after Alzheimer disease, with onset generally occurring between the ages of 50 and 60 years. Prevalence increases in line with age: 1% to 2% in individuals older than 65 years and 3% to 5% in individuals aged 85 or older.1,4,5
The main symptoms of PD are muscle rigidity, akinesia, resting tremor, and alterations in postural reflexes.6 Motor impairment not only affects kinematics (speed or time), but also variability of movement.6–8
As disease progresses, patients develop festinating gait, characterised by short, shuffling steps, “en bloc” turns, and increased risk of falls.6 The common phenomenon of freezing and the difficulty initiating or maintaining a normal walking pattern seem to be associated with problems in voluntarily generating an endogenous rhythm, which is necessary for such repetitive movements as walking.7,9,10 In fact, difficulty initiating gait is one of the most frequent problems in patients with PD. Furthermore, over half of these patients have difficulty turning, which is associated with falls and results in poorer quality of life.11–14 Gait initiation and turning are 2 critical stages of movement in patients with PD.
External feedback is the rehabilitation treatment of choice for gait disorders. The basal ganglia play a major role in controlling complex motor sequences, most of which are automatic. Auditory (metronome beats or rhythmic beats on the floor) and visual cues (footprints or perpendicular lines on the floor) have therefore been used to activate other pathways involved in gait, bypassing damaged circuits.8,15–17
In this study, we aimed to review the available evidence on the effectiveness of auditory cueing for improving gait initiation and turning in patients with PD.
Material and methodsSearch methodologyWe conducted a literature search to gather all original articles that may offer some insight into the topic, using the following databases: Brain, PubMed, Medline, CINAHL, Scopus, Science Direct, Web of Science, Cochrane Database of Systematic Reviews, La Biblioteca Cochrane Plus, CENTRAL, Trip Database, PEDro, DARE, OTseeker, and Google Scholar. Given that our study assesses 2 very specific stages of gait, we aimed to gather as many articles as possible, and therefore included all articles published between January 2007 and December 2016.
We used the following MeSH terms and keywords“Parkinson's disease,” “auditory cues,” “cueing,” “audio biofeedback,” “externally cued,” “sensory stimuli,” “sensory guides,” “freezing,” “gait,” “turning,” “turn,” and “gait initiation.” Where possible, we filtered search results to include only randomised controlled trials, original clinical studies, and annals of conferences. We also gathered relevant studies included in the reference lists of the articles gathered during the literature search. Articles had to be published in either Spanish or English.
Inclusion criteriaThe patients included in the studies had to meet the following criteria: (1) having a clinical diagnosis of PD according to the United Kingdom Parkinson's Disease Society Brain Bank criteria,18 (2) being able to walk independently, and (3) experiencing freezing (optional). Exclusion criteria were as follows: (1) history of hospital admission or surgery that may have altered the patient's walking pattern, and (2) presence of other neurological, vestibular, or balance problems. The intervention had to be based on auditory cueing, and the studies had to compare this intervention with other types of sensory cue, other rehabilitation treatments for PD, or a lack of treatment. Study results also had to focus specifically on turning and gait initiation.
Article selection and assessment of methodological qualityWe read the titles and abstracts of the articles gathered to determine which articles met the inclusion criteria. We then read the full texts to determine whether they were relevant to our study.
Data were extracted according to the Consolidated Standards of Reporting Trials.19,20 We extracted the following data from the included studies: inclusion and exclusion criteria, methodology, randomisation (where applicable), description of the study, blinding (where applicable), outcome measures, description of the therapeutic intervention, and results. We used the Jadad scale to ensure objective, critical assessment of the studies included. This scale evaluates methodological quality by analysing randomisation, patient and researcher blinding to treatment, and withdrawals and drop-outs. Higher scores indicate greater methodological quality; the maximum possible score is 5 points.21
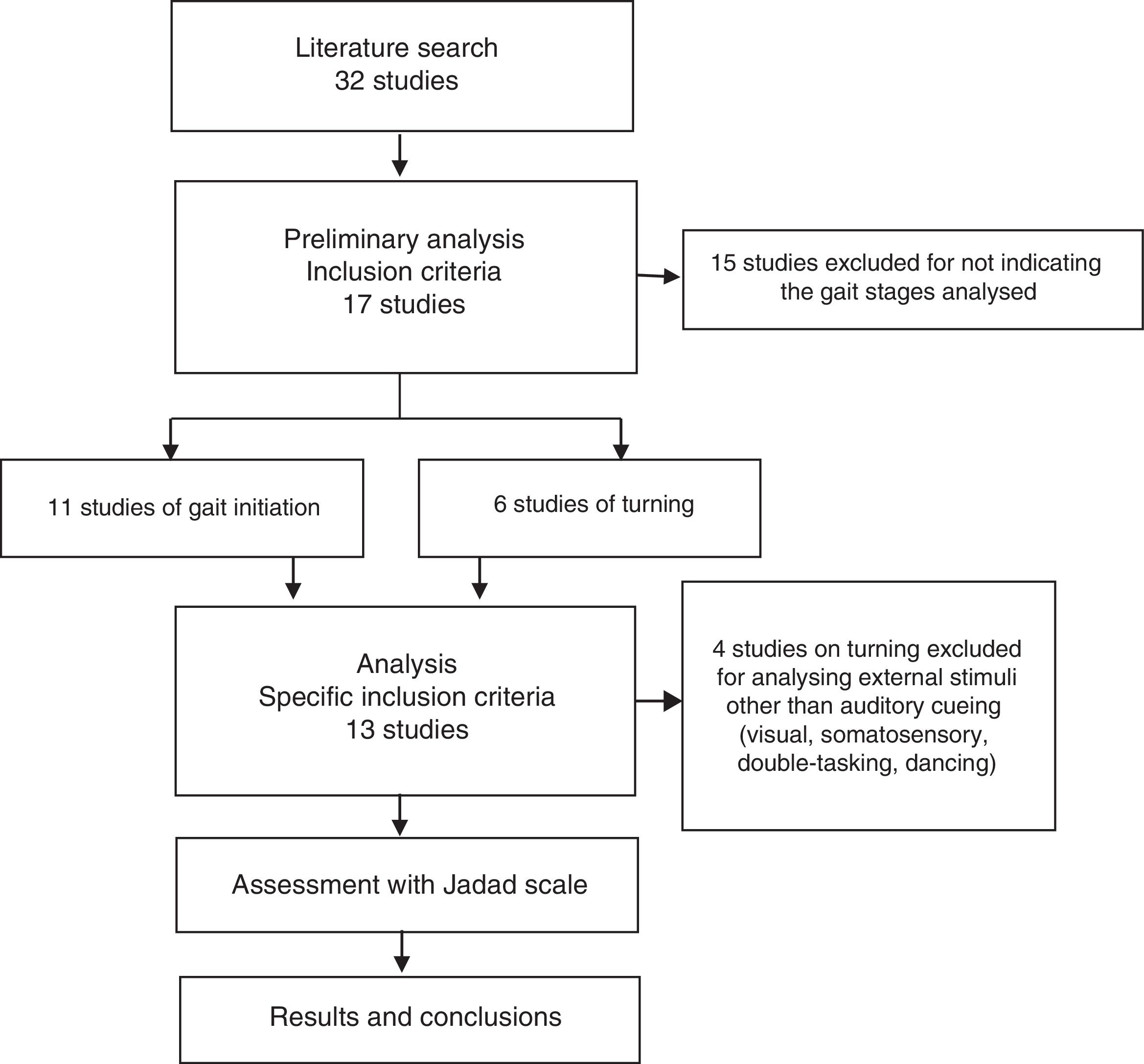
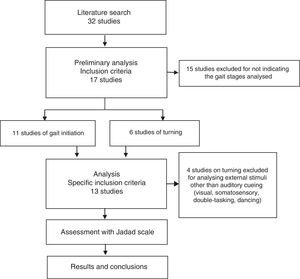
ResultsSearch resultsWe gathered a total of 32 articles related to the subject of our research. We excluded 15 studies that did not evaluate the effect of sensory stimuli on either turning or gait initiation.22–36 We excluded an additional 4 articles that did not meet the inclusion criteria.37–40 This left a total of 13 articles for our literature review (Fig. 1).41–53
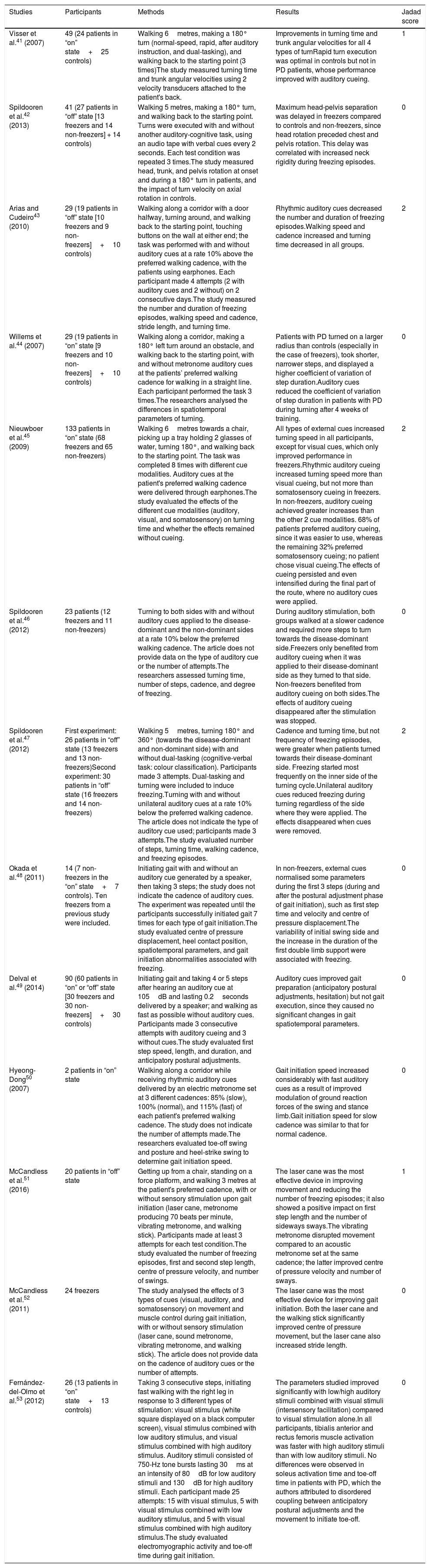
Table 1 describes the characteristics of the studies selected (which include a total of 546 participants), including authors, number of participants, and disease state (on/off) of the patients with PD, methodology, most remarkable results, and Jadad score.
Description of the studies included in our literature review.
| Studies | Participants | Methods | Results | Jadad score |
|---|---|---|---|---|
| Visser et al.41 (2007) | 49 (24 patients in “on” state+25 controls) | Walking 6metres, making a 180° turn (normal-speed, rapid, after auditory instruction, and dual-tasking), and walking back to the starting point (3 times)The study measured turning time and trunk angular velocities using 2 velocity transducers attached to the patient's back. | Improvements in turning time and trunk angular velocities for all 4 types of turnRapid turn execution was optimal in controls but not in PD patients, whose performance improved with auditory cueing. | 1 |
| Spildooren et al.42 (2013) | 41 (27 patients in “off” state [13 freezers and 14 non-freezers] + 14 controls) | Walking 5 metres, making a 180° turn, and walking back to the starting point. Turns were executed with and without another auditory-cognitive task, using an audio tape with verbal cues every 2 seconds. Each test condition was repeated 3 times.The study measured head, trunk, and pelvis rotation at onset and during a 180° turn in patients, and the impact of turn velocity on axial rotation in controls. | Maximum head-pelvis separation was delayed in freezers compared to controls and non-freezers, since head rotation preceded chest and pelvis rotation. This delay was correlated with increased neck rigidity during freezing episodes. | 0 |
| Arias and Cudeiro43 (2010) | 29 (19 patients in “off” state [10 freezers and 9 non-freezers]+10 controls) | Walking along a corridor with a door halfway, turning around, and walking back to the starting point, touching buttons on the wall at either end; the task was performed with and without auditory cues at a rate 10% above the preferred walking cadence, with the patients using earphones. Each participant made 4 attempts (2 with auditory cues and 2 without) on 2 consecutive days.The study measured the number and duration of freezing episodes, walking speed and cadence, stride length, and turning time. | Rhythmic auditory cues decreased the number and duration of freezing episodes.Walking speed and cadence increased and turning time decreased in all groups. | 2 |
| Willems et al.44 (2007) | 29 (19 patients in “on” state [9 freezers and 10 non-freezers]+10 controls) | Walking along a corridor, making a 180° left turn around an obstacle, and walking back to the starting point, with and without metronome auditory cues at the patients’ preferred walking cadence for walking in a straight line. Each participant performed the task 3 times.The researchers analysed the differences in spatiotemporal parameters of turning. | Patients with PD turned on a larger radius than controls (especially in the case of freezers), took shorter, narrower steps, and displayed a higher coefficient of variation of step duration.Auditory cues reduced the coefficient of variation of step duration in patients with PD during turning after 4 weeks of training. | 0 |
| Nieuwboer et al.45 (2009) | 133 patients in “on” state (68 freezers and 65 non-freezers) | Walking 6metres towards a chair, picking up a tray holding 2 glasses of water, turning 180°, and walking back to the starting point. The task was completed 8 times with different cue modalities. Auditory cues at the patient's preferred walking cadence were delivered through earphones.The study evaluated the effects of the different cue modalities (auditory, visual, and somatosensory) on turning time and whether the effects remained without cueing. | All types of external cues increased turning speed in all participants, except for visual cues, which only improved performance in freezers.Rhythmic auditory cueing increased turning speed more than visual cueing, but not more than somatosensory cueing in freezers. In non-freezers, auditory cueing achieved greater increases than the other 2 cue modalities. 68% of patients preferred auditory cueing, since it was easier to use, whereas the remaining 32% preferred somatosensory cueing; no patient chose visual cueing.The effects of cueing persisted and even intensified during the final part of the route, where no auditory cues were applied. | 2 |
| Spildooren et al.46 (2012) | 23 patients (12 freezers and 11 non-freezers) | Turning to both sides with and without auditory cues applied to the disease-dominant and the non-dominant sides at a rate 10% below the preferred walking cadence. The article does not provide data on the type of auditory cue or the number of attempts.The researchers assessed turning time, number of steps, cadence, and degree of freezing. | During auditory stimulation, both groups walked at a slower cadence and required more steps to turn towards the disease-dominant side.Freezers only benefited from auditory cueing when it was applied to their disease-dominant side as they turned to that side. Non-freezers benefited from auditory cueing on both sides.The effects of auditory cueing disappeared after the stimulation was stopped. | 0 |
| Spildooren et al.47 (2012) | First experiment: 26 patients in “off” state (13 freezers and 13 non-freezers)Second experiment: 30 patients in “off” state (16 freezers and 14 non-freezers) | Walking 5metres, turning 180° and 360° (towards the disease-dominant and non-dominant side) with and without dual-tasking (cognitive-verbal task: colour classification). Participants made 3 attempts. Dual-tasking and turning were included to induce freezing.Turning with and without unilateral auditory cues at a rate 10% below the preferred walking cadence. The article does not indicate the type of auditory cue used; participants made 3 attempts.The study evaluated number of steps, turning time, walking cadence, and freezing episodes. | Cadence and turning time, but not frequency of freezing episodes, were greater when patients turned towards their disease-dominant side. Freezing started most frequently on the inner side of the turning cycle.Unilateral auditory cues reduced freezing during turning regardless of the side where they were applied. The effects disappeared when cues were removed. | 2 |
| Okada et al.48 (2011) | 14 (7 non-freezers in the “on” state+7 controls). Ten freezers from a previous study were included. | Initiating gait with and without an auditory cue generated by a speaker, then taking 3 steps; the study does not indicate the cadence of auditory cues. The experiment was repeated until the participants successfully initiated gait 7 times for each type of gait initiation.The study evaluated centre of pressure displacement, heel contact position, spatiotemporal parameters, and gait initiation abnormalities associated with freezing. | In non-freezers, external cues normalised some parameters during the first 3 steps (during and after the postural adjustment phase of gait initiation), such as first step time and velocity and centre of pressure displacement.The variability of initial swing side and the increase in the duration of the first double limb support were associated with freezing. | 0 |
| Delval et al.49 (2014) | 90 (60 patients in “on” or “off” state [30 freezers and 30 non-freezers]+30 controls) | Initiating gait and taking 4 or 5 steps after hearing an auditory cue at 105dB and lasting 0.2seconds delivered by a speaker; and walking as fast as possible without auditory cues. Participants made 3 consecutive attempts with auditory cueing and 3 without cues.The study evaluated first step speed, length, and duration, and anticipatory postural adjustments. | Auditory cues improved gait preparation (anticipatory postural adjustments, hesitation) but not gait execution, since they caused no significant changes in gait spatiotemporal parameters. | 0 |
| Hyeong-Dong50 (2007) | 2 patients in “on” state | Walking along a corridor while receiving rhythmic auditory cues delivered by an electric metronome set at 3 different cadences: 85% (slow), 100% (normal), and 115% (fast) of each patient's preferred walking cadence. The study does not indicate the number of attempts made.The researchers evaluated toe-off swing and posture and heel-strike swing to determine gait initiation speed. | Gait initiation speed increased considerably with fast auditory cues as a result of improved modulation of ground reaction forces of the swing and stance limb.Gait initiation speed for slow cadence was similar to that for normal cadence. | 0 |
| McCandless et al.51 (2016) | 20 patients in “off” state | Getting up from a chair, standing on a force platform, and walking 3 metres at the patient's preferred cadence, with or without sensory stimulation upon gait initiation (laser cane, metronome producing 70 beats per minute, vibrating metronome, and walking stick). Participants made at least 3 attempts for each test condition.The study evaluated the number of freezing episodes, first and second step length, centre of pressure velocity, and number of swings. | The laser cane was the most effective device in improving movement and reducing the number of freezing episodes; it also showed a positive impact on first step length and the number of sideways sways.The vibrating metronome disrupted movement compared to an acoustic metronome set at the same cadence; the latter improved centre of pressure velocity and number of sways. | 1 |
| McCandless et al.52 (2011) | 24 freezers | The study analysed the effects of 3 types of cues (visual, auditory, and somatosensory) on movement and muscle control during gait initiation, with or without sensory stimulation (laser cane, sound metronome, vibrating metronome, and walking stick). The article does not provide data on the cadence of auditory cues or the number of attempts. | The laser cane was the most effective device for improving gait initiation. Both the laser cane and the walking stick significantly improved centre of pressure movement, but the laser cane also increased stride length. | 0 |
| Fernández-del-Olmo et al.53 (2012) | 26 (13 patients in “on” state+13 controls) | Taking 3 consecutive steps, initiating fast walking with the right leg in response to 3 different types of stimulation: visual stimulus (white square displayed on a black computer screen), visual stimulus combined with low auditory stimulus, and visual stimulus combined with high auditory stimulus. Auditory stimuli consisted of 750-Hz tone bursts lasting 30ms at an intensity of 80dB for low auditory stimuli and 130dB for high auditory stimuli. Each participant made 25 attempts: 15 with visual stimulus, 5 with visual stimulus combined with low auditory stimulus, and 5 with visual stimulus combined with high auditory stimulus.The study evaluated electromyographic activity and toe-off time during gait initiation. | The parameters studied improved significantly with low/high auditory stimuli combined with visual stimuli (intersensory facilitation) compared to visual stimulation alone.In all participants, tibialis anterior and rectus femoris muscle activation was faster with high auditory stimuli than with low auditory stimuli. No differences were observed in soleus activation time and toe-off time in patients with PD, which the authors attributed to disordered coupling between anticipatory postural adjustments and the movement to initiate toe-off. | 0 |
All patients had idiopathic PD and were able to walk 10metres without assistance. Non-freezers scored 0 on item 3 of the Freezing of Gait Questionnaire. Only 3 studies indicated the mean time of disease onset. Disease progression time ranged between one and 16 years. Patients were either in the “on” or the “off” state.
Most studies used a metronome for auditory cueing, although some used audio recordings, earphones, or speakers. In most studies, participants made 3 attempts for each task or test condition, during both gait initiation and turning, to obtain objective values for the kinematic variables assessed. The order of attempts was random; participants were allowed to rest between attempts. Most studies did not specify the duration of treatment.
The most frequently used assessment and/or classification scales were the Unified Parkinson's Disease Rating Scale, especially section III; the Hoehn and Yahr scale (most patients were in stages 2 [bilateral involvement without impairment of balance] or 3 [mild to moderate bilateral disease, some postural instability but physically independent]); the Mini–Mental State Examination (>24 points); and the Freezing of Gait Questionnaire.
The most frequently used cadences for the rhythmic auditory cues were either 100 steps per minute (10% above the patients’ usual cadence), 10% below the patient's preferred cadence, or a comfortable cadence for walking in a straight line.
Gait kinematics was analysed with the Vicon 3D motion capture system, retroreflective markers, force platforms, surface electromyography, and specific software for detecting freezing episodes and determining first heel contact position.
The main findings about the effectiveness of acoustic cueing during gait initiation and turning in patients with PD are as follows: (1) the effectiveness of acoustic cueing improves at higher cadences, both in gait initiation (significant increase in gait initiation speed with the fastest cadence used [115%]50 and faster initiation of tibialis anterior and rectus femoris muscle activity with auditory startle stimuli53) and in turning (decreased number and duration of freezing episodes, increased speed and cadence, and shorter turning time at 10% above the patient's usual walking cadence43); (2) at the patient's preferred walking cadence, auditory cues reduced only the variation coefficient of step duration during turning44; and (3) with acoustic cues set 10% below the preferred walking cadence, patients with PD walked at a slower cadence, resulting in longer turning time.46 Gait initiation speed for slow cadence (85%) was similar to that observed for normal cadence (100%).50
Auditory cueing seems to improve preparation for gait initiation by improving anticipatory posture adjustments and reducing start hesitation. Unilateral auditory cueing has been found to reduce freezing during turning when auditory cues are applied to the side most severely affected by PD.46 According to another study, however, unilateral auditory cueing reduces freezing during turning regardless of the side receiving the auditory stimulus.47 The effects of auditory cueing seem to disappear once stimulation stops.46,47
Methodological quality of the studies includedThe studies included in our review are of poor methodological quality according to the Jadad scale: 3 studies scored 2, 2 studies scored 1, and the remaining 8 scored 0 (Table 1).21
DiscussionTo our knowledge, this is the first systematic review of the effectiveness of auditory cueing for improving gait initiation and turning in patients with PD. The small number of articles retrieved and the low methodological quality prevent us from making recommendations with a high level of evidence. However, the studies analysed suggest that high-cadence auditory cueing achieves better results in both gait initiation and turning in these patients, reducing the likelihood of freezing and falls.
First applications, types of auditory stimulus, and neurophysiological basisThe effects of auditory cueing on gait in PD were first evaluated by Thaut et al.54 in 1996. In that study, PD patients had to walk at the pace of a rhythmic auditory stimulus. Subsequent studies seemed to confirm the improvements in walking speed, stride length, and walking cadence.7,55 The most frequent and effective method for auditory cueing in patients with PD is a metronome set 10% above the patients’ normal walking cadence.56–60
The most widely accepted hypothesis of how sensory cues improve gait patterns in PD is that these stimuli reach the premotor cortex and the supplementary motor area via a pathway that bypasses the basal ganglia, compensating for the impaired mechanisms.61–64 Furthermore, auditory stimuli may affect attention mechanisms and the execution of movements requiring greater planning.45,63
Auditory cueing for gait initiation and turningGait initiation requires anticipatory posture adjustment. PD is characterised by a lack of preparation and execution of stepping during gait initiation, which may explain abnormalities in the first step (slower speed and reduced stride length).48,49 External cues reduce abnormalities and decrease the duration of the postural adjustment phase of gait initiation in patients with PD, and increase strength and centre of pressure displacement during this phase.48
Turning is the motor action most frequently triggering freezing (63%), especially during quick 360° turns.42 Freezing may also appear during gait initiation (23%), when walking through narrow passages (12%), and when reaching a destination (9%).14 Freezing most frequently occurs halfway through a 180° turn, that is at 90°, when head-pelvis separation is greatest, and, possibly, the turn is more unstable.42 Studies suggest that cadence and turning time increase when the patient turns towards the most affected side; however, patients experience the same number of freezing episodes regardless of the side of turning.47 Auditory cueing has been found to improve “en bloc” turns, as well as other gait parameters.42,48
Auditory cueing and other types of sensory stimulationAuditory cues improve performance of different types of turns (normal turn, rapid turn, cued turn, dual-tasking) in patients with PD.41 Rhythmic auditory cueing achieves greater turn speed than visual cueing.45 Laser canes have been found to be the most effective cueing devices, reducing the number of freezing episodes and increasing stride length of the first step.51,52 In a case-control study into the effects of startle stimuli, intersensory facilitation (combined visual and auditory cueing) achieved significantly greater improvements in gait initiation than visual cueing alone.53
Our results suggest that auditory cueing (mainly 10% over the normal walking cadence) is more effective for improving walking cadence and turning speed, whereas visual cues are more effective for increasing stride length, an essential factor for gait initiation.64–68 The difficulty of maintaining the benefits of auditory cueing in the long term may be linked to the fact that external stimuli redirect attention towards gait processes and improve execution of gait, but the effects decrease over time,49 as shown by multiple studies.69–72
Our results suggest that a gait training programme with proprioceptive and exteroceptive stimuli at a frequency of 3 half-hour sessions per week may improve stride length, walking cadence, and walking speed and reduces the number of freezing episodes during gait initiation and turning, limiting the risk of falls and improving patients’ quality of life.64 However, this systematic review does have some methodological limitations, such as the fact that we only included studies published in English or Spanish, and the heterogeneous study designs and methodologies of most of the articles included, which prevent us from making recommendations with a high level of evidence.
ConclusionsGait initiation and turning frequently trigger freezing in patients with PD. Auditory cueing has been shown to improve temporal, spatial, and kinematic parameters in both stages. This rehabilitation treatment is most effective when using a metronome set 10% above the patient's normal walking cadence; this increases the speed of gait initiation, muscle activation, walking speed, and gait cadence, and reduces turning time and the duration of freezing episodes, especially with a high-intensity stimulus. Visual cueing, in contrast, has been shown to be more effective in increasing stride length, an essential factor in gait initiation.
Combining auditory cues with conventional rehabilitation for PD may improve motor function and reduce the risk of falls, improving quality of life. Further studies with better methodological quality are necessary to determine which patients are more likely to benefit from auditory cueing, which type of auditory cue should be applied, and the most effective cadence for use during gait initiation and turning in PD.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Gómez-González J, Martín-Casas P, Cano-de-la-Cuerda R. Effects of auditory cues on gait initiation and turning in patients with Parkinson's disease. Neurología. 2019;34:396–407.