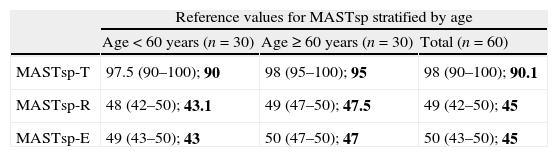Clinical validation of the Spanish version of the Mississippi Aphasia Screening Test (MASTsp) as a screening test for language disorders in patients who have suffered a stroke.
Materials and methodsA total of 29 patients who had suffered a stroke and had aphasia after a left hemispheric lesion were evaluated with the MASTsp, the Boston Diagnostic Aphasia Examination and the token test at baseline and after six months of rehabilitation. Two expert speech-therapists evaluated twelve aphasic patients to determine the inter-observer reliability. This sample was assessed twice in the same week to analyse the reproducibility of the test (test–retest reliability). Aphasic patients were compared with a matched sample of non-aphasic patients with vascular lesions in the right hemisphere (n=29) and a group of healthy subjects (n=60) stratified by age and educational level.
ResultsThe MASTsp showed a good convergent validity, interobserver validity, test–retest reliability and a moderate sensitivity to detect changes over time. A diagnostic cut-off <90 on the MASTsp total test score is proposed.
ConclusionsThe MASTsp is a valid tool for the detection and monitoring of language problems in patients with stroke.
Validación clínica de la versión en castellano del Mississippi Aphasia Screening Test (MASTsp) como batería de cribado de alteraciones lenguaje en pacientes que han sufrido un ictus.
Material y métodosUn total de 29 pacientes que habían sufrido un ictus y presentaban un cuadro afásico tras una lesión hemisférica izquierda fueron evaluados con el MASTsp, el test de Boston para el diagnóstico de la afasia y el test de las fichas, al inicio y tras 6s meses de rehabilitación. Doce de los pacientes afásicos fueron evaluados por dos logopedas expertos para comprobar la fiabilidad interobservador. Este mismo grupo (n=12) fue evaluado en dos ocasiones en la misma semana para comprobar la fiabilidad test-retest. Como grupo control se seleccionó una muestra pareada de sujetos no afásicos con lesión vascular en el hemisferio derecho (n=29) y un grupo de sujetos sanos (n=60) estratificado por edad y nivel educativo.
ResultadosEl MASTsp mostró una adecuada validez convergente y fiabilidad (interobservador y test-retest), siendo parcialmente sensible a detectar cambios a lo largo del tiempo. Se sugiere un punto de corte diagnóstico < 90 en la puntuación total de la prueba.
ConclusionesEl MASTsp es una medida válida para la detección y el seguimiento de los problemas de lenguaje en pacientes con ictus.
The percentage of patients presenting aphasia symptoms after suffering a stroke ranges between 21% and 38% according to a number of studies.1–4 This variation is attributable to differences in methodology between studies. On an epidemiological level, the incidence of aphasic patients in the general population is between 33 and 52 cases per 100000 inhabitants per year.5 Aphasia is not only significant due to being so common, but also because presence of aphasia after a stroke has been linked to poorer prognosis, in terms of both survival and disability (functional, social or occupational).2,5,6 The severity of the disability created by communication problems arising after a stroke, as well as the suddenness of their onset, means that early detection of such problems is absolutely crucial. Language therapy should be started in the acute phase in order to reestablish functional communication as early as possible. On this subject, a number of studies of patients with cerebrovascular lesions have demonstrated the importance of early, intensive language retraining through specific rehabilitation programmes.7–9
There are currently a number of test batteries on the market intended to provide an in-depth analysis of language deficits that may be present after a sudden or degenerative brain lesion.10–12 Some of the most commonly used testing tools include the Boston Diagnostic Aphasia Examination,13 Western Aphasia Battery,14 and the Multilingual Aphasia Examination.15 However, most of these tests are intended for diagnostic purposes only, and they have been traditionally used to classify the severity and semiological features of aphasia cases. In addition, most of these batteries entail a long testing time, which adds to overall fatigue in patients with more severe deficits. It also complicates administering the test to patients who are bedridden due to their clinical condition.
In order to resolve these problems, different screening tests have been published in the past few decades to provide rapid, effective detection of language anomalies. They can even evaluate functional communication in patients with a low level of alertness or a severely limited ability to communicate.16–18 However, few test batteries of this type have been validated in Spanish. As a result, doctors often recur to using subtests or selected elements from more extensive language assessment batteries in order to define an approximate profile corresponding to the patient's psycholinguistic situation.
Nakase-Thompson et al recently introduced and validated the Mississippi Aphasia Screening Test (MAST) as a tool for detecting potential alterations in the different components of language in English-speaking stroke patients.19,20 MAST enables rapid determination of which key aspects of language will require more in-depth analysis. Based on that expanded analysis, general and specific objectives within a speech therapy programme may be established. According to published validation studies, its accuracy has been proven in patients with language disorders secondary to a range of different sudden cerebral events.20 It has also been validated as a tool for evaluating communicative level in patients who are just coming out of minimally conscious states,21 which makes it especially interesting for specialist neurorehabilitation units like our own. Compared to the very few screening tests available in Spanish, including the recent “bedside assessment of language” test,8 MAST does not include elements that would be unfamiliar in our culture, requires no outside material in order to complete the evaluation, and does not focus specifically on classifying the type of aphasia. This means that its structure helps us assess progress over time and plan specific treatment objectives.
Although this test had already been validated in other languages,20,22 a normative study has not yet been carried out in our population. Furthermore, there are no results demonstrating the test's ability to detect changes over time, which would be especially interesting in samples of patients who have undergone therapeutic interventions.
We present a normative study of the Spanish language version of MAST (MASTsp) as a tool for assessing the aphasic population after a first stroke. Our objective is to determine MAST's validity and reliability as a tool for detecting the possible effects of a stroke on a patient's different language dimensions, and to evaluate this new tool's ability to detect changes following a rehabilitation programme.
Patients and methodsSampleCandidates for participation in this study consisted of a total of 126 consecutive patients who between December 2007 and January 2009 suffered a left hemisphere stroke (confirmed by neuroimaging tests) and were treated in a specialist neurorehabilitation unit. Patients meeting the following criteria were excluded from the sample: (a) low level of consciousness (vegetative state and/or minimally conscious state); (b) severe cognitive decline that would interfere with administration of the test; (c) premorbid illiteracy; (d) severe visual and/or auditory deficit that would prevent proper completion of the test, and (e) behavioural disorders and/or lack of cooperation with the speech therapist. Of the resulting sample (n=46) we selected only those patients who presented a language disorder according to the results of an assessment by a specialist. The final sample contained 29 patients who presented signs of aphasia following either an ischaemic (n=10) or haemorrhagic (n=19) left hemispheric lesion.
To demonstrate MASTsp's ability to differentiate between the communicative abilities of aphasic and non-aphasic patients, we established a paired sample of 29 subjects with right hemispheric vascular lesions (confirmed by neuroimaging tests) and for whom a language assessment had ruled out aphasia. All non-aphasic patients had been admitted to the same neurorehabilitation unit during the same period of time.
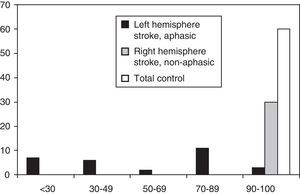
A third group, consisting of 60 healthy subjects (total control group) divided into 2 different age categories (45–60 and 61–80 years) and 3 educational levels (primary, secondary and tertiary), provided normative values for the healthy population. Members of this group participated voluntarily. The group consisted of 24 women and 36 men, with a mean [SD] age=60[7.5] years and mean [SD] years of school attended=12.1[5.3]. Since the correlation between MAST scores and age and educational level had already been demonstrated in prior studies, we selected a subgroup of 30 subjects (adjusted control group) adjusted for age and educational level of the aphasic subjects in order to compare their MASTsp values to those of the 2 patient samples (Table 1). All subjects in the adjusted control group were evaluated by a speech therapist and expert neurologist to exclude subjects whose first language was not Spanish, subjects with visual/auditory disorders or cognitive disorders, and any with preexisting communication or cognitive disorders (Mini-Mental score<24) (Fig. 1).
Sociodemographic variables.
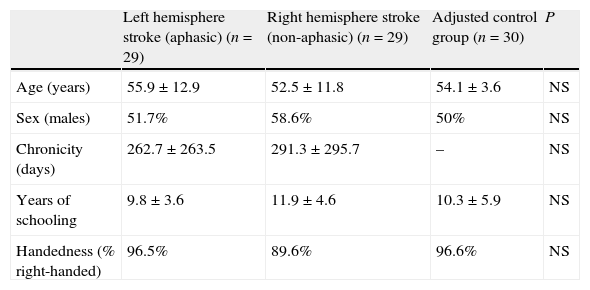
| Left hemisphere stroke (aphasic) (n=29) | Right hemisphere stroke (non-aphasic) (n=29) | Adjusted control group (n=30) | P | |
| Age (years) | 55.9±12.9 | 52.5±11.8 | 54.1±3.6 | NS |
| Sex (males) | 51.7% | 58.6% | 50% | NS |
| Chronicity (days) | 262.7±263.5 | 291.3±295.7 | – | NS |
| Years of schooling | 9.8±3.6 | 11.9±4.6 | 10.3±5.9 | NS |
| Handedness (% right-handed) | 96.5% | 89.6% | 96.6% | NS |
Data are presented as mean (SD) or as percentages (%).
All aphasic patients were evaluated by a medical extern at the beginning of the study using the Spanish version of MAST (MASTsp). The doctor also applied an assessment protocol (described below) which included the scales normally used for this population. Following initial evaluation, the 29 aphasic patients participated in a multidisciplinary rehabilitation programme which included 3–5 weekly sessions of language rehabilitation with an expert speech therapist. All aphasic patients were reevaluated with the same test battery 6 months after inclusion in the programme. Results were used to determine the test's ability to detect significant clinical changes over time. The group of right hemisphere stroke patients who were non-aphasic and the total control group were evaluated with MASTsp at the time of inclusion only.
To evaluate interobserver reliability, 12 of the aphasic patients were evaluated by 2 different expert speech therapists. This same patient group (n=12) was evaluated on 2 different occasions, with a mean [SD] of 5[2.9] days between tests in order to determine test-retest reliability.
Evaluation scalesAll aphasic patients were evaluated during the same day with a psycholinguistic test battery containing the following, in addition to MASTsp:
- –
Boston Diagnostic Aphasia Examination (BDAE)10,13: this test detects potentially altered language areas. For purposes of our study, only the following subsections were administered: (a) commands: assesses ability to understand 5 verbal commands (score range 0–15); (b) vocabulary: 60 stimulus cards showing everyday objects which the subject must name (score range 0–60); (c) oral reading of sentences: assesses grapheme–phoneme decoding (score range 0–10); (d) writing: evaluates mechanics, recovery, syntax and correct content (score range 0–11); (e) repetition: assesses auditory analysis, control over speech and audio verbal memory (score range 0–10) and (f) degree of severity: evaluates functional competence for communication and assesses the severity of deterioration for both expressive and receptive language (score range 0–5).
- –
Token test23: enables assessment of language comprehension. This easy-to-administer test makes use of 20 tokens in different sizes, 5 different colours and 2 shapes (squares and circles). The test consists of 6 subsections with increasingly complex tasks, based on the length of the series and the level of abstraction. The maximum score is 36 points; very severe deficit is represented by scores of 0–8; severe deficit, 9–16; moderate deficit, 17–24; and mild deficit, 25–28.
Once the first evaluation phase had been completed, language-related criteria were used to establish the type of aphasia and degree of severity for each of the patients. Subjects were then evaluated using MASTsp. The original version of MAST19,20 was translated from English to Spanish (Castilian) using a translation–retranslation method, and slight modifications reflecting the idiosyncrasies of Spanish were subsequently introduced. The version presented here maintains both the original structure of the 9 subtests and the original scoring system with a minimum score of 0 (suggesting severe aphasia) and a maximum of 100 (a normal individual). This version of MASTsp contains 4 receptive subtests (MASTsp-R) and 5 expressive subtests (MASTsp-E) with overall scores ranging from 0 to 50 for each of 2 subsections. The structure and partial scores for each subsection are listed in Appendix 1. The completed MASTsp provides a global index that measures aphasic syndrome severity (MASTsp-T), obtained by adding the MASTsp-R and MASTsp-E subsection scores.
Statistical analysisDescriptive analysis was used for demographic variables and to describe MASTsp values for each group included in the study (left hemisphere stroke with aphasia, right hemisphere stroke without aphasia, and the 2 control groups). Spearman's rank order correlation was used to determine the association between MASTsp scores in the total control group and demographic variables with a potential influence on the test score (age and years of school attended). For the convergent validity study, MASTsp values obtained from the aphasic patient sample and scores from the BDAE and token tests were correlated using Spearman's rank test. A comparative study of patient groups and the adjusted control group was performed using analysis of variance (ANOVA), Student t-test or the chi-square test for comparisons between demographic and clinical variables. Non-parametric methods (Kruskal–Wallis test and Mann–Whitney test) were used to compare MAST scores due to their non-normal distribution. The diagnostic accuracy of MASTsp was evaluated using ROC curve analysis. In addition, following the methodology used in previous studies of the same tool, we also calculated empirical cut-off points corresponding to the 5th percentile of each of the MAST scores for the total control group. The scale's ability to detect significant clinical changes in the aphasic patients sample over time (6 months of treatment) was evaluated by calculating the standardised effect size (SES) and standardised response mean (SRM). Interobserver and test-retest reliability were determined using the Pearson correlation coefficient and the intraclass correlation coefficient (ICC). Statistical significance was set at P<0.05. All analyses were performed using SPSS statistical software version 15.0.
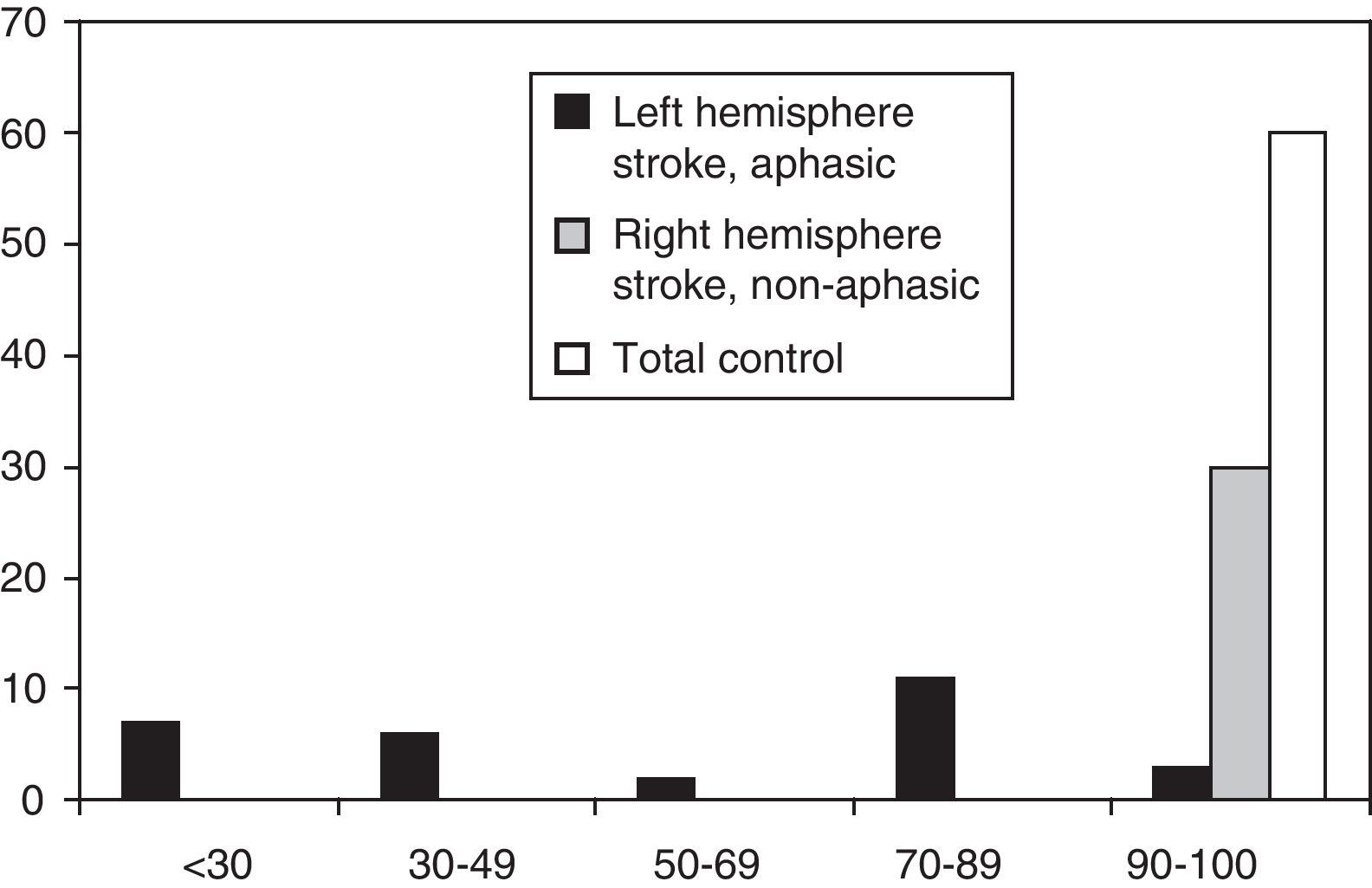
ResultsDescriptive studyFig. 1 shows the distribution of MASTsp-T scores for each of the 3 groups included in the study. Distribution in the total control group and the non-aphasic group was clearly asymmetrical (skewness: −1.1±0.3 and −2.3±0.4, respectively) with a median of 98 (range, 90–100) in the total control group and 100 (range, 92–100) in the non-aphasic patient group. However, the curve was different for the aphasic patient group (skewness: −0.04±0.4), with a median value of 52 (range, 14–100). The same distribution is observed for MASTsp-R and MASTsp-E scores (medians: 40 [range, 4–50] and 28 [range, 0–50], respectively) in the aphasic group. It was also observed among non-aphasic patients (medians: 50 [range, 46–50] and 50 [range, 45–50], respectively) and in the total control group (medians: 49 [range, 42–50] and 50 [range, 43–50], respectively).
In the total control group, age was significantly correlated with the MASTsp-T score (r=0.26; P<.05) and the MASTsp-R score (r=0.29; P<.05). Years of school attended were correlated with the MASTsp-R score (r=0.28; P<.05). In line with the correlations observed, normative values for the control group were stratified by age and education as shown in Table 2.
Reference values for MASTsp in the total control group, stratified by age and years of schooling.
| Reference values for MASTsp stratified by age | |||
| Age<60 years (n=30) | Age≥60 years (n=30) | Total (n=60) | |
| MASTsp-T | 97.5 (90–100); 90 | 98 (95–100); 95 | 98 (90–100); 90.1 |
| MASTsp-R | 48 (42–50); 43.1 | 49 (47–50); 47.5 | 49 (42–50); 45 |
| MASTsp-E | 49 (43–50); 43 | 50 (47–50); 47 | 50 (43–50); 45 |
| Reference values stratified by years of schooling | |||
| <13 years (n=30) | ≥13 years (n=30) | Total (n=60) | |
| MASTsp-T | 98 (90–100); 91.1 | 98 (90–100); 90 | 98 (90–100); 90.1 |
| MASTsp-R | 48.5 (42–50); 43.1 | 49 (45–50); 45.5 | 49 (42–50); 45 |
| MASTsp-E | 50 (46–50); 46.5 | 49 (43–50); 43 | 50 (43–50); 45 |
Data are presented as medians (range) and 5th percentile (in bold).
MASTsp: MAST (Spanish-language version); MASTsp-R: receptive language index; MASTsp-E: expressive language index; MASTsp-T: total index.
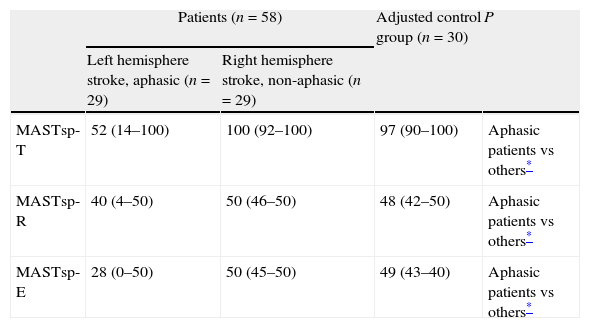
Since we used a paired-sample method for our aphasic patient group, we found no statistically significant differences among patient groups and the adjusted control group for any of the variables (Table 1). The 2 patient groups showed no differences in estimated time elapsed between the event causing the lesion and the testing date. MASTsp scores for both expressive and receptive language, and logically for total scores as well, were significantly lower in aphasic patients than in non-aphasic patients and in adjusted control-group subjects (P<.001). No statistically significant differences were found between the adjusted control group and the non-aphasic patient group (Table 3).
Comparative study (Kruskal–Wallis test and Mann–Whitney test) of the 2 patient groups and the adjusted control group.
| Patients (n=58) | Adjusted control group (n=30) | P | ||
| Left hemisphere stroke, aphasic (n=29) | Right hemisphere stroke, non-aphasic (n=29) | |||
| MASTsp-T | 52 (14–100) | 100 (92–100) | 97 (90–100) | Aphasic patients vs others* |
| MASTsp-R | 40 (4–50) | 50 (46–50) | 48 (42–50) | Aphasic patients vs others* |
| MASTsp-E | 28 (0–50) | 50 (45–50) | 49 (43–40) | Aphasic patients vs others* |
Data are presented as medians (ranges).
MASTsp: MAST (Spanish-language version); MASTsp-R: receptive language index; MASTsp-E: expressive language index; MASTsp-T: total index.
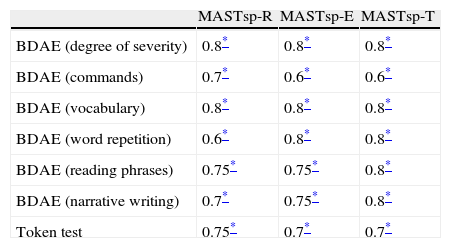
Table 4 shows the correlation matrix of the 58 areas evaluated by MASTsp and the BDAE and token test subsections in the 29 aphasic patients evaluated at baseline and at the end of the rehabilitation programme. Overall, the correlation matrix shows good convergent validity, especially for MASTsp-T values. Obviously, the magnitude of the MASTsp-R correlation increases for those subtests evaluating verbal or written comprehension, and the magnitude of MASTsp-E increases for subtests evaluating fluency and verbal expression.
Spearman correlation matrices between MASTsp scores and the psycholinguistic test battery at the rehabilitation programme beginning and end points.
| MASTsp-R | MASTsp-E | MASTsp-T | |
| BDAE (degree of severity) | 0.8* | 0.8* | 0.8* |
| BDAE (commands) | 0.7* | 0.6* | 0.6* |
| BDAE (vocabulary) | 0.8* | 0.8* | 0.8* |
| BDAE (word repetition) | 0.6* | 0.8* | 0.8* |
| BDAE (reading phrases) | 0.75* | 0.75* | 0.8* |
| BDAE (narrative writing) | 0.7* | 0.75* | 0.8* |
| Token test | 0.75* | 0.7* | 0.7* |
MASTsp: MAST (Spanish-language version); MASTsp-R: receptive language index; MASTsp-E: expressive language index; MASTsp-T: total index; BDAE: Boston diagnostic aphasia examination.
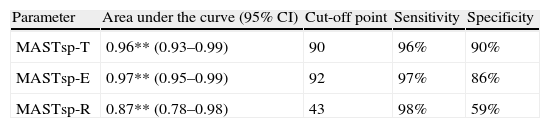
As in earlier studies,22 MASTsp sensitivity and specificity were evaluated using 5th percentile values from the control group as empirical cut-off points (Table 2). Using these cut-off points, the sensitivity of MASTsp-T (correct detection of anomalous MASTsp-T values in aphasic patients) was 89.6% (CI, 80%–99%); 3 of the 29 patients with aphasia due to a left hemispheric lesion scored higher than 90 (Fig. 1). Test specificity (correct detection of normal values in non-aphasic stroke patients) was 100% (CI, 98%–100%); none of the non-aphasic patients scored lower than 90 (Fig. 1). As an alternative, the sensitivity and specificity of MASTsp values for detecting aphasic subjects were also evaluated by means of a ROC curve analysis. All curves showed statistically significant values for the area under the curve, with acceptable 95% confidence limits (Table 5). MASTsp-E values, and to an even greater extent, MASTsp-T values, showed sufficient sensitivity and specificity (>85%) for the proposed cut-off points, while MASTsp-R values were clearly inferior.
Analysis using ROC curves. Aphasic left-hemisphere stroke patients compared to all other groups.
| Parameter | Area under the curve (95% CI) | Cut-off point | Sensitivity | Specificity |
| MASTsp-T | 0.96** (0.93–0.99) | 90 | 96% | 90% |
| MASTsp-E | 0.97** (0.95–0.99) | 92 | 97% | 86% |
| MASTsp-R | 0.87** (0.78–0.98) | 43 | 98% | 59% |
MASTsp-R: receptive language index; MASTsp-E: expressive language index; MASTsp-T: total index.
*P<.01.
The interobserver reliability study for the sample of aphasic patients delivered excellent results for MASTsp-T (69±27 vs 68.9±27.1; r=0.99, P<.001 and ICC=0.99; P<.001); MASTsp-R (40.8±8.9 vs 40.9±8.8; r=0.99; P<.001 and ICC=0.99; P<.001); and MASTsp-E (28±20.2 vs 28.2±20; r=0.9; P<.001 and ICC=0.99; P<.001). Values for MASTsp-T, MASTsp-R and MASTsp-E in the aphasic patient group upon repeating the test were 69±27, 41.2±9.5, and 28.3±20.1, respectively. The ICC obtained in the test–retest study was 0.99 for the 3 MASTsp indices.
Sensitivity to changeAlthough MASTsp-T, MASTsp-R and MASTsp-E scores improved during the 6-month rehabilitation period by means (SD) of 10 (13), 4.7 (7.8) and 5.2 (8.8), respectively, the sensitivity indices for detecting changes in score showed values that would traditionally be considered low (<0.5 on the SES scale) or moderate (>0.5 and <0.8 on the SRM scale). This was true for MASTsp-T (SES=0.35 and SRM=0.77), MASTsp-E (SES=0.3 and SRM=0.6), and MASTsp-R (SES=0.35 and SRM=0.6).
Patients also showed improvement according to the other scales used. In the “degree of severity” section on the Boston test, patients improved a mean (SD) of 0.7 (0.9); on the token test, 4.8 (7.1); and on the vocabulary subsection of the Boston test, 6.4 (11.2). Sensitivity indices showed similar results to those obtained with MASTsp, with low values according to the SES scale (degree of severity=0.46; token test=0.4 and vocabulary=0.35), with medium values on the SRM (degree of severity=0.77; token test=0.67 and vocabulary=0.57).
DiscussionOur results confirm the validity of MASTsp as a screening tool for language problems in patients who have suffered an ischaemic or haemorrhagic stroke. According to the data shown here, this scale has sufficient sensitivity and specificity to distinguish between patients with aphasia following a stroke and stroke patients/healthy subjects without aphasia. Normative data presented here also coincide with data for this tool that have been published in other languages. This shows that this tool has transcultural validity when properly adapted in Spanish.19,20,22 With this in mind, the effect of age and educational level in our sample on MASTsp results has already been described in earlier studies. Our results show a correlation between age and the total MASTsp score similar to that described by Nakase-Thompson et al.20 However, those authors also found a correlation with the MAST-E score, while our study showed the correlation with the MASTsp-R score. The change in correlation direction—negative in the Nakase-Thompson study and positive in our case—may be due to a selection bias in the control group. Additionally, the fact that MAST values were not normalised may have allowed small individual variations to cause significant changes in the intensity or direction of the correlations. Likewise, the correlation between years of schooling and scores on MASTsp-R which we found in our study had also been described in prior studies showing a broader correlation including MAST-T and MAST-E.19,20,22 Differences found in some of the indices listed above with respect to previous studies may be due to both the different population characteristics of countries in which they were conducted, and to differences between samples pertaining to each of the 3 studies. To illustrate, the Kostalova et al22 study included a significantly larger sample of control patients than our own, and the mean age of patients in the Nakase-Thompson et al20 study was clearly lower than that which we describe here. Even allowing for these limitations, it seems that age and years of schooling are variables which must be considered when evaluating patient scores obtained by using this tool.
As expected, and in line with previous findings, patients in the aphasia group scored lower than the other two groups (non-aphasic patients and control subjects) in terms of total score and in the receptive and expressive language sub-indices. The degree of sensitivity of MASTsp, measured using both ROC curve analysis and proposed empirical cut-off points (5th percentile), corroborates results obtained in previous studies.20,22 The lower diagnostic sensitivity found for the MASTsp receptive language index could be related to the period of time represented by our sample, and the recognised tendency for aphasia patients’ receptive language skills to improve more than their expressive skills over time. At the same time, the reliability and the convergent validity of MAST had both been described previously with excellent results. In our case, interobserver reliability and test–retest scores were excellent, both for total and partial scores, as was described in previous studies. The current validity study showed significant correlations between MASTsp scores and those obtained by using 2 classic tools for evaluating aphasia patients: BDAE and the token test. These data, added to the fact that MAST is user-friendly and short, indicate that MAST is a useful screening test for language disorders in this population. Similar results to those from our study were reported by the study to validate the Czech version of MAST (MASTcz).22 The study compared MASTcz scores with scores obtained by evaluating an aphasia group using the Western Aphasia Battery. Without being intended to substitute any of the traditional scales, MASTsp seems to provide sufficient information in order to design an initial approach for speech therapy. It allows us to determine which aspects of language must be examined with more extensive test batteries in order to identify the right strategy for speech therapy.
No prior studies of this tool included a longitudinal analysis evaluating the sensitivity of MAST to detecting significant clinical changes over time. Our results show that since MASTsp was designed as a screening test, it is limited in that area. While MASTsp index values in the sample included in our study improved throughout the follow-up period, the tool was not powerful enough to detect them. We typically saw similar results with the rest of the tests in the battery we used. The fact that chronicity in our sample was heterogeneous could account in part for the low sensitivity. MAST is principally intended as an assessment of function, as part of the World Health Organization's triple aim model. This limits its ability to detect patient improvement. Traditionally, we have assumed that the process of recovering deficits, including psycholinguistic deficits, which take place with cerebral injury slows with the passage of time after the event causing the initial injury.24,25 Further studies in samples with less chronicity will be able to demonstrate the value of this tool, and meanwhile, we will be mindful of the fact that deficit and loss of function are not always the same in neurorehabilitation.
Conflicts of interestThe authors have no conflicts of interest to declare.
APHASIA SCREENING TEST (MAST)
Name: Date:
Naming:/10
- (1)
Bolígrafo [Pen]
- (2)
Mano [Hand]
- (3)
Pulgar [Thumb]
- (4)
Reloj [Watch]
- (5)
Techo [Ceiling]
Score: Each item is scored according to the following criteria:
2 points: item named correctly. May include 1 instance of phonemic paraphasia.
0 points if answer contains more than 1 instance of phonemic paraphasia.
Automatic speech:/10
- (1)
Count to ten
- (2)
Tell me the days of the week
- (3)
Más vale pájaro en mano… [A bird in the hand…]
- (4)
Perro ladrador…[A barking dog…]
- (5)
No por mucho madrugar… [Rome wasn’t built in a…]
Score: Each item is scored according to the following criteria:
Items 1–2:
2 points given for each item completed correctly.
1 point given if half of the sequence is correct.
0 points given if the patient cannot complete half of the sequence.
Items 3–5:
2 points given for each item completed correctly.
0 points given if there is an error.
Repetition:/10
- (1)
Tarro [pot]
- (2)
Zanahoria [carrot]
- (3)
Abecedario [alphabet]
- (4)
Debajo del viejo puente de madera [Under the old wooden bridge]
- (5)
La plateada luna brilla en la oscura noche [The silver moon shines in the dark sky]
Score: Each item is scored according to the following criteria:
Items 1–3:
2 points given for correct restatement of words.
0 points given if there is an error.
Items 4 and 5:
2 points given if the complete phrase is repeated correctly.
1 point given if half of the phrase is correct.
0 points given if the patient cannot complete half of the phrase.
Yes/No accuracy: 20
- (1)
Is your name_____________? (insert incorrect name)
- (2)
Is your name_____________? (insert correct name)
- (3)
Are we in______________? (incorrect location)
- (4)
Are we in______________? (correct location)
- (5)
Do you wear a glove on your foot?
- (6)
Am I touching my eye? (clinician touches his/her nose)
- (7)
Does Monday come before Tuesday?
- (8)
Does summer come after spring?
- (9)
Is a chicken bigger than a spider?
- (10)
Do you put your shoe on before your sock?
Score: Each item is scored according to the following criteria:
2 points given for correct answers.
0 points given for incorrect answers.
Object recognition:/10
- (1)
Watch
- (2)
Keys
- (3)
Book
- (4)
Paper
- (5)
Pen
Score: Each item is scored according to the following criteria:
2 points given for correct answers.
0 points given for incorrect answers.
Following instructions:/10
- (1)
Touch your nose
- (2)
Open your mouth
- (3)
With your left hand, touch your right eye.
- (4)
Point to the floor, then touch your nose.
- (5)
Before opening your mouth, touch your ear.
Score: Each item is scored according to the following criteria:
Items 1 and 2:
2 points given for correct execution of the instructions.
0 points given if there is an error.
Items 3–5:
2 points given for correct execution of the actions.
1 point given if the order of the actions is inverted.
0 point given if only half of the actions are carried out.
Reading instructions:/10
- (1)
Open your mouth.
- (2)
Make a fist.
- (3)
Point to the floor, then point to the ceiling.
- (4)
With your right hand, touch your left knee. Alternative: with your left hand, touch your right knee.
- (5)
Point to your left ear and then make a fist.
Items 1 and 2:
2 points given for correct execution of the actions.
0 points given if there is an error.
Items 3–5:
2 points given for correct execution of the actions.
1 point given if the order of the actions is inverted.
0 point given if only half of the actions are carried out.
Writing/Spelling:/10
- (1)
Silla. [Chair]
- (2)
Girar. [Turn]
- (3)
Aeroplano. [Aeroplane]
- (4)
Ordenador. [Computer]
- (5)
Bajo el puente negro. [Under the black bridge]
Score: Each item is scored according to the following criteria:
2 points given for each word correctly written.
1 point given if there are 1 or 2 spelling errors.
0 points given if there are more than 2 spelling errors.
Verbal fluency:/10
Score:
10 points: verbal fluency is normal.
5 points: sentence structure contains some abnormalities.
0 points: verbal fluency is very abnormal.
This study has not been presented, either partially or in full, at the Annual Meeting of the SEN or at any other meetings or congresses. No funding of any type was received for this study.
Please cite this article as: Romero M, et al. Utilidad clínica de la versión en castellano del Mississippi Aphasia Screening Test (MASTsp): validación en pacientes con ictus. Neurología. 2012;27:216–24.