Leukotriene receptor antagonists (LTRAs) are used as a therapeutic alternative in asthmatic patients. Different animal studies indicate that LTRAs can decrease intimal hyperplasia after vascular injury, and have a protective role in cerebral ischemia.
ObjectiveThe aim of this study was to assess the role of leukotriene receptor antagonists in preventing the cardiovascular and ischemic stroke in humans.
Material and methodA matched case–control study with a follow up period of three years has been conducted, investigating the effect of the LTRAs in the myocardial infarct (MI) risk, and in the ischemic stroke (IS) risk in asthmatic patients from San Cecilio University Hospital of Granada, and from two Primary Health Care Centers of Granada.
Results59 cases with MI and 108 cases with IS were included in the study, each of them with an equal number of controls matched by age and sex in each of the two Health Care Centers. Unlike for MI risk, the treatment with LTRAs was associated with a slight trend in reducing the risk of stroke, in both of the primary care controls (Odds ratios: 0.74 (0.37–1.47); 0.82 (0.4–1.67), for the first, and the second Health Centers Controls, respectively), but without reaching a statistical significance.
ConclusionsThe results did not confirm a protective effect of LTRAs on cardiovascular risk as suggested by different animal studies.
Los antagonistas del receptor de leucotrienos (LTRAs) se utilizan como alternativa terapéutica en pacientes asmáticos. Diferentes estudios en animales sugieren que los LTRAs pueden disminuir la hiperplasia de la íntima después de una lesión vascular, ejerciendo así un papel protector sobre la isquemia vascular.
ObjetivoEl objetivo de este estudio fue evaluar el papel del antagonista del receptor de leucotrienos en la prevención de la cardiopatía isquémica y los accidentes cerebrovasculares en humanos.
Material y métodoSe realizó un estudio de casos y controles retrospectivos para investigar el efecto de la LTRAs sobre el riesgo de infarto agudo de miocardio (IAM) y accidente cerebrovascular isquémico (ACV). Los casos fueron pacientes ingresados en el Hospital Universitario San Cecilio de Granada; para cada caso se tomaron dos grupos de controles apareados por sexo y edad, entre pacientes adscritos a dos Centros de Atención Primaria de Salud de Granada con diagnóstico de asma.
ResultadosSe incluyeron 59 casos de IAM y 108 casos de ACV, e igual número de controles apareados por sexo y edad de cada uno de los dos Centros de Salud. El tratamiento con LTRAs se asoció con un aumento no significativo del riesgo de IAM (OR = 1,74; IC 95% 0,61- 4,96), y al contrario, con una ligera reducción del riesgo de accidente cerebrovascular, tanto cuando se estudió cada uno de los grupos de controles de forma independiente (OR = 0,74; IC 95% 0,37-1,47 para el primer centro; OR = 0,82; IC 95% 0,4 -1,67 para el segundo centro), como cuando se combinaron los dos grupos de controles (OR 0,63; IC 95% 0,32 -1,26). En ningún caso se alcanzó una significación estadística.
ConclusionesLos resultados no permiten confirmar el efecto protector sobre el riesgo cardiovascular sugerido por estudios previos realizados en animales.
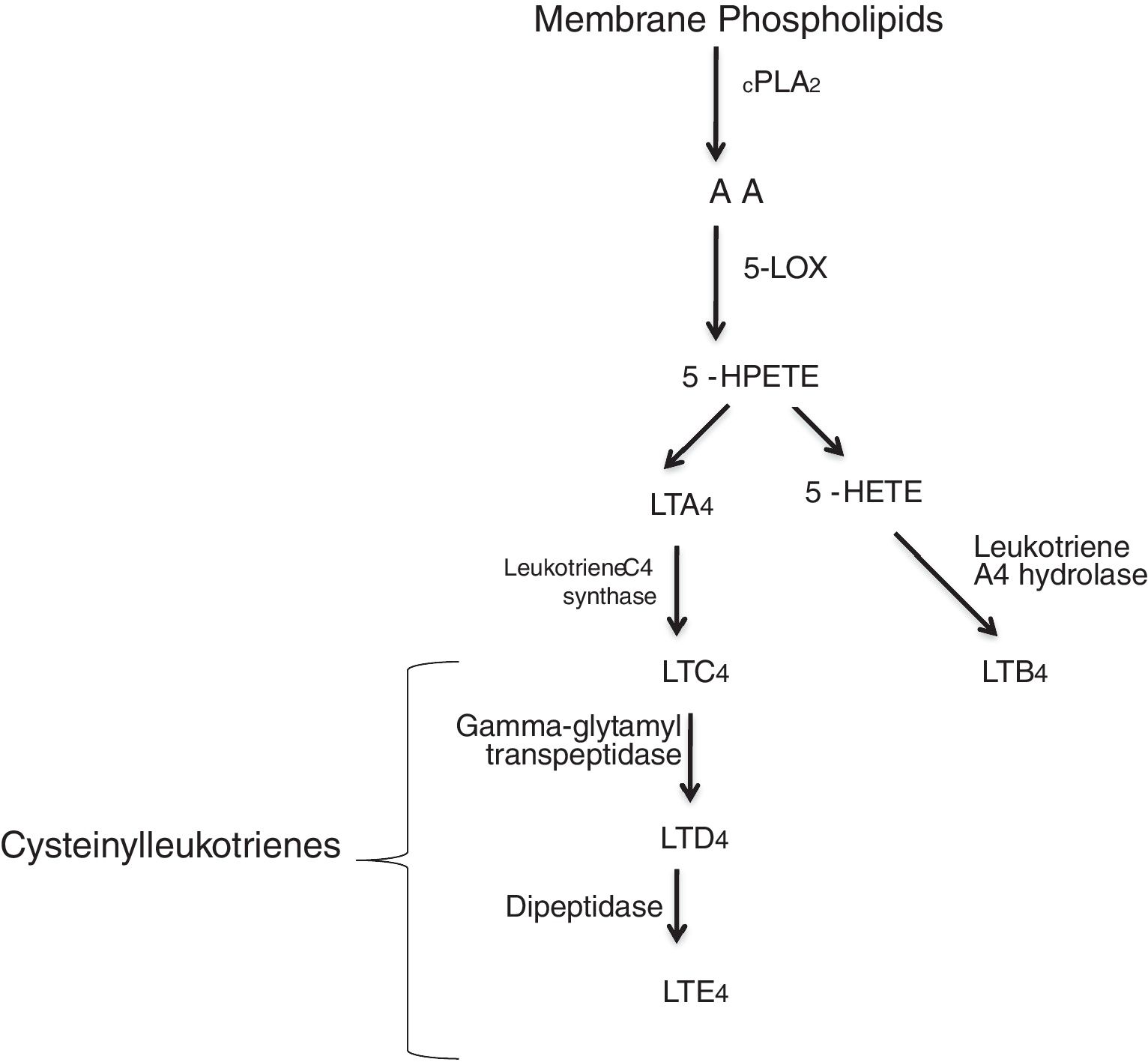
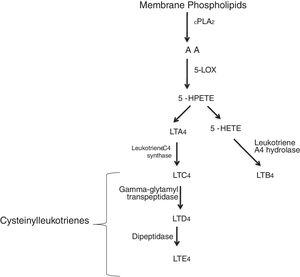
Leukotrienes are arachidonic acid (AA) mediators of inflammation synthesized by the 5-lipoxygenase (5-LO), that are involved in different inflammatory disease (Fig. 1), such as cardiovascular disease. They increase the capillary permeability, cause vasoconstriction and reduce the coronary blood flow.1,2 Some of these cardiovascular events in which leukotrienes are generated are: myocardial infarction (MI), ischemic stroke (IS), atherosclerosis or aortic aneurysm. Several studies have reported an increased activity of the 5-lipoxygenase pathway in different cardiovascular diseases.3,4
5-LO pathway. Leukotrienes are produced via 5-LO pathway. They are classified in leukotriene B4 (LTB4) and cysteinyl leukotrienes (cysteinyl-LTs: LTC4, LTD4, LTE4). Abbreviations: cytosolic phospholipase A2 (cPLA2), arachidonic acid (AA), 5-lipoxygenase (5-LO), 5-hydroperoxyeicosatetraenoic (5-HPETE), 5-hydroxyeicosatetraenoic acid (5-HETE), leukotriene A4 (LTA4), leukotriene B4 (LTB4), leukotriene C4 (LTC4), leukotriene D4 (LTD4), leukotriene E4 (LTE4).
Two different types of leukotrienes are known: the non peptidic leukotrienes (LTB4), and the peptidic leukotrienes or cysteinyl-leukotrienes (cysLT) (LTC4, LTD4 and LTE4). The cysteinyl leukotrienes play an important role in asthma, and in the 1990s, leukotriene receptor antagonists were used as a therapeutic alternative in asthmatic patients. Soon after, their use was expanded to the chronic obstructive pulmonary disease (COPD), allergic rhinits and urticaria patients.5 However, certain patients do not respond to the treatment, and this may be attributed to different substances that can mediate the inflammatory process.6 Cysteinyl leukotrienes increase the permeability of the endothelial cells7 and are involved in the recruitment of leukocytes in different inflammatory events as: cardiovascular disease, cancers, etc. Numerous studies have reported a significant role of cysteinyl leukotrienes as a cause of damage, secondary to myocardial injury,8,9 while others have reported a less significant role.10,11 Most interestingly, cysteinyl leukotrienes have been found in damaged arteries,12,13 therefore leukotriene receptor antagonists (LTRAs) may be potential drugs for cardiovascular disease. Both cysteinyl leukotrienes, and their receptors can be synthesized and expressed in atherosclerosis.1
Leukotriene receptor antagonists and Cardiovascular (CV) diseaseZafirlukast, Montelukast and Pranlukast were designed as antagonists of the CysLT1.10,14,15 Furthermore, montelukast, is one of the most potent antagonists used in patients with different levels of asthma, and was approved in 2003 by the FDA for use in allergic rhinitis, and in 2005 for the perennial rhinitis.16 In an in vivo study conducted in rats, montelukast, a leukotriene receptor antagonists, has been shown to reduce vascular reactive oxygen species synthesis, thus improving the endothelial cell function17 and inhibiting the atherosclerotic damage and intimal hyperplasia.16 On the other hand, another in vivo study in rabbits has shown that montelukast inhibits MCP-1 (monocyte chemoattractant protein-1). Montelukast may also have an anti-atherogenic effect.18
Different studies indicate that leukotriene receptor antagonists can decrease intimal hyperplasia after vascular injury, and have a protective role in cerebral ischemia.19–21 Moreover, they can have a protective role in the CV and cerebrovascular events through their antiapoptotic and anti-inflammatory functions.22
Different evidence indicate that LTRAs drugs, such as montelukast and zafirlukast can prevent the progression of atherosclerosis, and thus can be useful in reducing the chances of suffering cardiovascular or cerebrovascular disease.17,18,21,22 In experimental models (in vivo), LTRAs have shown to play a role in reducing the blood-brain barrier permeability and brain injuries.23–26 A national cohort study conducted in Sweedish population with a follow up period of three years demonstrated that montelukast might reduce the risk for recurrent myocardial infarction in male subjects (HR,0.65; 95% CI, 0.43–0.99), as well as recurrent stroke (HR, 0.62; 95% CI, 0.38–0.99) in patients taking montelukast,5 suggesting a potential role of montelukast for secondary prevention of CV disease.
The protective effect of LTRAs drugs on cardiovascular diseases, would be of particular interest for the clinical use of these drugs. Independent population-based studies are needed to further evaluate this association.
Aim of the studyThe main objective of our study was to estimate the effect of leukotriene receptor antagonists in preventing the myocardial infarction and ischemic stroke. The second objective was to determine the frequency of treatment with montelukast and with other leukotriene receptor antagonists in a group of hospitalized asthmatic patients with myocardial infarction and ischemic stroke (cases), and in a group of asthmatic patients without cardiovascular disease (controls). The third objective of the study was to analyze other cardiovascular risk factors, that could have acted as potential confounding factors.
Ethics approvalThe authorization was requested from the Ethics Committee of the province of Granada, which approved the study protocol, after assessing the need for the informed consent. All the information collected was included in an anonymous database, in which each subject is identified by an alphanumeric code.
MethodsDesign: A matched case–control study has been conducted to investigate the effect of the LTRAs in the myocardial infarct risk, and in the ischemic stroke risk.
Follow up period: 2012–2015
Field of study: Reference area of the San Cecilio University Hospital of Granada.
Definition of cases and controlsCases of MI: Patient, who were recovered during the follow up study period (2012–2015) in the University Hospital of Granada (CHUGR), whose main diagnosis is acute myocardial infarction, and also having asthma among their secondary diagnoses (n=59).
Cases of IS: Patient, who were recovered during the follow up study period (2012–2015) in the University Hospital Complex of Granada (CHUGRA), whose main diagnosis is ischemic stroke, and also having asthma among their secondary diagnoses (n=107).
Selection of the cases: A list of subjects that fulfilled the criteria of “the case” were requested from the database of University Hospital Complex of Granada. 59 cases with MI were included in the study with 59 respective controls for each of the two Health Centers, versus 108 cases with IS and 108 respective controls for each of the two Health Care Centers. The list was initially requested for the year 2015, but since, there was a small number of subjects that fulfilled the diagnostic criteria, it was extended to the year 2014, 2013 and 2012.
Selection of the controlsControls from the Primary Health Care Centers: We requested to the Metropolitan Granada Sanitary District a list of asthmatic patients from two Primary Health Centers of Granada, respectively the “Zaidín Sur” and “La Chana” Health Centers. Subsequently, a matched control was selected for each case, in each of the Health Centers, maintaining the following conditions: same sex and age that the case or, when this latter was not possible with an age range of not more than two years difference.
Data collectionBased on the Andalusian Health Unique History Number (NUHSA), the patient's history was taken from DIRAYA system of the Andalusian Health Service, with the hospital access to collect information on the cases from the hospital, referred to the period of hospitalization. Through the primary DIRAYA system access we recovered the information on the current active pharmacological prescription for the selected controls from both the Primary Health Care Centers. The following variables were recovered:
- •
Age: age range not more than four years difference
- •
Sex
- •
Drug use
- •
Use of Montelukast: It is considered affirmative only when montelukast was used for at least three months before the cardiovascular event had occurred. For the controls any use was accepted.
- •
First day of use of Montelukast
- •
Total duration of the use of Montelukast
- •
Smoking
- •
Date of discharge from the hospital
- •
Patient discharge Unit
- •
Principal diagnosis: MI; IS
- •
Secondary diagnosis: Diabetes Mellitus, Hypertension, Hypertriglyceridemia.
The study variables were estimated through the frequency distribution or by measuring the central tendency and dispersion, for qualitative and quantitative variables respectively.
Both an unvaried and multivariate conditional logistic regression, have been used to analyze the effect of leukotriene receptor antagonists and the potential confounders on the MI and IS risk, differentiating the group of cases and the multiple groups of controls that have been selected: controls from the Primary Health Care Centers.
For the multivariate adjustment, all the variables studied in a model were initially included. In the absence of convergence, a stepwise model was chosen, with a probability of 0, 20. Once the significant variables were selected by the system, the input of the variable “leukotriene receptor antagonists” was forced, in order to obtain adjusted estimates.
All the statistical analysis were performed with the Stata 14.0 statistical package.
ResultsThe case–control study characteristics are reported in Tables 1 and 2. Two health centers were randomly chosen. Our data demonstrate a homogeneity of the age between cases and controls.
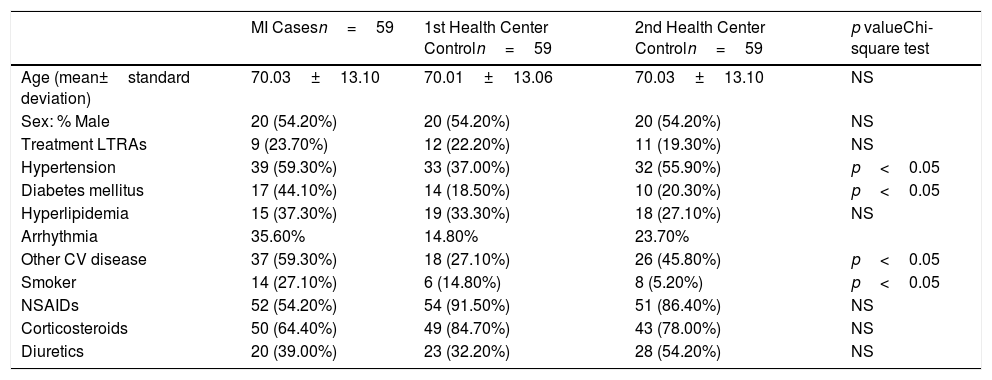
Description of the study group of controls characteristics: myocardial infarction cases.
| MI Casesn=59 | 1st Health Center Controln=59 | 2nd Health Center Controln=59 | p valueChi-square test | |
|---|---|---|---|---|
| Age (mean±standard deviation) | 70.03±13.10 | 70.01±13.06 | 70.03±13.10 | NS |
| Sex: % Male | 20 (54.20%) | 20 (54.20%) | 20 (54.20%) | NS |
| Treatment LTRAs | 9 (23.70%) | 12 (22.20%) | 11 (19.30%) | NS |
| Hypertension | 39 (59.30%) | 33 (37.00%) | 32 (55.90%) | p<0.05 |
| Diabetes mellitus | 17 (44.10%) | 14 (18.50%) | 10 (20.30%) | p<0.05 |
| Hyperlipidemia | 15 (37.30%) | 19 (33.30%) | 18 (27.10%) | NS |
| Arrhythmia | 35.60% | 14.80% | 23.70% | |
| Other CV disease | 37 (59.30%) | 18 (27.10%) | 26 (45.80%) | p<0.05 |
| Smoker | 14 (27.10%) | 6 (14.80%) | 8 (5.20%) | p<0.05 |
| NSAIDs | 52 (54.20%) | 54 (91.50%) | 51 (86.40%) | NS |
| Corticosteroids | 50 (64.40%) | 49 (84.70%) | 43 (78.00%) | NS |
| Diuretics | 20 (39.00%) | 23 (32.20%) | 28 (54.20%) | NS |
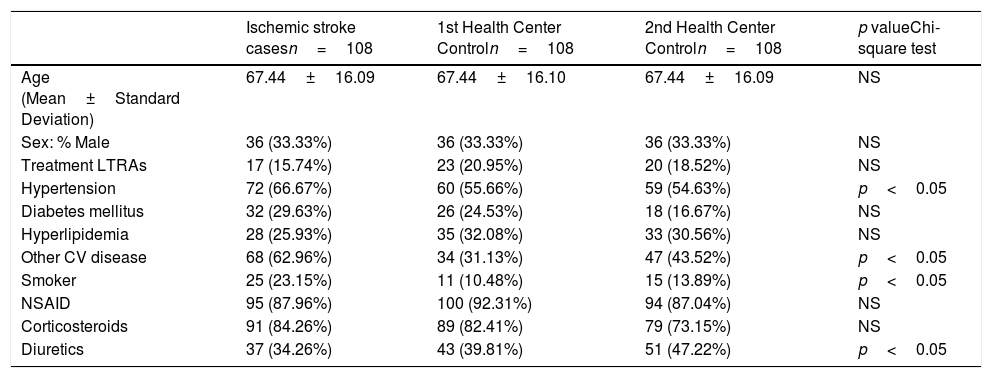
Description of the study group of controls characteristics: Ischemic stroke cases.
| Ischemic stroke casesn=108 | 1st Health Center Controln=108 | 2nd Health Center Controln=108 | p valueChi-square test | |
|---|---|---|---|---|
| Age (Mean±Standard Deviation) | 67.44±16.09 | 67.44±16.10 | 67.44±16.09 | NS |
| Sex: % Male | 36 (33.33%) | 36 (33.33%) | 36 (33.33%) | NS |
| Treatment LTRAs | 17 (15.74%) | 23 (20.95%) | 20 (18.52%) | NS |
| Hypertension | 72 (66.67%) | 60 (55.66%) | 59 (54.63%) | p<0.05 |
| Diabetes mellitus | 32 (29.63%) | 26 (24.53%) | 18 (16.67%) | NS |
| Hyperlipidemia | 28 (25.93%) | 35 (32.08%) | 33 (30.56%) | NS |
| Other CV disease | 68 (62.96%) | 34 (31.13%) | 47 (43.52%) | p<0.05 |
| Smoker | 25 (23.15%) | 11 (10.48%) | 15 (13.89%) | p<0.05 |
| NSAID | 95 (87.96%) | 100 (92.31%) | 94 (87.04%) | NS |
| Corticosteroids | 91 (84.26%) | 89 (82.41%) | 79 (73.15%) | NS |
| Diuretics | 37 (34.26%) | 43 (39.81%) | 51 (47.22%) | p<0.05 |
The frequency of treatment with LTRAs is 23.70% in cases with MI, and 22.20% and 19.30% from the first and the second Primary Health Center controls, respectively.
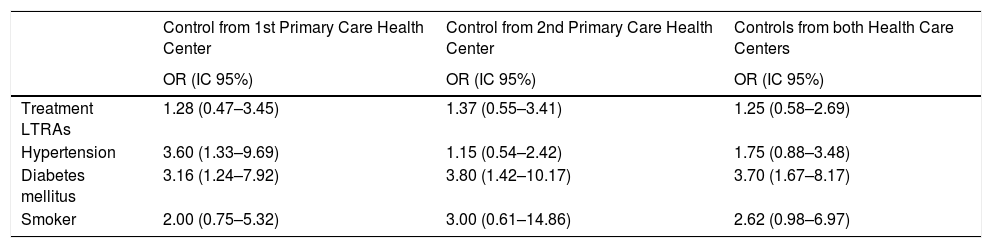
When analyzing the factors associated with MI (Table 3), a slight trend in reducing the risk effect between the association of LTRAs and MI stands out, for the primary care controls, despite the health center (Odds ratio: 1.28 and 1.37 respectively), but without reaching a statistical significance. The results show that there is also found a significant association between diabetes mellitus, hypertension, and tobacco, with the MI risk.
Association between different risk factors and myocardial infarction.
| Control from 1st Primary Care Health Center | Control from 2nd Primary Care Health Center | Controls from both Health Care Centers | |
|---|---|---|---|
| OR (IC 95%) | OR (IC 95%) | OR (IC 95%) | |
| Treatment LTRAs | 1.28 (0.47–3.45) | 1.37 (0.55–3.41) | 1.25 (0.58–2.69) |
| Hypertension | 3.60 (1.33–9.69) | 1.15 (0.54–2.42) | 1.75 (0.88–3.48) |
| Diabetes mellitus | 3.16 (1.24–7.92) | 3.80 (1.42–10.17) | 3.70 (1.67–8.17) |
| Smoker | 2.00 (0.75–5.32) | 3.00 (0.61–14.86) | 2.62 (0.98–6.97) |
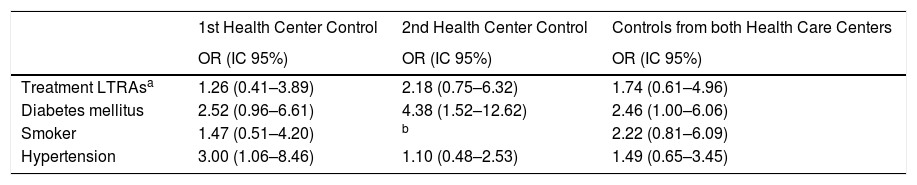
In the adjusted analysis of the different factors associated with MI (Table 4), data suggest a higher risk of the myocardial infarction when using LTRAs in the analysis adjusted for hypertension, diabetes mellitus and smoking in each of the two Health Centers controls, but without reaching a statistical significance.
Adjusted association between different risk factors and myocardial infarction.
| 1st Health Center Control | 2nd Health Center Control | Controls from both Health Care Centers | |
|---|---|---|---|
| OR (IC 95%) | OR (IC 95%) | OR (IC 95%) | |
| Treatment LTRAsa | 1.26 (0.41–3.89) | 2.18 (0.75–6.32) | 1.74 (0.61–4.96) |
| Diabetes mellitus | 2.52 (0.96–6.61) | 4.38 (1.52–12.62) | 2.46 (1.00–6.06) |
| Smoker | 1.47 (0.51–4.20) | b | 2.22 (0.81–6.09) |
| Hypertension | 3.00 (1.06–8.46) | 1.10 (0.48–2.53) | 1.49 (0.65–3.45) |
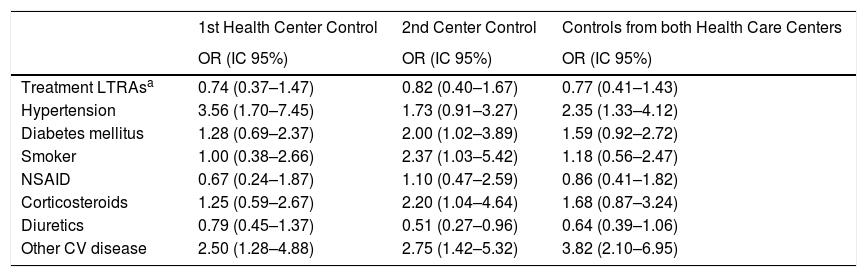
Unlike for myocardial infarction risk, the treatment with LTRAs was associated with a lower risk of stroke, in both of the primary care controls (Odds ratios: 0.74 (0.37–1.47); 0.82 (0.4–1.67); 0.77 (0.41–1.43)) for the first, second, and both Health Centers Controls, respectively), but without reaching a statistical significance. In the second Primary Health Center, a strong and significant association was found between the ischemic stroke risk and hypertension, or other cardiovascular diseases (arrhythmia, angina pectoris, valve problems, cardiomyopathy, etc.). Data from the first Primary Health Care Center have shown a significant association between the risk for ischemic stroke and hypertension, diabetes mellitus, smoking and other CV disease. The use of diuretics was associated with a protective effect in all Primary Health Care Centers (Table 5).
The crude analysis of the association between different risk factors and ischemic stroke.
| 1st Health Center Control | 2nd Center Control | Controls from both Health Care Centers | |
|---|---|---|---|
| OR (IC 95%) | OR (IC 95%) | OR (IC 95%) | |
| Treatment LTRAsa | 0.74 (0.37–1.47) | 0.82 (0.40–1.67) | 0.77 (0.41–1.43) |
| Hypertension | 3.56 (1.70–7.45) | 1.73 (0.91–3.27) | 2.35 (1.33–4.12) |
| Diabetes mellitus | 1.28 (0.69–2.37) | 2.00 (1.02–3.89) | 1.59 (0.92–2.72) |
| Smoker | 1.00 (0.38–2.66) | 2.37 (1.03–5.42) | 1.18 (0.56–2.47) |
| NSAID | 0.67 (0.24–1.87) | 1.10 (0.47–2.59) | 0.86 (0.41–1.82) |
| Corticosteroids | 1.25 (0.59–2.67) | 2.20 (1.04–4.64) | 1.68 (0.87–3.24) |
| Diuretics | 0.79 (0.45–1.37) | 0.51 (0.27–0.96) | 0.64 (0.39–1.06) |
| Other CV disease | 2.50 (1.28–4.88) | 2.75 (1.42–5.32) | 3.82 (2.10–6.95) |
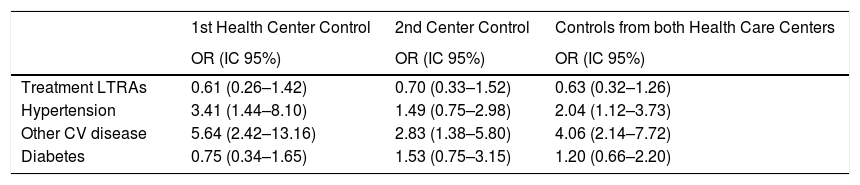
In the adjusted models for IS (Table 6), introducing all the variables in the table without convergence problems, we detected a slight trend in reducing the IS risk by using LTRAs, but without reaching a statistical significance. In both primary care controls, a protective effect is observed for LTRAs treatment (Odds ratio: 0.61 (0.26–1.42); 0.70 (0.33–1.52); 0.63 (0.32–1.26)) respectively. Hypertension and the presence of other cardiovascular disease was significantly associated with an increased ischemic stroke risk.
Adjusted association between different risk factors and ischemic stroke.
| 1st Health Center Control | 2nd Center Control | Controls from both Health Care Centers | |
|---|---|---|---|
| OR (IC 95%) | OR (IC 95%) | OR (IC 95%) | |
| Treatment LTRAs | 0.61 (0.26–1.42) | 0.70 (0.33–1.52) | 0.63 (0.32–1.26) |
| Hypertension | 3.41 (1.44–8.10) | 1.49 (0.75–2.98) | 2.04 (1.12–3.73) |
| Other CV disease | 5.64 (2.42–13.16) | 2.83 (1.38–5.80) | 4.06 (2.14–7.72) |
| Diabetes | 0.75 (0.34–1.65) | 1.53 (0.75–3.15) | 1.20 (0.66–2.20) |
The association between asthma and cardiovascular disease is shown by epidemiological studies, but it is still unclear whether it is a casual association, if it is due to side effects of the drugs used or to its proper bronchial inflammatory process, which brings to a possible endothelial dysfunction in asthmatic patients.
The bronchial and systemic inflammatory nature of asthma, has provided arguments that asthmatic patients may be at a higher risk for cardiovascular and cerebrovascular diseases.
However, asthma is associated with different risk factors (body mass index increased in some phenotype, hypertension, diabetes mellitus) that could justify this association. Furthermore, drugs used to treat asthma may increase the risk for cardiovascular disease.
In our previous systematic review that comprised data from 28 original research studies, 26 conducted on animals and two in humans, the potential role of LTRAs in reducing the MI and IS risk was consistently demonstrated.
However, in the present study the current data demonstrate that leukotriene receptor antagonists have a slight trend in reducing the effect for myocardial infarction, situation somehow inverse in the ischemic stroke risk, indicating a moderate protection of LTRAs in ischemic stroke risk, but in both cases with no statistical significance.
The frequency of use of leukotriene receptor antagonists is around 20%. The two series of controls chosen in primary care presented frequencies of 19.30% and 22.20% respectively. These are asthmatic patients, listed in the Primary DIRAYA system, with no diagnosed cardiovascular pathology, matched by age and sex with the respective cases. The frequency of treatment with LTRAs among the cases was 23.70% and 15.74% for MI and IS respectively.
Despite not having found other studies reporting the prevalence of treatment with LTRAs in asthmatic patients, we should consider that age is associated with greater resistance to treatment, as described by Dunn et al.27 Hence, it is possible that our patients, with a mean age of 70 years, have a higher prevalence of use of this type of drugs and consequently greater resistance to them. We did not report the obesity due to scarce registering in the patient histories database.
However, when comparing the cases with selected population of controls from the lists of asthmatic patients from primary care health centers, we found a protective effect, more pronounced when adjusted for the confounding factors. This effect does not have a statistical significance, probably because the low intensity of use of LTRAs treatment requires a larger sample size, but would be in line with Riccioni G, et al. findings.27
However, we cannot exclude the existence of information bias. Despite this, most of the variables studied are in line with their well known scientific effects. In some cases, it is the treatment that determines that there are variables that have a greater effect, which must be interpreted carefully, since there may be differences in the quality of registering some variables.
Strengths and weaknesses of the studyThe main limitation of the study is its retrospective design, and the study sample. This can influence the evaluation of the temporal sequence of the association, particularly due to the difficulty to define the time of exposure to the LTRAs. The lack of reliable information on the dates of prescription and use of drugs is a limitation for our analysis. In order to solve this problem, the cohort of asthmatic patients followed in primary care would have to be monitored, which would be very expensive in time and resources if it is considered prospectively, but it could be important to do it retrospectively if we could save this information from the computer system displaying a reliable source of information. We hope that in the future, once the XXI recipe is fully implemented, this kind of studies can be precisely carried out.
Another important limitation is the small sample size. Selecting the MI cases, in which asthma is a secondary diagnosis, this population is greatly reduced. We did not want to go back in time, earlier to the year 2012, due to the lack of information in the electronic history system, which would be an additional difficulty to access the information, as well as for possible changes in the use of the pharmacological treatments under study.
ConclusionsThe results did not confirm a protective effect of LTRAs on cardiovascular risk. Data from animal studies on LTRAs in preventing CV and cerebrovascular risk are very promising, however our results did not confirm a protective effect of LTRAs. Therefore, we believe that further prospective studies, should be carried out to further test this hypothesis.
FundingNo sources of funding were used to assist in the preparation of this research paper.
Conflict of interestThe authors have no conflicts of interest that are directly relevant to the content of this research paper.
The author would like to thank Dr. Esther Thomas for the help on accessing the Zaidin Sur Health Center patient database.