This study evaluates the cost-effectiveness of 5-aminolevulinic acid (5-ALA, Gliolan®) in patients undergoing surgery for malignant glioma, in standard clinical practice conditions in Spain.
Material and methodsCost-effectiveness ratios were determined in terms of incremental cost per complete resection (CR) and incremental cost per additional quality-adjusted life year (QALY), based on data collected in the VISIONA observational study.
ResultsIncremental cost with 5-ALA versus conventional surgery using white light only amounts to €4550 per additional CR achieved and €9021 per QALY gained. A sensitivity analysis shows these results to be robust.
ConclusionMalignant glioma surgery guided by 5-ALA fluorescence entails a moderate increase in hospital costs compared to current surgical practice and can be considered a cost-effective innovation.
Evaluar el coste-efectividad del ácido 5-aminolevulínico (5-ALA, Gliolan®) en pacientes intervenidos quirúrgicamente de glioma maligno, en condiciones de práctica médica habitual en España.
Material y métodosSe determinaron las ratios de coste incremental por resección completa (RC) y de coste incremental por año de vida ajustado por calidad (AVAC) ganado, sobre la base de los datos recogidos en el estudio observacional VISIONA.
ResultadosEl coste incremental con 5-ALA frente a la cirugía convencional con luz blanca asciende a 4.550 € por RC adicional conseguida y a 9.021 € por AVAC ganado. Estos resultados se muestran consistentes en un análisis de sensibilidad.
ConclusiónLa cirugía del glioma maligno guiada por fluorescencia con 5-ALA conlleva un incremento de costes moderado respecto a la práctica quirúrgica actual y muestra una relación coste-efectividad favorable.
Malignant gliomas (grades III and IV according to the WHO classification system) constitute a relatively infrequent type of tumour with an approximate annual incidence rate of 6 per 100000 persons. However, prognosis is poor for this type of brain tumour since no curative treatment is currently available.1–3 The standard therapeutic approach entails wide-margin resection of the tumour without affecting eloquent areas, followed by radiotherapy and chemotherapy.4
Studies have shown that complete resection (CR) of the part of the tumour showing contrast uptake in the MRI study is associated with better survival.5–9 However, identifying tumour margins during surgery is extremely difficult, so CR cannot be achieved in many patients.5
Fluorescence induced by 5-aminolevulinic acid (5-ALA, Gliolan®), a drug delivered orally approximately 3hours before anaesthesia, outlines tumour margins more clearly, which can substantially improve surgery outcomes.6,7 Use of this technique was endorsed by the neuro-oncology working group of the Spanish Society of Neurosurgery in a recent consensus document.10 However, since this new therapeutic approach requires additional healthcare resources, researchers need to determine whether it is cost-effective. Our study evaluates the technique's cost-effectiveness in the context of the Spanish healthcare system. To this end, we determined the incremental cost-effectiveness of 5-ALA surgery in terms of cost per additional CR achieved compared to current CR rate, and incremental cost-utility in terms of cost per quality-adjusted life year gained.
Material and methodsCost-effectivenessWe calculated the cost-effectiveness ratio of 5-ALA guided surgery as follows:
Cost-utility analysis is a variant of cost-effectiveness analysis in which achieved effectiveness is expressed in additional quality-adjusted life years (QALY). In our study, we estimated the incremental cost-utility ratio as follows:
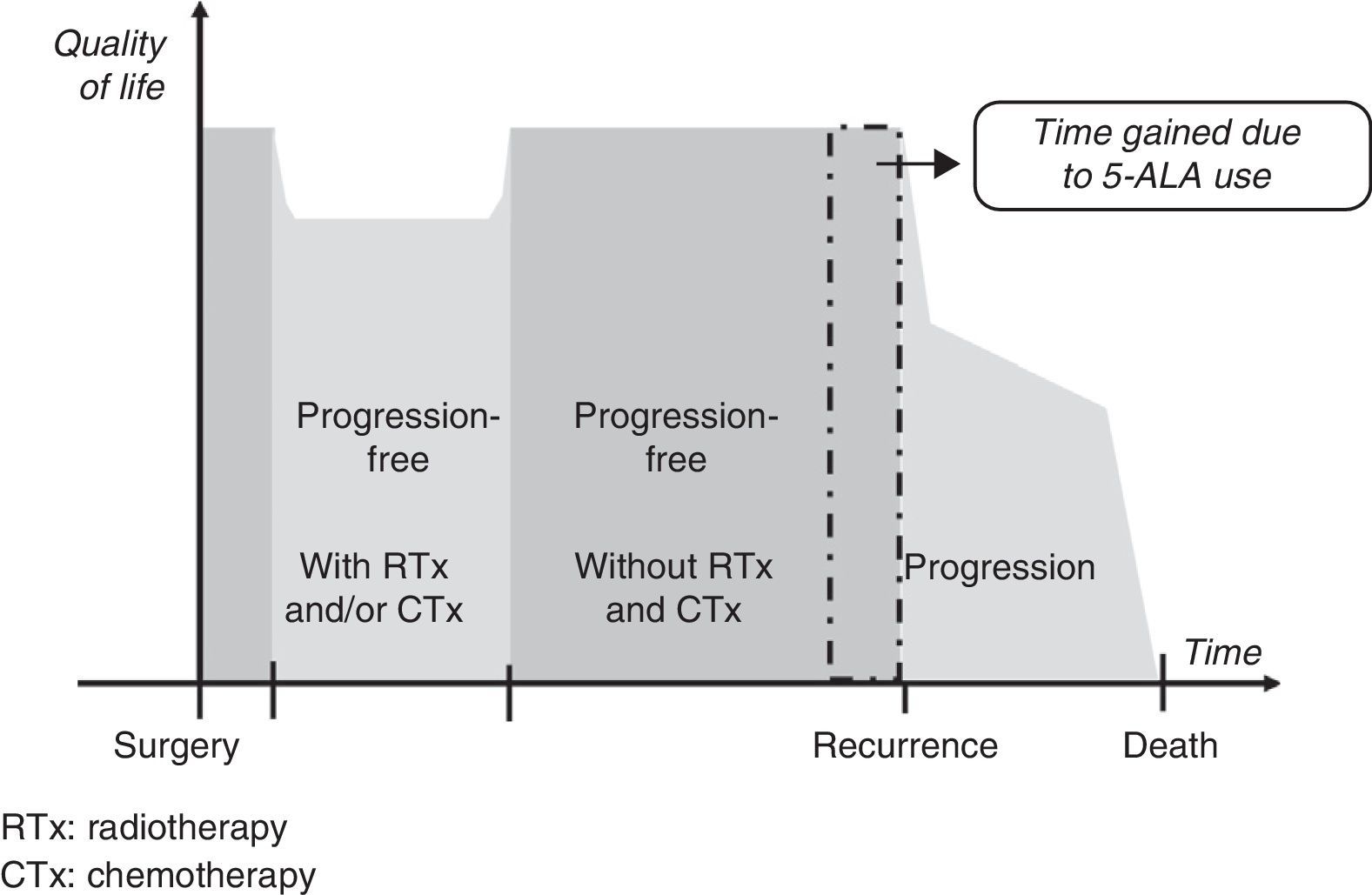
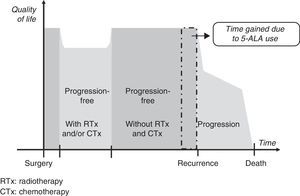
Gain in QALY was regarded as the increase in progression-free survival (PFS) resulting from resection of grade IV malignant gliomas using 5-ALA compared to that achieved using white light, adjusted for quality of life in patients who were progression-free after having undergone surgery, radiotherapy, and chemotherapy. This definition is based on a diagram of disease progression and treatment (Fig. 1). This model differentiates 3 phases after surgery in which levels of quality of life11 may differ substantially: a stage including radiotherapy and/or chemotherapy, a progression-free stage with no radiotherapy or chemotherapy, and a final stage initiating after recurrence.
We suggest that a higher CR rate might result in more prolonged PFS in patients undergoing fluorescence-guided surgery, but it will not affect life expectancy or quality once recurrence occurs. For this reason, our working hypothesis was that there would be no differences between the 2 groups included in our analysis in terms of clinical parameters and use of healthcare resources between time of surgery and the end of radiotherapy and chemotherapy, or between detection of recurrence and the patient's death. It was consequently suggested that the only difference between the 2 patient groups would be an increase in progression-free time in the group undergoing 5-ALA guided surgery.
Source of clinical dataClinical data used in the present analysis were obtained from the VISIONA study database, a recent retrospective observational study conducted in Spain that compared fluorescent-guided malignant glioma resection with 5-ALA to conventional resection under white light. Full details from this project were recently published in another article.12 In this study, researchers selected a group of Spanish hospitals offering neurosurgery in which tumour resection with 5-ALA was a frequent surgical procedure, as well as another group of hospitals in which this treatment option was not available. They gathered data from 251 evaluable patients treated surgically in or after July 2008, when 5-ALA was made available in Spain for compassionate use if requested from the Spanish regulatory authorities. Inclusion and exclusion criteria were formulated so as to include patients meeting the requirements for 5-ALA to be clinically indicated. This meant that use of 5-ALA did not depend on the patient's characteristics, but rather on whether that procedure was available at the hospital where he or she underwent surgery.
Increased effectivenessOur economic analysis required data on the clinical impact of this procedure, measured in terms of CR rate and PFS time. CR rate was defined as the percentage of patients whose grade III and IV gliomas were resected with 5-ALA-guided surgery or traditional white-light based microsurgery and who displayed no contrast enhancement in a postoperative MRI scan taken prior to radiotherapy. Those cases in which MRI-based evaluations of residual tumour presence were inconclusive were regarded as incomplete resections.
For PFS analysis, we included only those patients with grade IV gliomas since survival rates for patients with grade III gliomas are quite different. PFS time was defined as the number of months elapsed between surgery and disease progression or death by any cause. Disease progression was defined as the appearance of new contrast-enhanced lesions larger than 1cm, increase in tumour size of 25% or more in CT or MRI scans, clinical or neurological deterioration, or needing higher doses of corticosteroids. We used the Kaplan-Meier method to analyse PFS time and estimate the restricted mean survival time for each group in the analysis.
Health utilities after glioma surgery, which are needed for the quality of life adjustment applied to PFS time gained, were taken from a recent article by Rogers et al.11 These authors measured utility values using what is known as the ‘standard gamble method’.13
Incremental costsThe additional costs of 5-ALA guided surgery essentially amount to the cost of using Gliolan®: a 1.5g vial of that product is priced at €980 (wholesale), according to the drug database maintained by the Spanish General Council of Official Pharmacists’ Associations. We emphasise that 5-ALA Gliolan® should only be used by experienced neurosurgeons proficient in malignant glioma surgery, thoroughly knowledgeable about functional brain anatomy, and who have completed a training course in fluorescence-guided surgery, according to the current guidelines established by the European Medicines Agency for this drug. The drug company that markets Gliolan® in Spain offers training courses, as is required by the risk management plan for 5-ALA. As a result, training costs are not borne by the Spanish healthcare system and have therefore not been included in the present study.
Since the incremental costs are paid at the beginning of the analysed period (at time of surgery), no discounting has been applied.
Sensitivity analysisSince some of the key parameters used in this economic analysis are not precisely known, we performed a sensitivity analysis to recalculate cost-effectiveness and cost-utility ratios by introducing variations in the values of these parameters. Firstly, we calculated the ratios based on the results from the main analysis of the study conducted by Stummer et al.,6 which is the only controlled trial for 5-ALA in the literature. We also modified key parameters by −40% to +40%, since this interval includes not only the results from all subgroups in the VISIONA study but also those reported by Stummer et al.
Additionally, since not all neurosurgery departments in Spain are equipped with a surgical microscope suitable for fluorescence-guided surgery, we performed an additional analysis including these cases. In order for these departments to use 5-ALA, they first have to adapt their equipment by installing a specific module, which entails a significant additional cost. In departments having to acquire that module, the cost per procedure would increase by an amount based on the price of the module itself, the amortisation period, and the number of patients undergoing surgery in that department. More specifically, we calculated the cost in each case using the following formula:
Lastly, we calculated the results for a hypothetical case in which the values for each of the parameters were the least favourable for 5-ALA use.
ResultsThe VISIONA study included evaluable data from 131 5-ALA-guided surgery patients (8 with grade III glioma and 123 with grade IV glioma) and 120 evaluable patients treated surgically under white light (15 with grade III glioma and 105 with grade IV glioma). There were some statistically significant differences between groups regarding baseline characteristics: in the white-light group, there were more patients with good functional status (KPS 90–100) as well as a higher percentage of patients with an uncertain degree of encroachment on an eloquent area. In the group of patients undergoing 5-ALA guided surgery, preoperative tumour volume was slightly larger and more tumours had infiltrated the ependyma.12
We found no statistically significant differences with regard to time of onset and duration of radiotherapy and chemotherapy. Nonetheless, the total radiotherapy dose was slightly higher in the white-light group. In both groups, 90% of patients with grade IV glioma underwent chemotherapy with temozolomide.
CR was achieved in 67% of patients undergoing 5-ALA guided surgery compared to 45% in the white-light group (P=.001, chi-square test). The percentage of progression-free patients with grade IV glioma was higher in the 5-ALA group at all times during the first 24 months after surgery (P=.034, Breslow test). Furthermore, patients in this group gained 1.5 additional months of PFS (P=.067, t test), and the difference between medians was 2.1 months. Twenty-two percent of the observations were censored and only 10 patients were listed as living and progression-free at 24 months. The percentage of progression-free patients at 6 months, which was the main variable used in the VISIONA study for determining its sample size, was 69% in the 5-ALA group and 48% in the white-light group (P=.002, chi-square test).
Regarding quality of life, the utility value for a progression-free state (without receiving radiotherapy or chemotherapy) was calculated at 0.887 on a scale from 0 (death) to 1 (optimal state of health). Patients in the 5-ALA guided group gained a mean of 0.11 QALY compared to patients in the white-light group (=0.887×1.5/12).
A single 1.5g vial of Gliolan® was used in 97% of patients in this group, whereas 3% required 2 doses. Since the price per vial is €980, 5-ALA guided surgery entails a mean additional pharmacological cost of €1010 per patient compared to that of the white-light group.
The incremental cost-effectiveness ratio is therefore €4550 per additional CR compared to that of conventional white-light surgery group, and incremental cost-utility ratio amounts to €9021 per QALY gained.
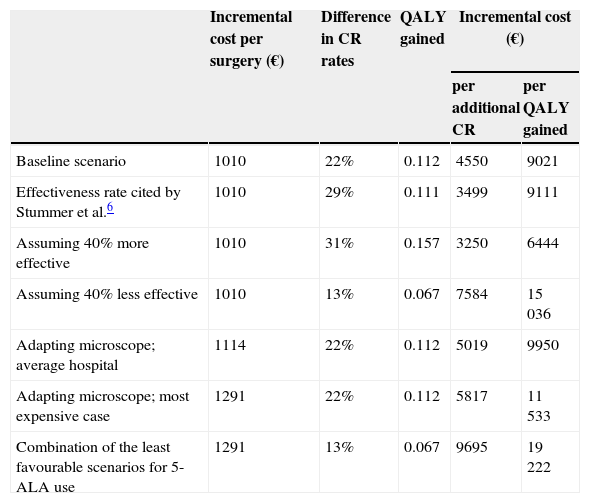
Sensitivity analysisResults varied proportionally when we contemplated other alternative situations for the effectiveness parameters, as shown in Table 1. For example, if we were to perform our analysis using data from the clinical trial by Stummer et al.6 instead of data from the VISIONA study, incremental cost-effectiveness ratio would be €3499 per additional CR, and incremental cost-utility ratio would come to €9111 per QALY gained.
Sensitivity analysis
| Incremental cost per surgery (€) | Difference in CR rates | QALY gained | Incremental cost (€) | ||
|---|---|---|---|---|---|
| per additional CR | per QALY gained | ||||
| Baseline scenario | 1010 | 22% | 0.112 | 4550 | 9021 |
| Effectiveness rate cited by Stummer et al.6 | 1010 | 29% | 0.111 | 3499 | 9111 |
| Assuming 40% more effective | 1010 | 31% | 0.157 | 3250 | 6444 |
| Assuming 40% less effective | 1010 | 13% | 0.067 | 7584 | 15036 |
| Adapting microscope; average hospital | 1114 | 22% | 0.112 | 5019 | 9950 |
| Adapting microscope; most expensive case | 1291 | 22% | 0.112 | 5817 | 11533 |
| Combination of the least favourable scenarios for 5-ALA use | 1291 | 13% | 0.067 | 9695 | 19222 |
Since some centres do not have fluorescence modules for surgical microscopes, we contacted the main providers of this tool to inquire about their price, which ranges from €30000 to €45000. We assumed a depreciation period of at least 8 years. Lastly, considering the degree to which personnel must be familiar with this surgical technique, hospitals offering the procedure should perform it a minimum of 20 times per year. According to these figures, the additional cost per surgery would not exceed €281 (€45000/[8 years×20 procedures per year]). As a result, even in a department performing the minimum number of 5-ALA guided surgeries, the mean total cost including pharmacological costs would not exceed €1291 per procedure. In this extreme case, the incremental cost-effectiveness ratio amounts to €5817 per additional CR, and incremental cost-utility ratio would be €11 533 per QALY gained.
On the other hand, for an average department that acquires a fluorescence module at a standard price, writes it off in 12 years, and operates on 30 patients per year, incremental cost-effectiveness and cost-utility ratios will be €5019 per additional CR and €9950 per QALY gained, respectively.
Lastly, Table 1 also includes a hypothetical scenario in which the least favourable value to employing 5-ALA is adopted for each of the different parameters.
DiscussionFluorescence induced by 5-ALA is an innovative option for the surgical treatment of malignant gliomas. We have calculated the cost-effectiveness of this new technology with a view to the main objective of 5-ALA guided surgery: removing the tumour as completely as possible. To this end, we calculated incremental cost-effectiveness ratio per CR compared to white-light surgery. Since the ultimate aim of surgery is to improve survival rates and patients’ quality of life, we also believed it pertinent to assess cost-utility in terms of incremental cost per QALY gained.
Both ratios indicate that 5-ALA guided surgery is substantially cost-effective. The incremental cost per additional CR (€4550) is not very high when we consider hospitalisation costs resulting from glioma surgery. According to the data published by Spain's inter-regional healthcare coordination fund (Fondo de Cohesión Sanitaria), costs for the diagnosis-related group most frequently associated with craniotomy are estimated at €18000.14 Taking into account that CR is achieved in 45% of cases without using 5-ALA, according to the VISIONA study, the mean cost per CR achieved currently amounts to about €40000 (≈€18000/0.45).
With regard to the cost per QALY gained, the estimated value we obtain (about €9000) is well below the cost-effectiveness threshold usually considered acceptable in Spain.15 The United Kingdom's National Institute for Health and Care Excellence, another reference, normally places the threshold for public funding of new drugs at £30000 per QALY. However, more flexible criteria have been applied recently in cases of malignant gliomas.
Results from the main analysis were shown to be robust in the sensitivity analysis although the latter reflects the impact of larger variations than had been expected based on available clinical data. The range of values we studied includes not only the results observed in all subgroups analysed in the VISIONA study, but also those reported by Stummer et al. That study cannot be overlooked since it is the only prospective randomised controlled multicentre phase III trial in the literature comparing malignant glioma surgery using 5-ALA-induced fluorescence to conventional surgery. It should also be emphasised that results from the VISIONA study are consistent with the study by Stummer et al. regarding improvements in CR and PFS.
In order to allow for variability in activity levels and available equipment in Spanish hospitals, our sensitivity analysis also contemplated the situation for hospitals that currently lack a surgical microscope adapted for fluorescence-guided microsurgery. Even in the scenario least favourable to 5-ALA use, the additional costs per procedure, which derive from adapting the existing equipment, are not excessively high and therefore do not have a substantial impact on cost-effectiveness and cost-utility ratios.
The main limitation of our analysis is directly related to the limitations of the VISIONA study, our source of clinical data. Since VISIONA is a retrospective observational study, its results depend on the quality and quantity of the data obtained, which will invariably be lower than the quality and quantity of data from a prospective controlled study. Additionally, the 2 patient groups were not completely homogeneous with respect to some of the factors that may impact outcome variables. However, any such differences would have served as a bias for 5-ALA surgery.
On the other hand, the VISIONA study has several advantages compared to the study by Stummer et al. Firstly, data were collected from normal clinical practice in Spanish hospitals and therefore constitute a better basis for predicting the economic impact of 5-ALA surgery. Secondly, treatment for patients with malignant glioma has evolved since the study by Stummer et al. was published. At present, patients undergo radiotherapy and chemotherapy with temozolomide after surgery, whereas patients included in the study by Stummer et al. only underwent surgery and radiotherapy. The percentage of 5-ALA patients with PFS at 6 months after surgery in the VISIONA study is higher than that reported in the study on temozolomide efficacy by Stupp et al.,16 which defines the current standard. This finding is especially relevant for confirming that outcomes showing benefits for 5-ALA apply to the current situation, since it supports the idea that the utility of CR does not decrease when effective chemotherapy treatment is used.
Regarding our statistical analysis, we should highlight that the difference in PFS between the 5-ALA and the white-light groups was estimated exclusively on the basis of empirical observations from the VISIONA study. We did not extrapolate survival curves, so our implicit assumption was that patients with a PFS>2 years would not experience further gains in PFS time due to 5-ALA use. This is clearly a very conservative hypothesis.17
Lastly, in order to transform increases in PFS achieved with 5-ALA into gains in QALY, PFS increases were weighted using utilities taken from a quality of life study conducted in the United Kingdom. In any case, we are aware that the method used to measure utility values in this study may be questioned, and that the study results do not necessarily reflect the Spanish population's preferences regarding health states. Our sensitivity analysis has shown that potential discrepancies between countries are very unlikely to have a relevant impact on the results of this pharmacoeconomic analysis. Nevertheless, future studies should evaluate quality of life in Spanish patients at different stages of treatment for malignant glioma.
In conclusion, we can state that employing fluorescence-guided malignant glioma surgery with 5-ALA will result in moderate cost increases compared to the cost of current surgical practice, and that this technique is cost-effective.
FundingThis study was financed by Laboratorios Gebro Pharma, S.A.
Conflicts of interestJordi Galván is employed by Laboratorios Gebro Pharma, S.A. John Slof and Ricardo Díez Valle have received professional fees from Laboratorios Gebro Pharma, S.A.
Please cite this article as: Slof J, Díez Valle R, Galván J. Análisis coste-efectividad de la cirugía del glioma maligno guiada por fluorescencia con ácido 5-aminolevulínico. Neurología. 2015;30:163–168.