Cases of cerebral venous sinus thrombosis have been reported in individuals vaccinated against COVID-19 with non-replicating adenoviral vector vaccines. We issue our recommendations on the diagnosis and management of patients presenting this complication.
MethodsThe multidisciplinary working group, led by the Spanish Federation of Medical and Scientific Associations (FACME) and including representatives of several scientific societies, reviewed the available evidence from the literature and reports of the European Medicines Agency. We establish a definition for suspected cases and issue diagnostic and treatment recommendations regarding vaccine-induced immune thrombotic thrombocytopaenia.
ResultsWe define suspected cases as those cases of cerebral venous sinus thrombosis occurring between 3 and 21 days after the administration of non-replicating adenoviral vector vaccines, in patients with a platelet count below 150 000/μL or presenting a decrease of 50% with respect to the previous value. Findings suggestive of vaccine-induced immune thrombotic thrombocytopaenia include the presence of antibodies to platelet factor 4, D-dimer levels 4 times greater than the upper limit of normal, and unexplained thrombosis. The recommended treatment includes intravenous administration of non-specific human immunoglobulin or alternatively plasmapheresis, avoiding the use of heparin, instead employing argatroban, bivalirudin, fondaparinux, rivaroxaban, or apixaban for anticoagulation, and avoiding platelet transfusion.
ConclusionsNon-replicating adenoviral vector vaccines may be associated with cerebral venous sinus thrombosis with thrombocytopaenia; it is important to treat the dysimmune phenomenon and the cerebral venous sinus thrombosis.
Se han reportado casos de trombosis venosas cerebral en personas vacunadas frente a COVID-19 con vacunas vectoriazadas con adenovirus no replicantes. Aportamos recomendaciones sobre el diagnóstico y manejo de pacientes con esta complicación.
MétodoEl Grupo de Trabajo multidisciplinar, liderado por la Federación de Asociaciones Científico Médicas Españolas (FACME) y representado por distintas sociedades científicas, revisó la evidencia disponible publicada en la literatura y en los informes de la Agencia Europea del Medicamento. Se estableció una definición de caso sospechoso y recomendaciones diagnóstico-terapéuticas de la trombocitopenia trombótica inducida por la vacunación.
ResultadosSe considera caso sospechoso aquella trombosis venosa cerebral ocurridas entre 3 y 21 días tras la administración de vacunas no replicantes de adenovirus que presenten un valor de plaquetas inferior a 150.000 plaquetas por μL o un descenso del 50% respecto de la cifra previa. Los datos sugestivos de trombocitopenia trombótica inducida por la vacunación incluyen la presencia de anticuerpos anti-factor plaquetario tipo 4, la elevación de dímero-D cuatro veces por encima del límite superior de la normalidad o la ausencia de justificación de la trombosis. En su tratamiento, se recomienda administrar inmunoglobulina humana inespecífica intravenosa o realizar plasmaféresis en su defecto, evitar el uso de heparina, empleando como anticoagulantes argatroban, bivalirudina, fondaparinux, rivaroxaban o apixaban, y evitar la transfusión de plaquetas.
ConclusionesLas vacunas de vectores no replicantes de adenovirus pueden asociarse a trombosis venosas cerebrales con trombocitopenia, en cuyo manejo es importante el tratamiento del fenómeno disinmune y de la trombosis venosa cerebral.
The coronavirus disease 2019 (COVID-19) pandemic has caused more than 3 million deaths worldwide1 and more than 77 000 in Spain,2 together with an excess mortality rate of up to 65% with respect to figures from the previous 10 years.3 The insufficient benefit of the different treatments studied4–6 has meant that all hope has been placed on vaccines. The benefits shown in the clinical trials conducted to date7–13 and the data on the current epidemiological situation are encouraging.14
On 7 March 2021, Austria reported the first 2 cases of venous thrombosis with atypical clinical manifestation in patients who have received the AstraZeneca vaccine (Vaxzevria). On 14 March 2021, Spain reported its first case. On 29 March 2021, after reviewing the evidence available at the time, the European Pharmacovigilance Risk Assessment Committee (PRAC) concluded that the number of thromboembolic events reported in vaccinated individuals was lower than that expected in the general population. However, Vaxzevria may be associated with cases of atypical thrombosis, such as disseminated intravascular coagulation or cerebral venous sinus thrombosis with a distinctive feature: onset together with thrombocytopaenia.15 A second assessment by the PRAC, whose conclusions were published on 14 April, concluded that this causal association was plausible, but that considering the limited available evidence, no risk factors could be identified in the reported cases, and no clinical practice recommendations were issued.16
The benefits of vaccination for the general population are unquestionable. However, it is essential that healthcare professionals be trained to adequately detect and manage such infrequent but severe adverse reactions as cerebral venous sinus thrombosis with thrombocytopaenia. The aim of this document is to provide practical recommendations on the diagnosis and management of patients with cerebral venous sinus thrombosis and thrombocytopaenia after vaccination with recombinant adenovirus vaccines (Vaxzevria by AstraZeneca and Janssen, although the association currently seems to be stronger with the AstraZeneca vaccine).17 Considering the changing situation and the constant publication of new information, it is important to refer to official recommendations, which are more regularly updated than this consensus statement.18
DevelopmentDefinition of suspected caseIn addition to the homogeneous pattern of onset in the first 3 weeks after vaccination, the characteristics considered when establishing the association with the vaccine include: (1) an observed frequency of 4.94 (95% confidence interval [CI], 2.36-8.45) times higher than that expected for a given population and period of time19; (2) greater severity than that observed in cases not associated with the vaccine, amounting to a mortality rate of up to 36.4%17,19,20; (3) association with thrombocytopaenia in a significant percentage of cases15,17,19; (4) the biological plausibility of immunological origin and the presence of pathophysiological mechanisms that may explain part of the phenomenon; and (5) the lack of an alternative hypothesis that may explain symptom onset.
Pathophysiological hypothesisThe association with thrombocytopaenia and the severity of the events somewhat resemble what occurs in heparin-induced thrombocytopaenia (HIT),21,22 although these patients had not received heparin.16The factor triggering these symptoms in vaccinated patients is yet to be determined. Thrombocytopaenia after administration of adenoviral vector vaccines,23,24 as well as autoimmunity, has been described in primates and rodents after intravenous administration of adenoviral vaccine.23,25–27 Presence of antibodies targeting platelet factor 4 (anti-PF4) has been reported in patients presenting thrombotic events after Vaxzevria administration.28,29
Thrombocytopaenia-associated thrombosisSeveral terms have been used, including vaccine-induced prothrombotic immune thrombocytopaenia (VIPIT), vaccine-induced immune thrombotic thrombocytopaenia (VITT),28–30 atypical heparin-induced thrombocytopaenia, and thrombosis with thrombocytopaenia syndrome.31 However, thrombocytopaenia was not described in some reported cases.16 This may be because: (1) thrombocytopaenia was not detected or reported, despite being present (eg, blood analysis was not performed); (2) the patient presented decreased platelet count with regard to baseline values, but not below 150 000 platelets/μL; or (3) symptoms were caused by a different phenomenon.
Working definitionFrom a practical point of view, we recommend searching for a venous thromboembolic event in any location, occurring between 3 and 21 days after the administration of a non-replicating adenoviral vector vaccine (Vaxzevria or Janssen).18,31
A complete blood count should be performed in suspected cases. Thrombocytopaenia is defined as a platelet count below 150 000 platelets/μL or a decrease of 50% with regard to the previous value, provided that this value is known and measurement was reasonably recent (previous 3 months). In the event of thrombocytopaenia, it is recommended to perform a blood smear to rule out pseudothrombocytopaenia as a result of platelet clumping.
If thrombocytopaenia is confirmed, anti-PF4 antibodies should be determined: presence of these antibodies has been described in patients with VITT30,32,33 and would lead to diagnosis of this condition and the appropriate treatment.18,34. ELISA antibody determination is more sensitive than the screening tests more commonly used in HIT (particle gel immunoassay, chemiluminescence), although it may not be available at all centres. A serum or plasma sample should be frozen for subsequent functional assays of platelet activation at a reference laboratory.18,34
A pronounced increase in D-dimer levels has also been reported.18,34 In this context, a value 4 times higher than the upper limit of normal should raise suspicion of VITT.18 In the event of negative results for anti-PF4 antibodies (especially when ELISA is not available) and normal D-dimer levels, in the absence of other possible causes of thrombocytopaenia, it is advisable to manage the thrombotic complication as in patients with VITT. In patients with normal platelet counts, there is insufficient information to diagnose VITT; therefore, close monitoring of platelet count is necessary.18
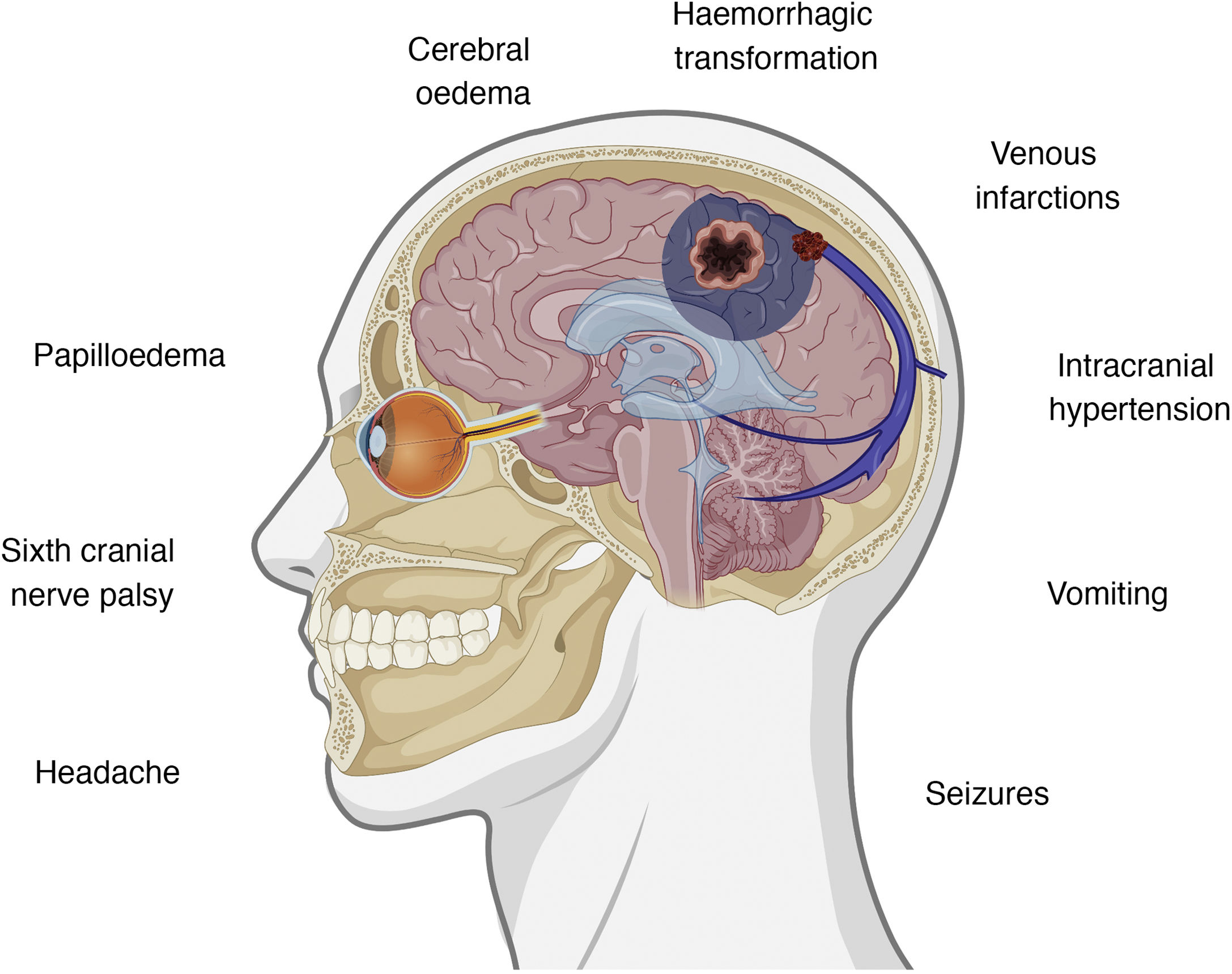
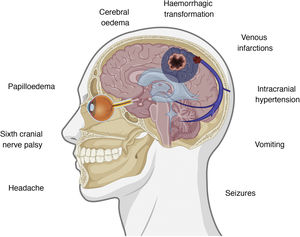
Clinical presentation of cerebral venous sinus thrombosisThe intracranial venous system drains blood from the brain and contributes to the reabsorption of cerebrospinal fluid (CSF) through arachnoid granulations.35 Interruption of flow may cause several symptoms, with clinical presentation varying according to the location of thrombosis and the effectiveness of the alternative drainage pathways. There is some correlation between clinical symptoms and certain intracranial abnormalities, namely intracranial hypertension, venous infarctions, and the presence of underlying haemorrhages.36,37Fig. 1 summarises the symptoms and pathophysiological events involved in cerebral venous sinus thrombosis.
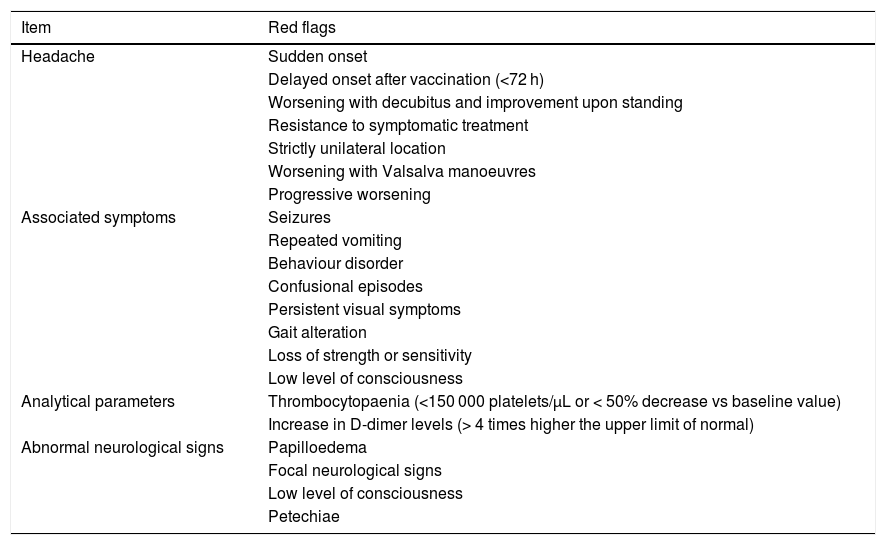
If some red flags are present in the appropriate clinical context, cerebral venous sinus thrombosis must be ruled out.38 The most frequent symptom of cerebral venous sinus thrombosis is headache, presenting in up to 88% of patients.38–40 Headache is also one of the most frequently reported symptoms after vaccination,7–13 occurring in up to 67% of vaccinated individuals; however, onset is usually immediate. The predictive value of red flags in this condition is not established; therefore, if none are present but some data are atypical or concerning in the opinion of the patient’s physician, this possibility should also be considered. Table 1 summarises the main red flags for cerebral venous sinus thrombosis.38–40
Red flags for suspicion of a possible cerebral venous sinus thrombosis following vaccination.
| Item | Red flags |
|---|---|
| Headache | Sudden onset |
| Delayed onset after vaccination (<72 h) | |
| Worsening with decubitus and improvement upon standing | |
| Resistance to symptomatic treatment | |
| Strictly unilateral location | |
| Worsening with Valsalva manoeuvres | |
| Progressive worsening | |
| Associated symptoms | Seizures |
| Repeated vomiting | |
| Behaviour disorder | |
| Confusional episodes | |
| Persistent visual symptoms | |
| Gait alteration | |
| Loss of strength or sensitivity | |
| Low level of consciousness | |
| Analytical parameters | Thrombocytopaenia (<150 000 platelets/μL or < 50% decrease vs baseline value) |
| Increase in D-dimer levels (> 4 times higher the upper limit of normal) | |
| Abnormal neurological signs | Papilloedema |
| Focal neurological signs | |
| Low level of consciousness | |
| Petechiae |
We recommend measuring anti-PF4 antibodies in a sample taken before administration of immunoglobulins.18,30,32–35 Positive results indicate VITT; however, negative results, especially when ELISA is not available, do not rule out VITT.
Diagnosis of cerebral venous sinus thrombosisRadiological examination of cerebral venous sinus thrombosis may vary depending on the patient’s symptoms and the radiological techniques available (computed tomography [CT], magnetic resonance imaging [MRI]).
In the event of acute onset, a non-contrast CT scan is frequently the first study to be performed. However, this study shows poor sensitivity, as it only displays indirect and suggestive alterations of cerebral venous sinus thrombosis in 30% of patients. Thus, if cerebral venous sinus thrombosis is suspected, non-contrast CT should be complemented with a contrast CT scan and three-dimensional venous reconstruction (CT venography)41–46.
In patients with subacute onset, MRI is the study of choice, provided that it is immediately available and the patient has no contraindication to the technique. The appropriate technical protocol includes sequences with and without contrast, complemented with an MRI venography. MRI is also useful in assessing the possible complications of cerebral venous sinus thrombosis, such as venous infarction, haemorrhage, and oedema.
Considering the high diagnostic sensitivity and specificity of non-invasive tests in the diagnosis of cerebral venous sinus thrombosis, direct catheter venography is rarely necessary, and should be used only when intravascular treatment is needed.
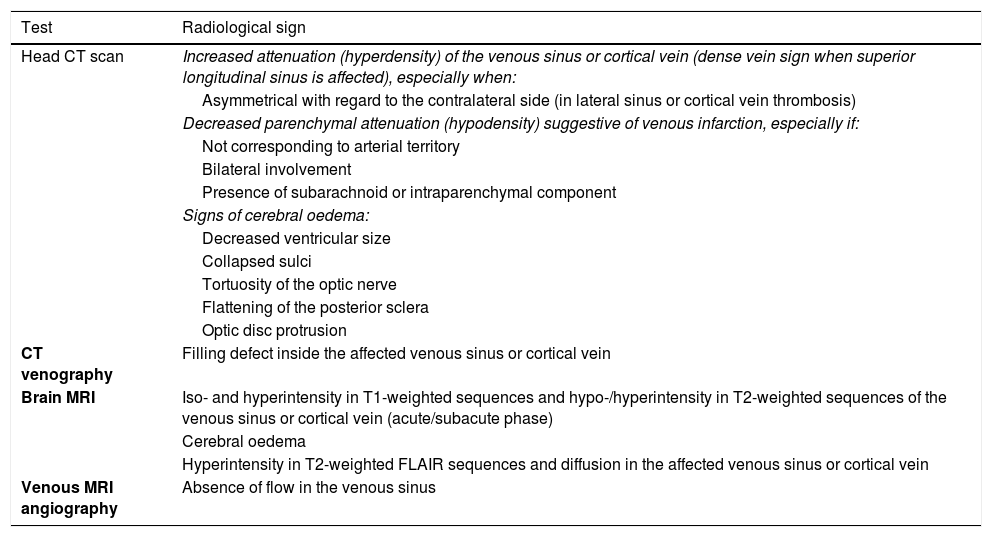
Despite its low sensitivity, a simple CT scan may reveal some signs of cerebral venous sinus thrombosis. Presence of these signs obliges us to consider this diagnosis, in the event that it was omitted earlier. Table 2 summarises the main radiological signs of cerebral venous sinus thrombosis.41–49
Direct and indirect radiological signs providing value and certainty in the diagnosis of cerebral venous sinus thrombosis.
| Test | Radiological sign |
|---|---|
| Head CT scan | Increased attenuation (hyperdensity) of the venous sinus or cortical vein (dense vein sign when superior longitudinal sinus is affected), especially when: |
| Asymmetrical with regard to the contralateral side (in lateral sinus or cortical vein thrombosis) | |
| Decreased parenchymal attenuation (hypodensity) suggestive of venous infarction, especially if: | |
| Not corresponding to arterial territory | |
| Bilateral involvement | |
| Presence of subarachnoid or intraparenchymal component | |
| Signs of cerebral oedema: | |
| Decreased ventricular size | |
| Collapsed sulci | |
| Tortuosity of the optic nerve | |
| Flattening of the posterior sclera | |
| Optic disc protrusion | |
| CT venography | Filling defect inside the affected venous sinus or cortical vein |
| Brain MRI | Iso- and hyperintensity in T1-weighted sequences and hypo-/hyperintensity in T2-weighted sequences of the venous sinus or cortical vein (acute/subacute phase) |
| Cerebral oedema | |
| Hyperintensity in T2-weighted FLAIR sequences and diffusion in the affected venous sinus or cortical vein | |
| Venous MRI angiography | Absence of flow in the venous sinus |
CT: computed tomography; MRI: magnetic resonance imaging.
Techniques providing diagnostic certainty are shown in bold.
Any individual presenting a thrombotic event following administration of a non-replicating adenoviral vector vaccine should be hospitalised, even if he/she is clinically stable and paucisymptomatic, as there are reports of greater severity than in conventional forms and cases of rapid clinical worsening.18 Management should be multidisciplinary and involve the specialists who treat this type of thrombosis at the centre and a haematologist with experience treating HIT. Treatment is based on 2 pillars: the dysimmune phenomenon and the cerebral thrombosis.
Treatment for vaccine-induced immune thrombotic thrombocytopaeniaIn the event of diagnosis or reasonable suspicion of VITT, administration of platelets is contraindicated unless there is clinically relevant active bleeding or some invasive procedure with a high associated risk of bleeding.18,34 Furthermore, we recommend blocking anti-PF4–mediated platelet activation and aggregation by administering intravenous non-specific human immunoglobulins dosed at 1 g/kg/day for 2 days or 0.4 g/kg/day for 5 days; no prior measurement of serum immunoglobulin levels is required. Use of this treatment has been reported in several cases to date.29,30,32,33,50 Alternatively, plasmapheresis with albumin replacement may be used if immunoglobulins are contraindicated. Although thrombosis is a well-known risk after immunoglobulin administration, it is infrequent, and the benefit seems to outweigh the risk.51,52
Treatment for cerebral venous sinus thrombosisCurrent evidence on the management of cerebral venous sinus thrombosis due to any cause is weak.53 In our context, treatment will be decided according to the presence or absence of suspected VITT. If it is suspected, and according to the working criteria defined above, administration of heparin is not recommended, either as treatment or in such procedures as hepsal flushes.18,34
Anticoagulant therapy for cerebral venous sinus thrombosis in the absence of suspected VITTThe main recommendations are those of the 2010 European guidelines,54 the 2011 American guidelines,55 and the 2017 European guidelines.56 The drugs for which most evidence is available are low molecular weight heparin (LMWH) and unfractionated heparin (UFH).57–63 One study revealed a lower mortality rate, a higher rate of complete recovery, and a lower rate of bleeding with LMWH.60 Another study showed lower rates of mortality and dependence in patients treated with LMWH than in those receiving UFH, as well as a lower rate of new intracerebral haemorrhages.61 A third study with a smaller sample size revealed no differences between the 2 treatments.62 A systematic review published in 2017 found a trend towards a higher mortality rate and better functional prognosis in patients treated with LMWH, with no differences in the rate of extracranial haemorrhage.63
Anticoagulant therapy for cerebral venous sinus thrombosis in patients with suspected VITTLMWH and UFH are not currently recommended,18–34 and an alternative anticoagulant should be used.63 There is little evidence on any of the alternative therapeutic options for cerebral venous sinus thrombosis.64 Argatroban seems to be an alternative in the treatment of HIT,65,66 although there is a lack of robust evidence on its use in the treatment of cerebral venous sinus thrombosis67; however, studies on ischaemic stroke suggest an adequate level of safety.68–71 Cases have been reported on the use of argatroban to treat VITT.30,50 Evidence on bivalirudin in the treatment of cerebral venous sinus thrombosis is also very limited,72 although a systematic review published in 2017 concluded that hirudin analogues (lepirudin and bivalirudin) presented similar rates of thrombotic and haemorrhagic complications to those of argatroban,73 which has also been used in cases of VITT.50 Its administration should be closely monitored.74 Fondaparinux75 is another agent proposed for the treatment of HIT,76 although evidence on its use for treating cerebral venous sinus thrombosis is limited to one case.77
The use of direct-acting anticoagulants may be considered in less severe forms. Rivaroxaban78–84 and apixaban84,85 are the drugs for which most evidence is available, although it is of low quality.86–89 Recommendation of these drugs is also justified by the fact that they do not require the concomitant use of heparin at treatment onset.75
There is limited evidence to recommend systematic endovascular or surgical treatment.90–93 These treatments may be considered in patients presenting poor response to pharmacological treatment at centres with experience in their use.56,57,94
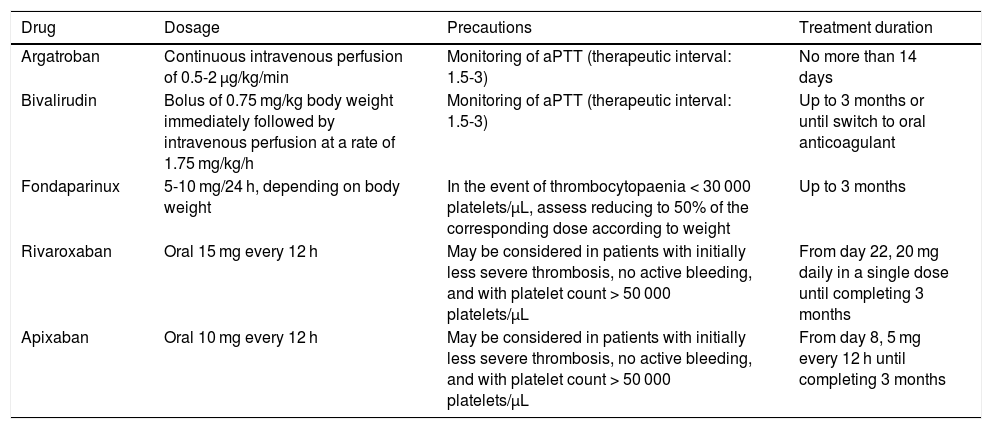
Table 3 describes the main drugs recommended, their dosage, and treatment duration.75 Considering that VITT is a provoked venous sinus thrombosis, the recommended anticoagulant treatment duration would be 3 months, but should be tailored to each patient.
Anticoagulant treatment in patients presenting cerebral venous sinus thrombosis after administration of non-replicating adenoviral vector vaccines and suspected vaccine-induced immune thrombotic thrombocytopaenia.
| Drug | Dosage | Precautions | Treatment duration |
|---|---|---|---|
| Argatroban | Continuous intravenous perfusion of 0.5-2 μg/kg/min | Monitoring of aPTT (therapeutic interval: 1.5-3) | No more than 14 days |
| Bivalirudin | Bolus of 0.75 mg/kg body weight immediately followed by intravenous perfusion at a rate of 1.75 mg/kg/h | Monitoring of aPTT (therapeutic interval: 1.5-3) | Up to 3 months or until switch to oral anticoagulant |
| Fondaparinux | 5-10 mg/24 h, depending on body weight | In the event of thrombocytopaenia < 30 000 platelets/μL, assess reducing to 50% of the corresponding dose according to weight | Up to 3 months |
| Rivaroxaban | Oral 15 mg every 12 h | May be considered in patients with initially less severe thrombosis, no active bleeding, and with platelet count > 50 000 platelets/μL | From day 22, 20 mg daily in a single dose until completing 3 months |
| Apixaban | Oral 10 mg every 12 h | May be considered in patients with initially less severe thrombosis, no active bleeding, and with platelet count > 50 000 platelets/μL | From day 8, 5 mg every 12 h until completing 3 months |
aPTT: activated partial thromboplastin time.
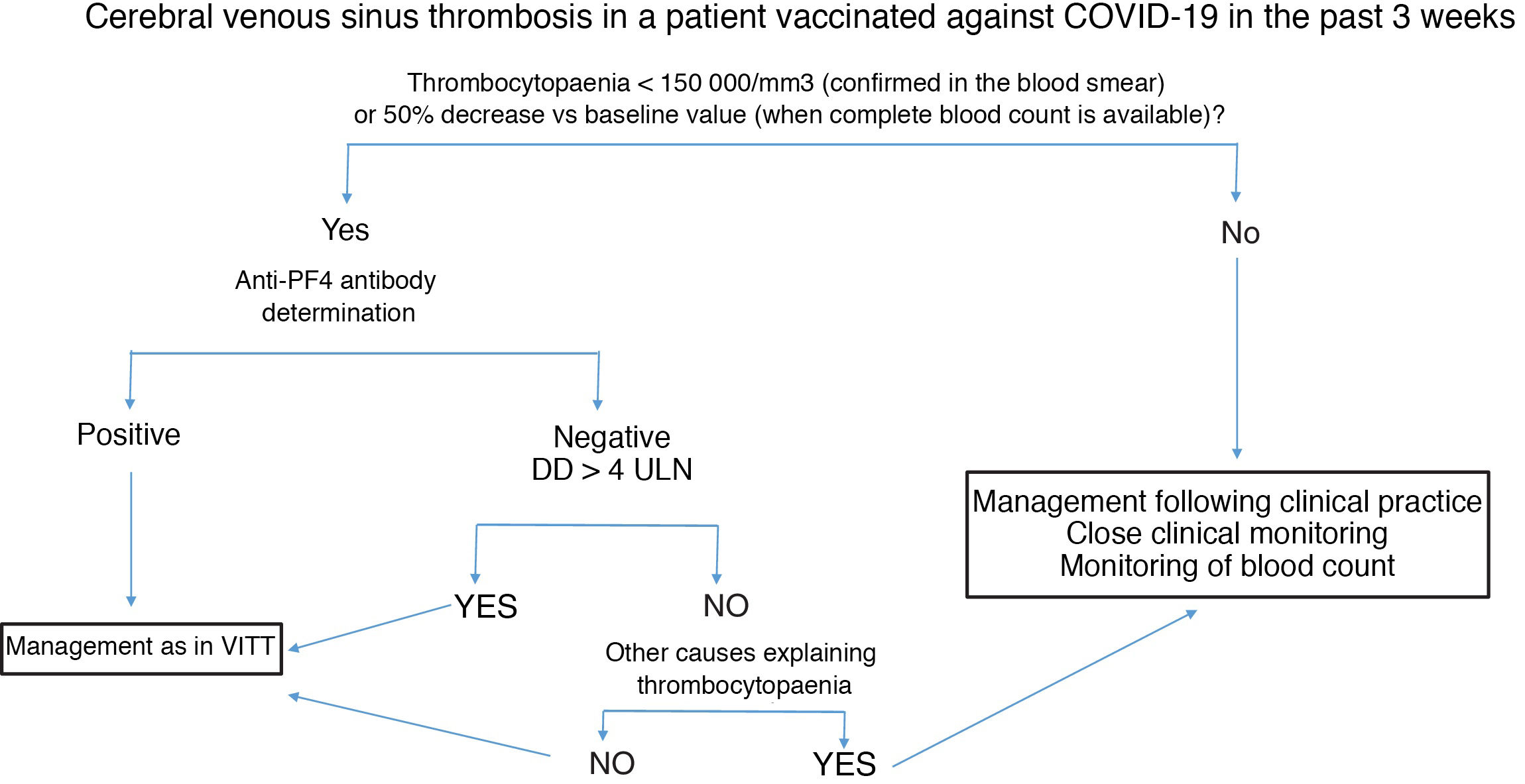
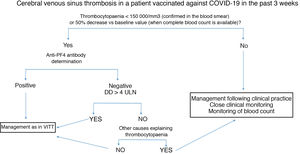
Fig. 2 summarises the diagnostic and therapeutic management of patients presenting cerebral venous sinus thrombosis after vaccination against COVID-19.
Diagnostic and therapeutic management of patients presenting cerebral venous sinus thrombosis after vaccination against COVID-19. We recommend freezing a baseline serum sample for a subsequent functional study, prior to administration of immunoglobulins.
Anti-PF4: antibodies targeting platelet factor 4; DD: D-dimer; ULN: upper limit of normal; VITT: vaccine-induced immune thrombotic thrombocytopaenia.
Suspected cases of VITT after administration of any vaccine against COVID-19 should be notified to the Spanish Pharmacovigilance System (www.notificaram.es) as soon as possible. Reports should include as much information as possible, especially demographic data (age, sex, relevant personal history, thrombosis risk factors, history of COVID-19 and severity), vaccine-related data (date of vaccination, type and batch of vaccine), and clinical data (date of symptom onset and detailed description of the case). It is important to specify the date of diagnosis of the thrombotic complication, location, the diagnostic method, and the presence or absence of associated haemorrhage (location; volume would also be desirable). Regarding laboratory findings, platelet count and D-dimer levels at diagnosis and follow-up should be reported, as well as the results of anti-PF4 antibody measurement and the technique used. In doubtful cases, conventional aetiological study findings may be useful. Regarding treatment, reporting physicians should specify the drugs used, including doses, concomitant treatments, and degree of efficacy, together with short- and medium-term prognosis.
ConclusionsA higher than expected number of cases of cerebral venous sinus thrombosis have been reported in individuals who have received a non-replicating adenoviral vector vaccine. A causal relationship has been established with thrombotic events associated with thrombocytopaenia. Non-replicating adenoviral vector vaccines may rarely cause thrombosis with thrombocytopaenia in less frequent locations, such as the cerebral venous sinuses. These cases are characterised by thrombocytopaenia or a 50% decrease in platelet counts with regard to previous analyses, high D-dimer levels, and presence of anti-PF4 antibodies. In this context, we recommend treatment with immunoglobulins and anticoagulants that are less frequently used in cerebral venous sinus thrombosis, such as argatroban, bivalirudin, fondaparinux, rivaroxaban, or apixaban. Patients not presenting the factors mentioned above may be treated in the same way as cases of conventional cerebral venous sinus thrombosis, although close clinical and laboratory follow-up is particularly important. More evidence on the management of this complication is urgently needed.
AuthorsAll authors have made substantial contributions to the drafting, conception, and review of the manuscript, as well as to the definitive approved version of the study.
Conflicts of interestsThe authors have no conflicts of interest to declare.
Authors:
C. Avendaño-Solá, Servicio de Farmacología Clínica, Hospital Universitario Puerta de Hierro, Junta Directiva de la Federación de Asociaciones Científico Médicas de España (FACME), Majadahonda, Madrid, Spain.
R. de la Cámara, Servicio de Hematología, Hospital de La Princesa, Madrid, España.
M. Castellanos (ORCID: 0000-0003-3116-1352), Servicio de Neurología, Complejo Hospitalario Universitario A Coruña, Instituto de Investigación Biomédica A Coruña, A Coruña, Spain.
D. Ezpeleta (ORCID: 0000-0002-9226-4550), Servicio de Neurología, Hospital Universitario Quirónsalud Madrid, Pozuelo de Alarcón, Madrid, Spain.
D. García-Azorín (ORCID: 0000-0002-3132-1064), Unidad de Cefaleas, Servicio de Neurología, Hospital Clínico Universitario de Valladolid, Valladolid, Spain.
C. Iñiguez Martínez (ORCID: 0000-0003-3746-3001), Servicio de Neurología, Hospital Clínico Universitario Lozano Blesa, Instituto de Investigación Sanitaria de Aragón (IIS Aragón), Zaragoza, España.
R. Lecumberri, Servicio de Hematología, Clínica Universidad de Navarra, Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBER-CV), Instituto de Salud Carlos III, Pamplona, Navarra, Spain.
M. Marti de Gracia (ORCID: 0000-0001-7843-9417), Sección de Radiología de Urgencias, Hospital Universitario La Paz, Madrid, Spain.
E. Redondo Margüello (ORCID: 0000-0003-2791-979X), Centro de Salud y Vacunación Internacional, Madrid Salud, Ayuntamiento de Madrid, Madrid, Spain.
A. Rovira (ORCID: 0000-0002-2132-6750), Sección de Neurorradiología, Hospital Universitario Vall d’Hebron, Barcelona, Spain.
A. Sancho-López, Servicio de Farmacología Clínica, Hospital Universitario Puerta de Hierro Majadahonda, Vocal SEFC, Grupo de Vacunas de la Federación de Asociaciones Científico Médicas Españolas (FACME), Majadahonda, Madrid, Spain.
P. Garrido (ORCID: 0000-0002-5899-6125), Servicio de Oncología Médica, Hospital Universitario Ramón y Cajal, Instituto Ramón y Cajal de Investigación Sanitaria (IRYCIS), Federación de Asociaciones Científico Médicas Españolas (FACME), Madrid, Spain.
All authors are members of the FACME multidisciplinary working group on the management of cerebral venous sinus thrombosis associated with COVID-19 vaccination, and are listed in the Appendix A.
Please cite this article as: García-Azorín D, FACME ad-hoc working group, Recomendaciones diagnóstico-terapéuticas del grupo de trabajo de expertos de FACME ad-hoc sobre el manejo de la trombosis venosa cerebral relacionada con la vacunación frente a COVID-19. Neurología. 2021;36:451–461.
E-mail address: dgazorin@ucm.es (D. García-Azorín).