The cerebellum has been linked to multiple functions, such as motor control, cognition, memory, and emotional processing. As for its involvement in the sensory systems, the role of the cerebellum in the sense of smell remains unclear. We suggest that sexually naive male rats will present increased neuronal activity in the cerebellar vermis after being stimulated with almond odour or oestrous odour from receptive females.
MethodsWe compared activity in the cerebellar vermis using Fos immunoreactivity after olfactory stimulation. Stimulation took place for 60min in a cube-shaped acrylic chamber with a double bottom. Stimuli were clean woodchip bedding, bedding with almond extract, and bedding taken from a cage of receptive females. Male rats were subsequently anaesthetised with intraperitoneal sodium pentobarbital. Cerebellar tissue was fixed with paraformaldehyde for later immunohistochemical analysis.
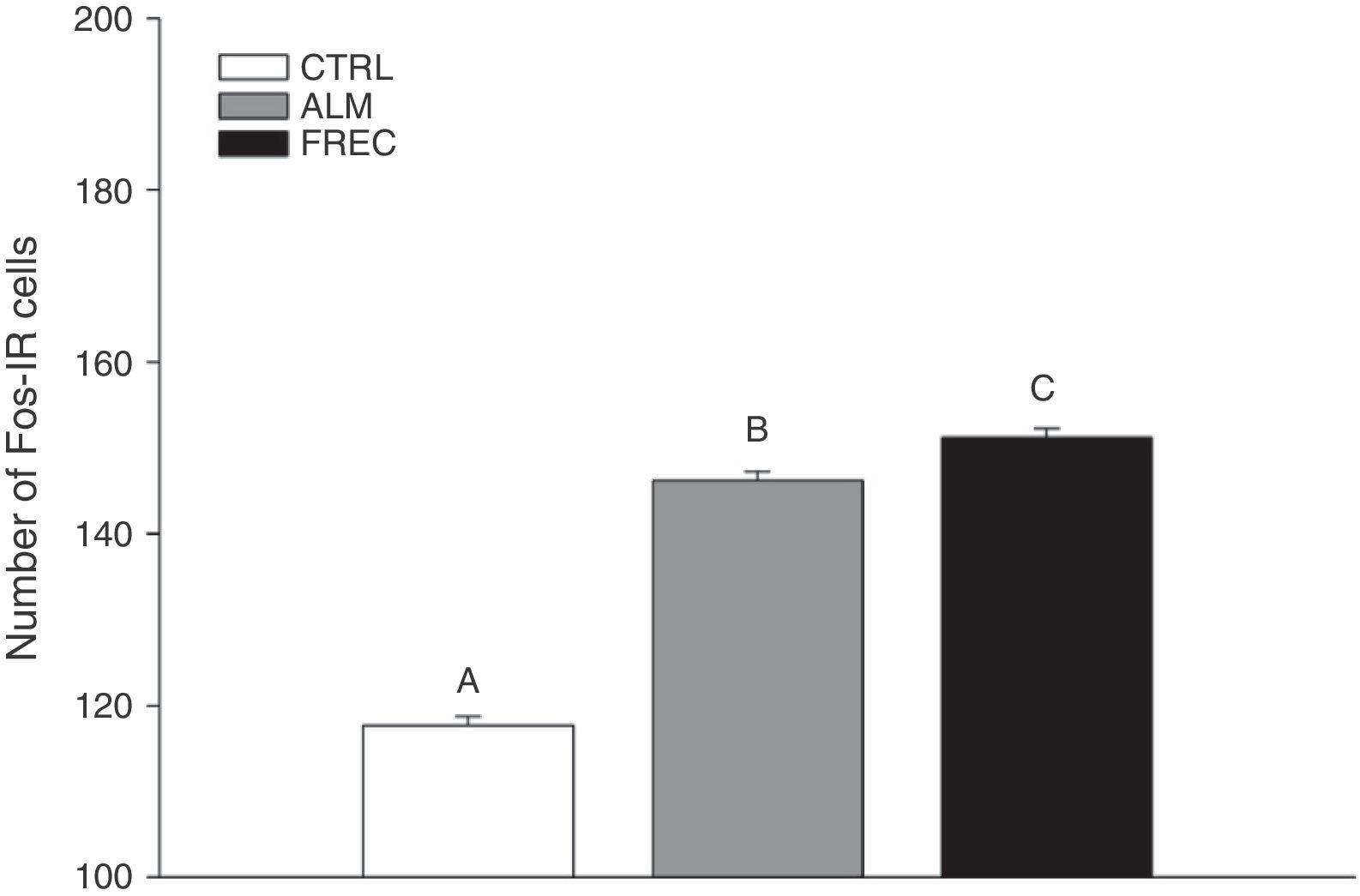
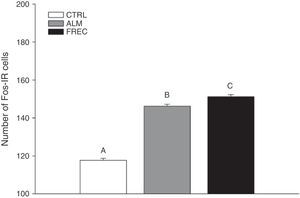
ResultsThe number of Fos immunoreactive cells in all lobes of the cerebellar vermis was similar between groups stimulated with almond extract and with oestrous odour, and higher than in the clean woodchip group.
ConclusionsStimulation of the main olfactory system (almond) and the accessory system (oestrous odour) increases Fos protein production in the granular layer of the cortex of the cerebellar vermis in naive male rats.
El cerebelo es una estructura que se ha vinculado con múltiples funciones, tales como control motor, cognición, memoria y procesamiento emocional. En cuanto a su relación con los sistemas sensoriales no está claro el papel que desempeña en relación con el sentido del olfato. Suponemos que las ratas macho sexualmente inexpertas presentarán un incremento en la actividad neuronal del vermis cerebelar tras ser estimuladas con olor de hembra receptiva y almendra.
MétodosComparamos la actividad de la capa granular del vermis cerebelar mediante la inmunorreactividad a Fos tras estimulación olfativa. Esta estimulación se realizó durante 60min en cámaras cúbicas de acrílico con un doble fondo. Los estímulos fueron aserrín limpio, con esencia de almendra y proveniente de la cama de hembras receptivas. Finalmente los machos fueron anestesiados con pentobarbital sódico intraperitoneal. El tejido cerebelar fue fijado con paraformaldehído para su posterior análisis inmunohistoquímico.
ResultadosEl número de células inmunorreactivas a Fos en todos los lóbulos del vermis cerebelar de los grupos estimulados con almendra y hembra receptiva fue similar, pero mayor comparado con el grupo de aserrín limpio.
ConclusionesLa estimulación olfativa del sistema olfatorio principal (almendra) y accesorio (hembra receptiva) incrementa la producción de proteína Fos en la capa granular de la corteza cerebelar del vermis de ratas macho sexualmente inexpertas.
The cerebellum is an organ that has been the subject of countless studies, and its functions include motor control, cognitive processing,1,2 and sensory processing.3–5 Scientists are also well aware of the link between the cerebellum and the senses of sight, hearing, and touch.6–10 Neuroimaging studies in humans and non-human primates have shown that the cerebellum is highly active during olfactory stimulation with odours related to food3,11 and sexual arousal.12 Meanwhile, immunohistochemical studies in rats have shown that cerebellar activity increases in the presence of stimuli related to reproductive conduct.13,14 We have already shown that execution of sexual behaviour in male rats causes an increase in the number of Fos-immunoreactive (Fos-IR) neurons in the cerebellum. In males achieving ejaculation, the number of activated neurons is similar to that seen in non-contact stimulation.13 In these tests, the males were able to see, hear, and smell the receptive females. This sensory stimulation results in activation of large numbers of granule cells, thereby indicating that sexual signals trigger activity in these neurons. Although these studies link the cerebellum with sensory stimulation, little is known about how this stimulation is affected by smell. The purpose of this study is to measure and compare Fos protein expression in the cerebellar vermis of sexually naive male rats after olfactory stimulation with clean woodchip bedding, almond-scented bedding, and bedding collected from a cage containing receptive females. We hypothesise that the stimuli consisting of almond and receptive female odours will increase the number of Fos-IR neurons.
Materials and methodsSubjects and housingThe study made use of 18 sexually naive male rats (weight 250-350g) and 6 ovariectomised females (weight 200-250g), all of which were Wistar rats. Animals were housed (by sex) in transparent acrylic boxes measuring 44cm×34cm×20cm (6 per box) with a 3-4cm layer of woodchip bedding in the bottom. They were kept in a room at a temperature of 22±2°C with an inverted light/dark cycle (12h×12h). Rats had ad libitum access to purified water and food (Rismart®) until the testing date. All handling and surgical procedures were carried out in accordance with the Mexican Society of Neuroscience guidelines on the use of animals in neuroscientific research.
Olfactory stimulationOlfactory stimulation in the male rats was performed as described by Portillo and Paredes,15 using sterile bedding (Rismart®, México) as a vehicle in a cube-shaped acrylic box (30cm×30cm×30cm). The box had a double bottom, and animals were placed in the upper part, which has a perforated acrylic base 4mm thick that forms a platform 5cm above the lower base. In the space between these bases, we placed a receptacle containing 80g of the olfactory stimulus: clean woodchip bedding for the control group (CTRL, n=6), bedding scented with almond extract (ALM, n=6), or bedding gathered from a cage containing receptive ovariectomised females with induced oestrus (FREC, n=6). Once the male rats were placed in the box, they were allowed to acclimate for 10min, after which the bedding receptacle was added to begin stimulation. The males then remained in the box with the stimulus for another 60min.
Immunohistochemical studyAt the end of the olfactory stimulation period, animals were anaesthetised with pentobarbital sodium (60mg/kg, IP). Researchers then performed transcardial perfusion with saline solution (0.9%; 300mL) followed by 4% paraformaldehyde (pH 7.4; 400mL) in a phosphate buffer (PB). Crania were removed and whole cerebella were extracted and fixed in paraformaldehyde for the next 12h. The vermis was isolated and cryoprotected in successive solutions of 10%, 20% and 30% sucrose (PB 0.1M, 10mL). Researchers then took sagittal sections measuring 40μm using a cryostat (Leica CM1850). Three sections from the central part of the vermis were analysed.
Sections were tested for Fos protein immunoreactivity as described by Manzo et al.13; they were incubated in the first polyclonal Fos antibody (Ab) diluted at 1:2000 in a 0.3% PBT solution and 3% normal goat serum (NGS). Sections were incubated for 48h at 4°C. Researchers added the anti-rabbit antibody (bridge Ab) in 0.3% PBT diluted at 1:250 and incubated the sections for 90min in constant agitation at room temperature. The avidin-biotin complex (ABC Kit) was then applied in PB 0.1M diluted at 1:200 for 90min in constant agitation at room temperature. Immunoreactivity was revealed in 20mL PB 0.1M with 5 drops of diaminobenzadine (0.1mg/100mL), 4 drops of nickel (0.05mg/5mL), and 3 drops of 30% hydrogen peroxide. Sections were finally mounted on gelatin-coated slides and left to dry for 2 days, after which they were dehydrated and cleaned with xylene (Fisher). Specimens were then coated with Permount (Fisher Scientific) and coverslips were provided to prepare them for inspection.
Histological and statistical analysisResearchers took digital images of the histological cerebellum sections and analysed them under a microscope (AX70 Olympus Optical Co., LTD, Japan) connected to a computer with Image Pro Plus 6.7 (Media Cybernetics). Using a 40× objective with a field of view of 20000μm2, we counted granule cells in 3 regions (proximal, medial, and distal) of each cerebellar lobule using a mean number of 3 slices per animal. Statistical analysis was performed using the Statistica program (Stat soft™) and JMP 6 (SAS 2005). As data did not show a normal distribution, they were classified by rank (rank transformation) so that they could be subsequently analysed using parametric statistical tests.16 We used fully nested ANOVA following a general linear model (GLM) since data were organised hierarchically. The model can be represented by the following formula: y=Group+Animal [Lobule]+Lobule [Region]+G×L+G×R+L×R+G×L×R+Error, where ‘y’ is the variable ‘response’ and G, L, and R are the Group, Lobule, and Region, respectively. Regions (proximal, medial, and distal) were nested within Lobule, and Lobule was nested within Animal, as suggested in the pseudoreplication analysis.17 A Tukey post hoc test was completed to determine any inter-group differences in lobules.
ResultsWe found significant differences between the groups (F2.1349=146.6; P<.0001). The Tukey post hoc test showed that the CTRL group (mean=124.02±1.39) had lower numbers of Fos-IR cells than either the ALM group (mean=150.03±1.39) or the FREC group (mean=155.13±1.39). The FREC group presented the highest number of Fos-IR cells (Fig. 1).
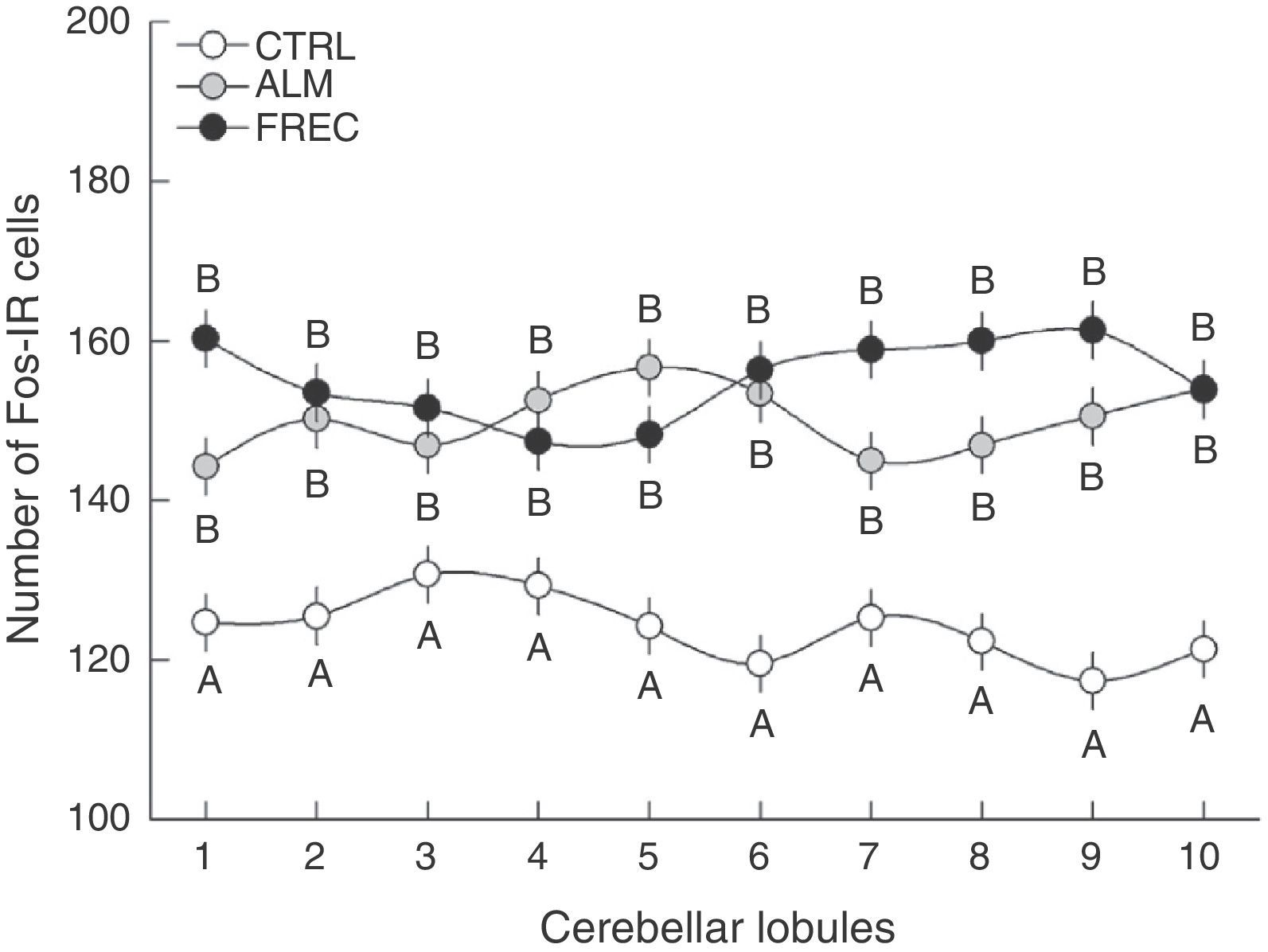
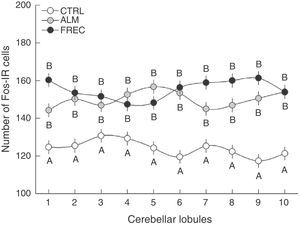
An analysis of group-lobule interaction effects found a significant difference (F18.1349=1.6555, P=.0458). The post hoc test revealed that all lobules from the CTRL group differed significantly from those in the ALM and FREC groups. Nevertheless, there were no statistically significant differences between the last 2 groups, but rather tendencies in lobules 1, 7, 8, and 9 (Fig. 2).
Number of Fos-IR cells (mean±SE) in the granular layer of each lobe after olfactory stimulation. Lobes in the ALM and FREC groups are significantly larger than those in the CTRL group (P=.04). Although a different activation pattern can be observed between the lobules of the ALM and FREC groups, these differences were not statistically significant.
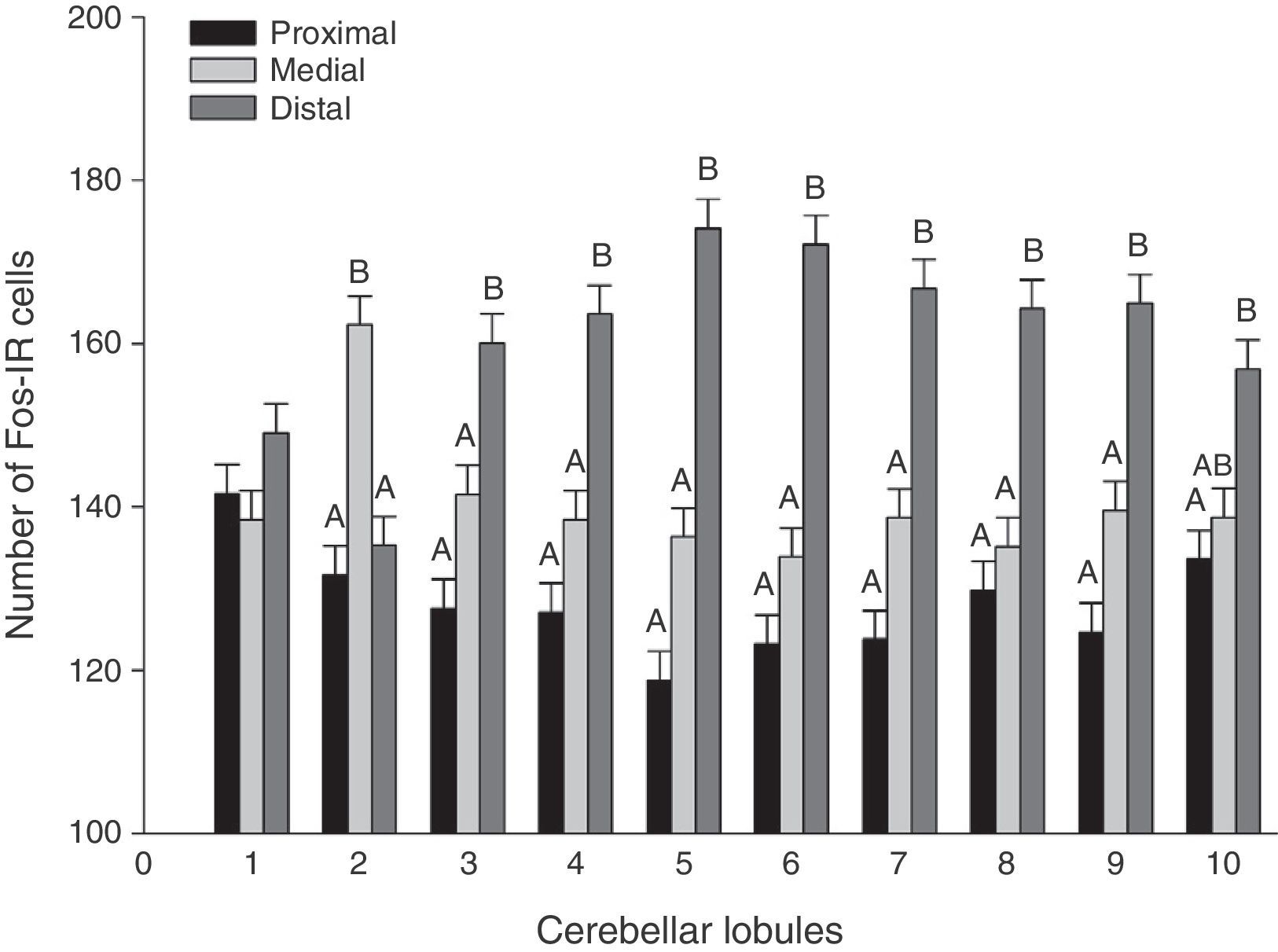
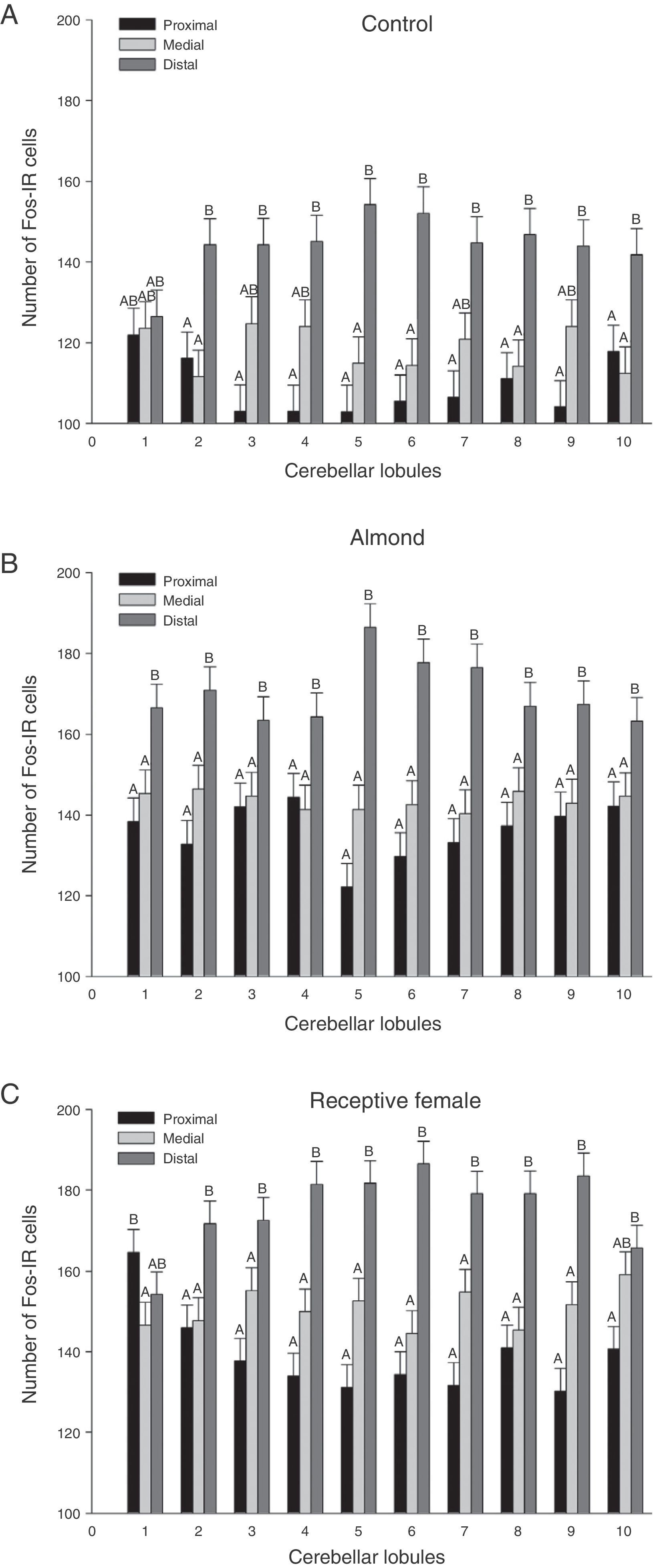
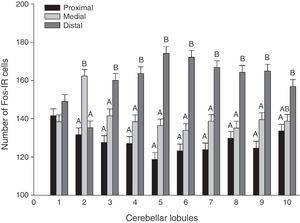
A global analysis of all 3 stimulus groups showed significant differences between lobule regions (F20.1349=21.02, P<.0001). The distal regions displayed a higher number of Fos-IR cells than either the medial or proximal regions in almost all lobules (Fig. 3). Analysis of the region-lobule interaction effects for each group determined the same tendency in activity; values differed significantly between regions (P<.0001) (Fig. 4).
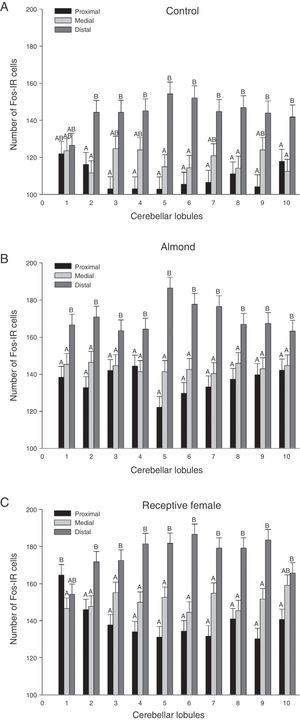
Graphic representation of Fos-IR neurons found in the 3 regions (proximal, medial, and distal) analysed for each group. (A) Control, (B) almond, and (C) receptive female. Letters indicate significant differences (P<.0001) between cerebellar regions. Regions that do not share the same letters differ from one another.
Our results show an increase in activity in the cerebellar cortex during olfactory stimulation of sexually naive male rats. Following the procedure described by Portillo and Paredes15 and Lledo et al.,18 we used the scents of almonds (ALM) and of receptive females (FREC) to activate the primary olfactory system in the first case and the accessory system (vomeronasal) in the second. We observe that regardless of the type of stimulus, or the olfactory system that was activated, activity in the granular layer of the cortex of the cerebellar vermis was higher in test subjects than in the control group (exposed to clean bedding). This idea is supported by other studies.3,11,19
Although both test stimuli (almond and receptive female) provoked a significant increase in cerebellar cortex activity compared to the control stimulus, we did observe an inter-group difference in the pattern of the magnitude of neuronal activation (this difference was not statistically significant). This difference might be explained by male rats having greater interest in the odour of receptive females, which in turn would increase exploratory movement and olfactomotor activity. This has been demonstrated in prior studies showing that the cerebellum may be activated without an odorant being present.3,11
On the other hand, Manzo et al.13 showed that the brain of male rats may be activated by multisensory stimuli (sight, smell, and sound) from receptive female rats, and that this cerebellar activity did not appear to be altered by the motor activity displayed by subjects during mating. Another possible explanation is that the cerebellum plays a role in the instinctive expectation of a sexual reward that arises with the perception of the sensory information contained in the odour of receptive females. The function of the cerebellum in emotions and in the expectation of sexual rewards has been confirmed by earlier studies.13,14 There have been no studies of cerebellar activity in sexually experienced rats stimulated with odours related to sexual behaviour.
The sagittal slices of the medial region of the cerebellar vermis show distinct neuronal activation patterns at the lobule level in each experimental group. However, since results for the group-lobule interaction effect are not statistically significant, we cannot claim that the lobules are more sensitive to one type of stimulus than to another. Despite the above, we did observe tendencies suggesting specific regionalisation in the medial part of the cerebellar vermis depending on the type of stimulus. Lobules 1, 7, 8, and 9 in subjects in the FREC group clearly display more Fos-IR cells than the same lobules from subjects in the ALM group. This may mean that the lobules are more susceptible to activation by projections from the accessory olfactory system than those from the primary system.
In turn, lobules 2, 3, 4, and 10, which showed similar number of immunoreactive cells in both groups, may be responsible for controlling olfactomotor behaviour.
The main olfactory bulb, together with the anterior olfactory nucleus, sends efferent signals to the piriform cortex, olfactory tubercle, cortical amygdala, and entorhinal cortex, as described by Lledo et al.18 On the other hand, the ventral tegmental area (VTA) contains neurons that project to the piriform cortex, the cerebellar cortex, and to the lateral cerebellar nuclei.20,21 The accessory olfactory bulb projects fibres from its mitral layer to the posterolateral and medial amygdala, which in turn originates the amygdalofugal pathway that connects to the ventromedial nucleus of the hypothalamus and the preoptic area. The posterolateral part of the amygdala projects via the stria terminalis towards the bed nucleus of the stria terminalis and the medial preoptic area.15 Potential interactions between the vomeronasal system and the cerebellum could occur by means of two pathways: (1) through hypothalamo-cerebellar fibres; or (2) through the VTA, which receives afferences from the medial amygdala.
A promising possibility that is supported by this experiment is that there is a link between both olfactory systems and the cerebellum, through the VTA. The piriform-VTA-pontocerebellum pathway corresponding to the primary olfactory system supports the idea proposed by Sobel et al.3 that the anterior cerebellar lobes regulate the olfactomotor aspect of olfaction. Furthermore, Ikai et al.20 described afferent fibres from the VTA innervating the rat cerebellar cortex, which is coherent with that observed by Sobel et al. in different cerebellar lobules in humans.3 This is also in line with observations from our experiment, in which vermal lobules 2 and 3 showed a significant increase in Fos-IR cells following contact with both stimuli. On the other hand, the vomeronasal system may be connected via a medial amygdala-VTA-cerebellar pathway.14 This pathway is supported by the results of Manzo et al.,13 who demonstrated that lobule 7 of the cerebellar vermis in male rats is more sensitive to a multisensory stimulus in the form of a receptive female rat. This result also supports our findings in that lobule 7 of the medial cerebellar vermis showed greater activation in subjects in the FREC group than in subjects in the ALM group. The fastigial nucleus-medial amygdala-VTA-cerebellum circuit3,14,20 suggests that there might be a feedback mechanism14 to regulate the sensitivity of the cerebellum when the vomeronasal system is stimulated. This in turn would explain why Fos-IR cell levels in the cerebellar vermis of male rats decrease with successive copulations even though sensory stimulation is persistent.13 Based on the above evidence, we suggest researching vermal lobule 7 as a region that is potentially sensitive to information provided by the vomeronasal system.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: García LI, García-Bañuelos P, Aranda-Abreu GE, Herrera-Meza G, Coria-Avila GA, Manzo J. Activación del cerebelo por estimulación olfativa en ratas macho sexualmente inexpertas. Neurología. 2015;30:264–269.