Women with breast cancer who receive aromatase inhibitors (AI) are at risk of bone mass loss and bone fracture. Many of these women have been previously treated with chemotherapy and/or tamoxifen, also with known deleterious bone effects. We studied the bone mineral status of a group of postmenopausal women with non-advanced breast cancer about to initiate AI treatment.
Between 2007 and 2010, 127 women aged 63±9 years were prospectively included. Clinical, epidemiological, analytical, radiological, and densitometric data were collected.
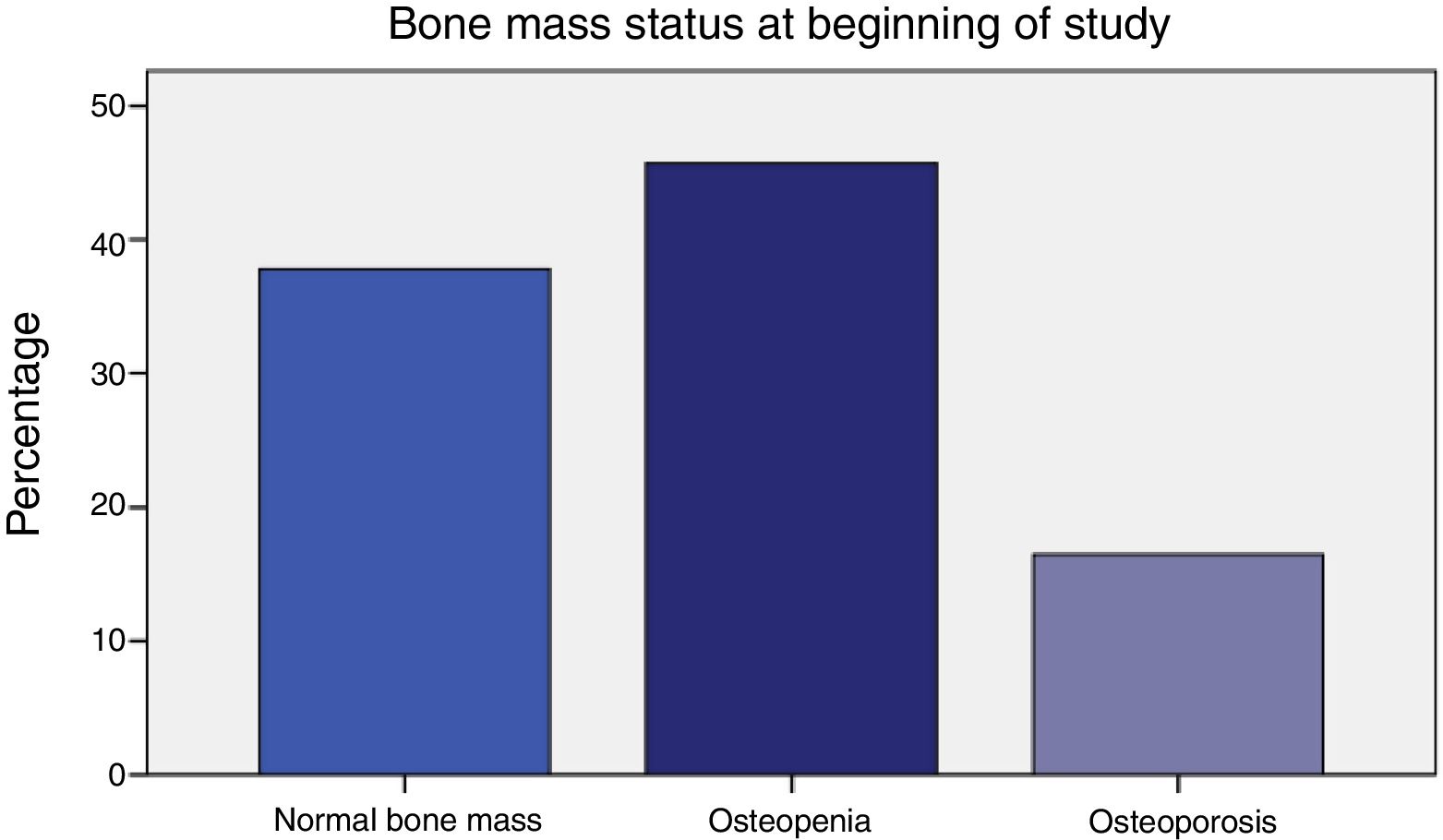
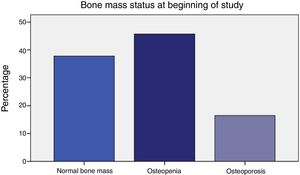
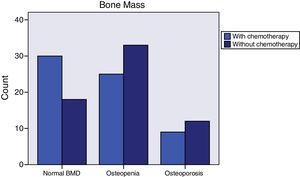
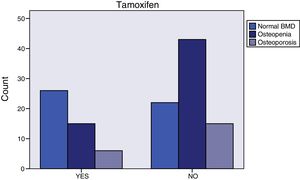
Prior to initiating AI treatment, the patients exhibited a high prevalence of bone mass loss (62.2%): 16.5% had osteoporosis and 45.7% osteopenia by OMS criteria. Besides, 7.4% of their dorso-lumbar spine x-rays revealed one or more vertebral fractures, all of which were found in patients with densitometry-defined osteoporosis or osteopenia. Surprisingly, 25-OH-vitamin D levels were normal (≥30 ng/ml) in 87.4% of the women. Obesity was prevalent (BMI 30±5). Their most common tumour was infiltrating ductal carcinoma (76.4%). The women who had received adjuvant chemotherapy (50%) were younger (59.1±8.3 vs. 66.2±8.2, p<.0001), and had greater total hip bone mass (.988±.138 vs. .935±.119, p=.048). The patients that had taken tamoxifen (37%) more often exhibited normal bone mass (55.3% vs. 27.5%, p=.009), and had greater bone mass at the femoral neck (.893±.113 vs. .826±.131, p=.003). In addition, they had lower bone turnover: lower osteocalcin (4.4±2.8 vs. 8.2±6.7, p<.0001) and deoxypyridinoline (7.1±2.4 vs. 8.3±3.3, p=.037).
This study shows that more than 60% of women about to initiate aromatase inhibitors for non-advanced breast cancer have low bone density and so high risk of fracture or re-fracture. These facts underscore the importance of screening their bone mass status and propose them preventive treatment.
Las mujeres con cáncer de mama que reciben inhibidores de la aromatasa (IA) tienen riesgo de padecer pérdida de masa ósea y fracturas óseas. Muchas de estas mujeres han sido previamente tratadas con quimioterapia y/o tamoxifeno, que tienen también efectos nocivos. Estudiamos el estado mineral óseo de un grupo de mujeres posmenopáusicas con cáncer de mama no avanzado, a punto de iniciar tratamiento de IA.
Entre 2007 y 2010 incluimos prospectivamente a 127 mujeres de 63 ± nueve años de edad. Se recopilaron los datos clínicos, epidemiológicos, analíticos y densitométricos.
Previamente al inicio de la terapia de IA, las pacientes reflejaron una alta prevalencia de pérdida de masa ósea (62,2%): el 16,5% tenía osteoporosis y el 45,7% osteopenia con arreglo a los criterios de la OMS. Además, en el 7,4% de los casos la radiografía de columna dorsolumbar reveló una o más fracturas vertebrales, todas ellas en las pacientes con osteoporosis u osteopenia, definidas mediante densitometría. De manera sorprendente, los niveles de 25-hidroxivitamina D fueron normales (≥ 30 ng/mL) en el 87,4% de las mujeres. La obesidad fue prevalente (IMC 30 ± 5). El tumor más común fue carcinoma ductal infiltrante (76,4%). Las mujeres que recibieron quimioterapia adyuvante (50%) eran más jóvenes (59,1 ± 8,3 vs. 66,2 ± 8,2, p < 0,0001), y tenían mayor masa ósea en la cadera (0,988 ± 0,138 vs. 0,935 ± 0,119, p = 0,048). Las pacientes que recibieron tamoxifeno (37%) reflejaron a menudo masa ósea normal (55,3 vs. 27,5%, p = 0,009), y tenían mayor masa ósea en el cuello del fémur (0,893 ± 0,113 vs. 0,826 ± 0,131, p = 0,003). Además, tenían menor recambio óseo: niveles más bajos de osteocalcina (4,4 ± 2,8 vs. 8,2 ± 6,7, p < 0,0001) y deoxipiridinolina (7,1 ± 2,4 vs. 8,3 ± 3,3, p = 0,037).
Este estudio refleja que más del 60% de las mujeres que van a iniciar terapia de inhibidores de la aromatasa para cáncer de mama no avanzado tienen baja densidad ósea, y por ello mayor riesgo de fractura o refractura. Estos hechos subrayan la importancia de cribar el estado de su masa ósea y proponer tratamientos alternativos.
Women with breast cancer are at higher risk for osteoporosis and fractures than the general population because of the treatments they receive,1–3 such as chemotherapy and different hormonal maneuvers .4,5 Aromatase inhibitors use is recommended for several years; hence, their side effects are more relevant .6-18 The increased fracture risk has been estimated to have an “odds ratio” (OR) of 1.47 .19,20
The aim of this study was to assess the bone health (bone mass, presence of vertebral fractures, and bone remodeling markers) in a group of postmenopausal women with non-advanced breast cancer who were to initiate adjuvant hormonal treatment with aromatase inhibitors (AI) regardless the previous use of other treatments like chemotherapy or tamoxifen.
Material and methodsAll postmenopausal women with non-metastatic breast cancer (stages I-III; TNM 6th ed. [TNM Classification of Malignant Tumors 2002], valid when the study began) who were to begin adjuvant hormonal treatment with an aromatase inhibitor (steroid or non-steroid) at the Clinical Oncology Service of the University Hospital of Canarias between April 2007 and September 2010 were included.
We excluded patients who declined to participate in the study, those who had a creatinine clearance (ClCr) value of less than 50ml/minute, those with another concomitant or prior neoplasm, and those who had been treated to increase bone mass in the two years preceding the study were excluded. Seven women who had received a single preventive dose of intravenous zoledronic acid before chemotherapy, more than 2 years before participating in our study, were included after a statistical evaluation that failed to detect differences in bone mass between them and the other women.
In total, 127 Caucasian women were included; all were postmenopausal, prior to being diagnosed with the tumor, or after adjuvant treatment for their breast cancer like chemotherapy. The participants signed an informed consent form before being enrolled.
Following diagnosis of their neoplasm, all participants had been assessed by the Gynecological Tumor Multidisciplinary Committee at our center, comprised of clinical and radiation oncologists, gynecologists, and pathologists and the most appropriate therapeutic approach was determined on a case-by-case basis. Treatment options included surgery, chemotherapy, hormone therapy, and/or radiotherapy, and the sequence of treatment was decided based on clinical guidelines or multicenter clinical trials underway during each year of the study.
Blood and urine levels of several bone remodeling markers were determined for each patient: osteocalcin, deoxypyridinolines (by electrochemoluminescence assay), RANKL, and osteoprotegerin (by enzyme immunoassay). Anteroposterior and lateral x-rays of the dorsal and lumbar spine were also taken and read blindly in search of vertebral fractures. Bone densitometry (DEXA) of the lumbar spine and hip (femoral neck and total hip) was also performed using a HOLOGIC 2000 (Waltham, Mass) densitometer from 2007 until 2008, whereas a LUNAR (GE HEALTHCARE) densitometer was used as of 2008. All densitometries were referenced on a database of healthy Spanish women. WHO criteria (1994) (osteoporosis if T-score is<-2.5 standard deviations (SD), osteopenia between -1 and -2.5 SD, and normal bone mass if>-1 SD) established definitions. T-scores were used because all the participants were menopausal; results were also expressed as an absolute value (g/cm2).
Data were analyzed using the SPSS software (versions 15.0 and 20.0) with Kolmogorov-Smirnov, chi-square, Student's t, and Wilcoxon tests. Results were expressed in terms of mean±standard deviation or median. A two-tailed test determined p values that were deemed statistically significant when <0.05.
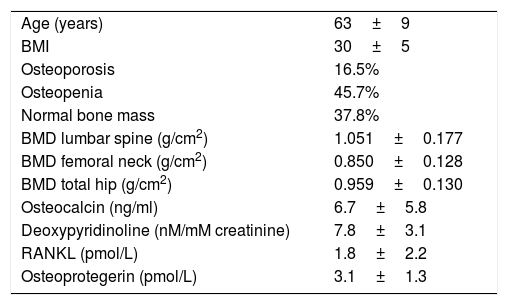
ResultsTable 1 displays women baseline characteristics: patients in this study had a high prevalence of osteoporosis (16.5%) and osteopenia (45.7%); more than 60% displayed bone mass loss (Figure 1).
Baseline characteristics.
| Age (years) | 63±9 |
| BMI | 30±5 |
| Osteoporosis | 16.5% |
| Osteopenia | 45.7% |
| Normal bone mass | 37.8% |
| BMD lumbar spine (g/cm2) | 1.051±0.177 |
| BMD femoral neck (g/cm2) | 0.850±0.128 |
| BMD total hip (g/cm2) | 0.959±0.130 |
| Osteocalcin (ng/ml) | 6.7±5.8 |
| Deoxypyridinoline (nM/mM creatinine) | 7.8±3.1 |
| RANKL (pmol/L) | 1.8±2.2 |
| Osteoprotegerin (pmol/L) | 3.1±1.3 |
A referred history of osteoporosis or osteopenia was present in 16% and 12% had suffered a fragility bone fracture. At least one parent was reported to have had a hip fracture by 6.9% of the participants. Women had had physiological menopause (75%), surgical menopause (15%) or secondary one after hormonal castration or chemotherapy (10%).
We got 121 lateral spine x-rays (95.3% of the women). Their analysis showed that 7.4% revealed one or several vertebral fractures. All women with vertebral fractures had densitometry-confirmed osteoporosis (55.6%) or osteopenia (44.4%). Participants with at least one vertebral fracture were generally older (16% over>65 years of age vs. 1.4%, p=0.004), had lower femoral neck bone mass (0.723±0.116 vs. 0.857±0.123, p=0.008) and total hip bone mass (0.844±0.096 vs. 0.968±0.129, p=0.008), albeit there were no significant differences in lumbar spine bone mass.
Only 46% consumed at least 3 servings per day of dairy products, although 87.4% had normal baseline 25-OH-vitamin D levels (≥30 ng/ml) with a mean of 47.6±18 ng/ml. Women with normal 25-OH-vitamin D levels had lower osteocalcin values (6.2±5.7 vs. 8.5±3.1, p=0.039). There were not significant differences between dairy products consumption and normal 25-OH-vitamin D levels.
Most of the participants were overweight or obese (BMI 30±5); 42% were sedentary, and 16.5% had diabetes. The obese women reported a history of fragility fracture less often (21.1% vs. 56.2%, p=0.006) and tended toward more frequent normal bone mass (60.4% vs. 39.6%, p=0.068). Diabetic subjects had higher BMI values (32.2±4.3 vs. 29.6±4.7, p=0.02), tended to have greater total hip bone mass (1.017±0.145 vs. 0.947±0.124, p=0.067), and had lower RANKL values (1.02±0.2 vs. 2.00±2.4, p=0.025). There were not bone mass differences between diabetic or nor diabetic women nor with active (58%) or sedentary (42%) ones.
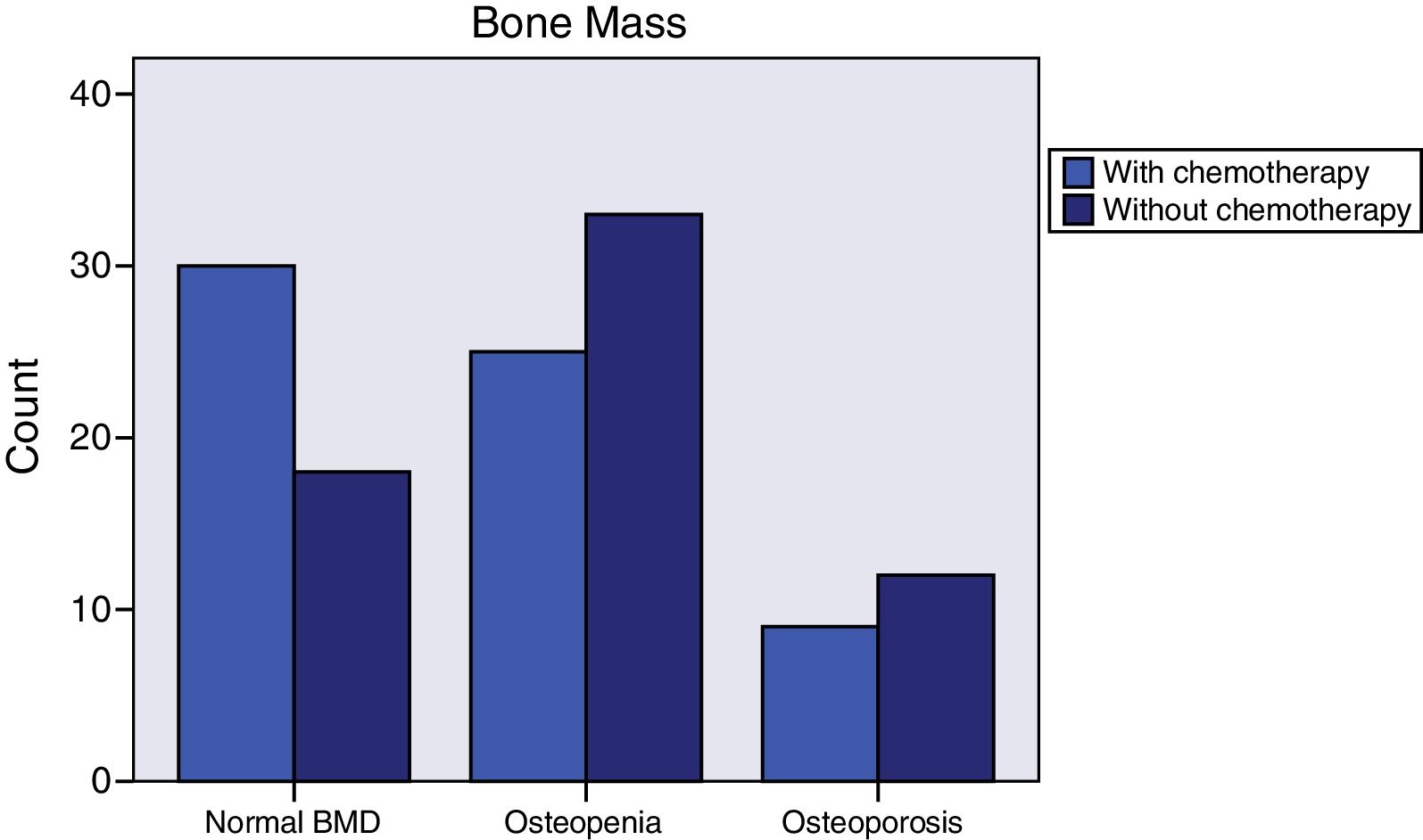
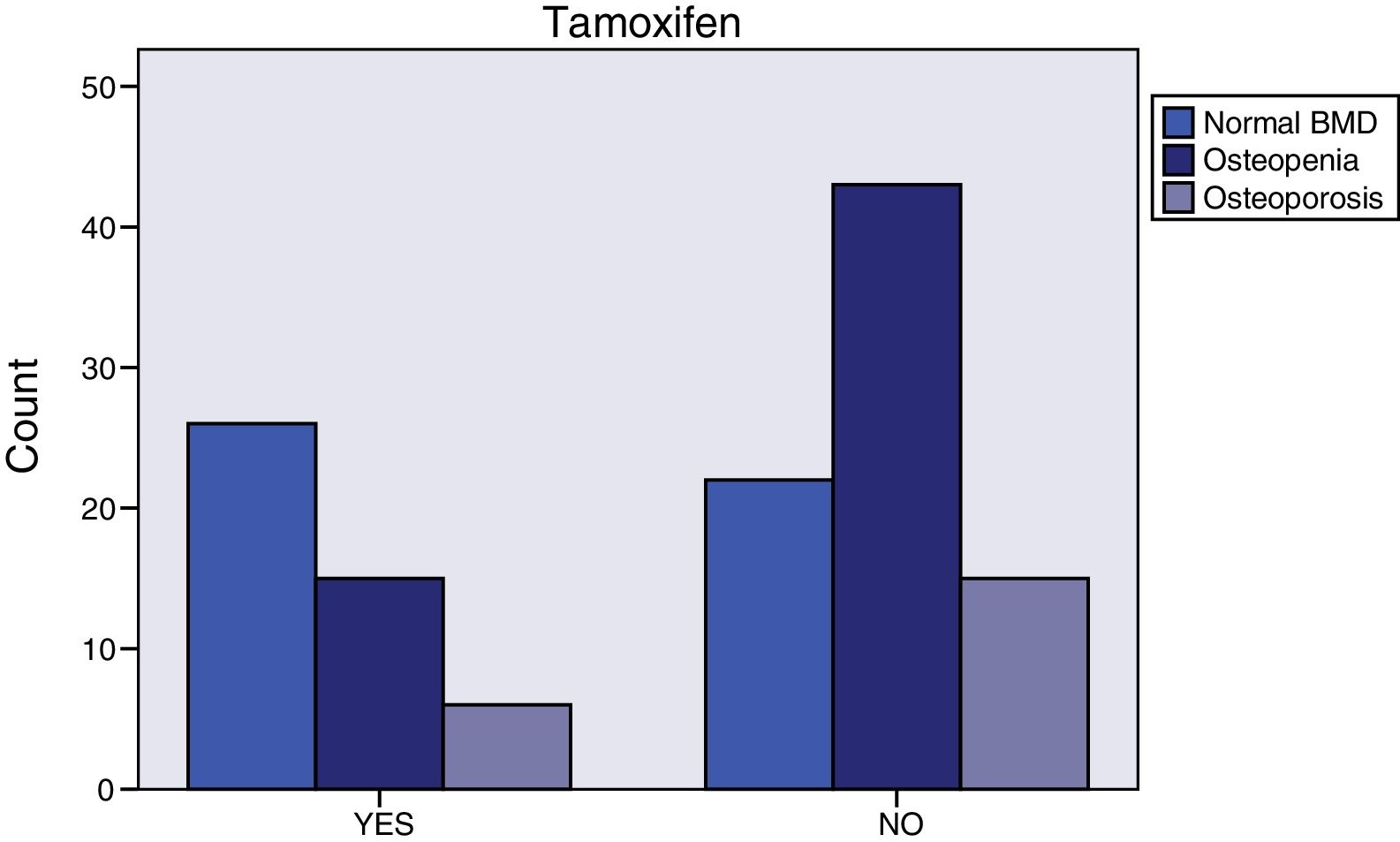
Breast cancer had been diagnosed between 1-69 months prior to initiating AI treatment. The most common histology was infiltrating ductal carcinoma (76.4%), followed by infiltrating lobular carcinoma (14.2%). Most of the cases (76%) were stage IIA. Almost all (99.2%) were surgically treated. 86% of the women received radiotherapy and 50% adjuvant chemotherapy. Those who had received chemotherapy tended to have normal bone mass more frequently (46.9% vs. 28.6%, p=0.064) (Figure 2), were younger (59.1±8.3 vs. 66.2±8.2, p<0.0001), and had greater total hip bone mass (0.988±0.138 vs. 0.935±0.119, p=0.048). The tumor tissue in all cases was hormone receptor positive and 3.1% were Her 2 positive. More than one third (37%) of the patients had taken tamoxifen for 27 months (12-62); fulvestrant had been administered to 6.3%, and 0.8% had received goserelin. Women treated with tamoxifen more often exhibited normal bone mass (55.3% vs. 27.5%, p=0.009) (Figure 3), higher 25-OH-vitamin D levels (56.2±17.8 vs. 42.4±17.0, p<0.0001), lower osteocalcin (4.4±2.8 vs. 8.2±6.7, p<0.0001) and deoxypyridinoline values (7.1±2.4 vs. 8.3±3.3, p=0.037), and greater femoral neck bone mass (0.893±0.113 vs. 0.826±0.131, p=0.003).
Current or prior use of drugs known to harm bone was reported by 18.1% of the subjects (9.4% antidepressants, 6.3% thyroid hormones, 1.6% anticonvulsants, and 0.8% steroids) but we did not find bone mass differences.
DiscussionMore than 60% of the women in this study had low bone mass (45,7% osteopenia and 16,5% osteoporosis). This proportion is lower than reported in the B-ABLE Study, conducted at a similar point in time in postmenopausal Catalan women who were beginning AI treatment and that detected osteopenia in 60% and osteoporosis in 22% prior to initiating AI therapy .21 This low bone mass is present before starting AI treatment, which underscores the importance of evaluating bone health in these women to assess the use of calcium, vitamin D, and biphosphonates or other drugs like denosumab with the aim of reversing and/or preventing AI-induced bone mass loss. These participants had prolonged exposure to estrogens that should have protected their bone mass; instead, they displayed a high prevalence of osteoporosis and fractures after having undergone chemotherapy, hormone maneuvers, and taking tamoxifen.
Their mean age was 63 years, similar to the B-ABLE sample .21 Dairy product consumption was scant (fewer than half of the patients had at least 3 servings of dairy products per day), which does not correlate with their 25-OH-vitamin D levels, which were normal in most cases. This contrasts with other studies conducted in women with breast cancer that have revealed much lower vitamin D levels (one in premenopausal women in northeastern United States 22 and another one in postmenopausal women in the Loire region of France 23. The B-ABLE Study showed that almost 90% had vitamin D deficiency 21. These discrepancies may be caused by the latitude at which our population resides (Canary Islands).
Most of the patients were overweight (BMI 30±5), a known risk factor for breast cancer, but a protective factor for osteoporosis. In fact, the obese women tended to have higher bone mass. Diabetic women, with higher BMI, also had greater baseline bone mass and lower RANKL levels, which would speak to a lower rate of bone resorption.
Women who had undergone chemotherapy tended to have greater bone mass, probably because they were younger. Additionally, those who had taken tamoxifen had greater bone mass, higher vitamin D levels, and greater slowing of bone metabolism, with lower baseline levels of osteocalcin and deoxypyridinolines, which is consistent with tamoxifen being considered bone mass-protective in postmenopausal women.
At least one vertebral fracture was seen in 7.4% of the sample and all of these patients had osteoporosis or osteopenia. Vertebral fractures were more common in women>65 years and in those with lower bone mass at the hip. Bouvard et al. found a higher prevalence of vertebral fracture (20%) in a similar group of women 23.
In short, this population of women who are going to be treated with AI already displays risk factors for fracture that will be augmented with the use of these drugs for several years, with an estimated yearly bone mass loss of 2-6% and increased overall fracture risk of at least 20-35% 24. This effect on bone mass has been proven for all aromatase inhibitors currently being used (anastrozole, letrozole, and exemestane).
Both bone densitometry and x-rays of the spine are indicated in these women, but recent studies show that the screening is low in this population 25–27.
ConclusionsIn our setting, postmenopausal women who are going to begin adjuvant treatment with an aromatase inhibitor already present low bone mass in more than 60% and a prevalence of vertebral fracture>7%, so the importance of both screening and treatment with the aim to prevent further fractures
Ethics approval and consent to participateAll participants signed an informed consent.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.