Identify lung sequelae of COVID-19 through radiological and pulmonary function assessment.
DesignProspective, longitudinal, cohort study from March 2020 to March 2021.
SettingIntensive Care Units (ICU) in a tertiary hospital in Portugal.
Patients254 patients with COVID-19 admitted to ICU due to respiratory illness.
InterventionsA chest computed tomography (CT) scan and pulmonary function tests (PFT) were performed at 3 to 6 months.
Main variables of interestCT-scan; PFT; decreased diffusion capacity of carbon monoxide (DLCO).
ResultsAll CT scans revealed improvement in the follow-up, with 72% of patients still showing abnormalities, 58% with ground glass opacities and 62% with evidence of fibrosis. PFT had abnormalities in 94 patients (46%): thirteen patients (7%) had an obstructive pattern, 35 (18%) had a restrictive pattern, and 58 (30%) had decreased DLCO. There was a statistically significant association between abnormalities in the follow-up CT scan and older age, more extended hospital and ICU stay, higher SAPS II and APACHE scores and invasive ventilation. Mechanical ventilation, especially with no lung protective parameters, was associated with abnormalities in PFT. Multivariate regression showed more abnormalities in lung function with more extended ICU hospitalization, chronic obstructive pulmonary disease (COPD), chronic kidney disease, invasive mechanical ventilation, and ventilation with higher plateau pressure, and more abnormalities in CT-scan with older age, more extended ICU stay, organ solid transplants and ventilation with higher positive end-expiratory pressure (PEEP).
ConclusionsMost patients with severe COVID-19 still exhibit abnormalities in CT scans or lung function tests three to six months after discharge.
Identificar secuelas pulmonares de COVID-19 através de evaluación radiológica y de función pulmonar.
Diseño: Estudio prospectivo, longitudinal, de cohortes de marzo de 2020 a marzo de 2021.
ÁmbitoUnidades de Cuidados Intensivos (UCI) en un hospital terciario en Portugal.
PacientesSe incluyeron un total de 254 pacientes con COVID-19 confirmada por ensayo de reacción en cadena de la polimerasa con transcriptasa inversa en tiempo real de hisopos nasales/faríngeos.
IntervencionesSe realizaron una tomografía axial computarizada (TAC) de tórax y pruebas de función pulmonar (PFP) a los 3 a 6 meses.
Principales variables de interésTAC; PFP; Disminución de capacidad de difusión de monóxido de carbono (DLCO).
ResultadosTodas TAC revelaron una mejora en el seguimiento, con el 72 % de los pacientes que aún mostraban anomalías, el 58 % con opacidades en vidrio esmerilado y el 62 % con evidencia de fibrosis. La PFP presentó anomalías en 94 pacientes (46 %): trece pacientes tenían un patrón obstructivo, 35 un patrón restrictivo y 58 tenían una DLCO disminuida. La regresión logística multivariada mostró más anomalías en la función pulmonar con hospitalización en la UCI más prolongada, enfermedad pulmonar obstructiva crónica (EPOC), enfermedad renal crónica, ventilación mecánica invasiva y ventilación con presión de meseta más alta, y más anomalías en la tomografía computarizada con mayor edad, estancia más prolongada en la UCI, y ventilación con mayor Titulación de Pression Positiva Expiratória (PEEP).
ConclusionesLa mayoría de los pacientes con COVID-19 grave aún presentan anomalías en las tomografías computarizadas o en las pruebas de función pulmonar de tres a seis meses después del alta.
In December 2019, a new coronavirus (SARS-CoV-2) emerged in Wuhan, China and quickly spread worldwide. As of August 17, 2022, more than 580 million cases were confirmed, and more than 6 million deaths were reported globally, posing a significant burden on healthcare systems1.
The manifestations and complications of acute Coronavirus Disease 2019 (COVID-19) have been extensively discussed. However, since the pandemic's beginning, it has also been apparent that many patients had persistent residual symptoms and organ impairment2. This has been known as post-COVID conditions or long COVID-19 3–5. Besides the impact on the quality of life and psychological status, it became essential to understand the possible long-term consequences of lung function and structure. The previous follow-up studies suggest various rates of abnormal pulmonary function testing (PFT) after recovery from COVID-19. The most frequent impaired parameter is the diffusing capacity for carbon monoxide (DLCO)6–9. Persistent lung damage has also been reported, with more than half of patients having some abnormality, mainly ground glass opacities, fibrotic changes and consolidations7,8. Age and the length of invasive mechanical ventilation during the Intensive Care Unit (ICU) stay were some of the factors associated with the severity of lung damage7. The presence of fibrosis raises the concern of chronic lesions, but more studies are needed.
While assessing a patient with respiratory symptoms months after a COVID-19 infection, familiarity with typical lung sequelae of COVID-19 will facilitate a more precise distinction between long-COVID and other lung conditions. On the individual side, follow-up imaging will assess improvement, which may correlate to present pulmonary symptoms.
The primary aim of this study was to evaluate structural and functional lung sequelae of COVID-19 through radiological and pulmonary function assessment.
Patients and methodsStudy design and populationInclusion criteria were age 18 years or older, hospitalisation for more than 24 hours, reverse transcription polymerase chain reaction (RT-PCR) confirmed SARS-CoV2 infection and inpatient treatment in an intensive care unit (with live discharge) in a Portuguese tertiary hospital. Exclusion criteria included death in the three months after discharge.
Patients eligible according to inclusion and exclusion criteria were invited to participate in the study. We included those who agreed to participate and signed informed consent.
Patients were assessed 3 to 6 months after discharge, and a chest computed tomography (CT) scan and pulmonary function tests (PFT) were performed. The primary variables of interest were the CT scan and PFT abnormalities.
Clinical data of patients at admission were collected retrospectively, including age, gender, smoking history, and comorbidities. The following comorbidities were assessed: hypertension, dyslipidemia, obesity, diabetes mellitus, chronic heart disease, chronic kidney disease, obstructive sleep apnea (OSA), chronic obstructive pulmonary disease (COPD), asthma, and solid organ transplant. Data on hospital stay and intensive care unit, the patients’ SAPS II and APACHE II scores and the type of respiratory support needed, namely high flow nasal cannula (HFNC), non-invasive ventilation, invasive mechanical ventilation, and extracorporeal membrane oxygenation (ECMO) were collected. Characteristics of invasive mechanical ventilation, specifically days of invasive mechanical ventilation, worst PaO2/FiO2 ratio, highest PEEP, highest plateau pressure, highest PaCO2 and highest driving pressure, were collected.
The SAPS II and APACHE II score are two scoring systems widely use to estimate mortality in ICU patients. An increasing score is associated with an increasing risk of hospital death.
Radiological assessmentAll patients underwent an unenhanced chest CT scan 3 to 6 months after discharge. All CT acquisitions were obtained with the patient in a supine position and with breath-holding following inspiration. Images were obtained in a spiral acquisition mode with a 1 mm slice thickness, and multiplanar reformats were performed in the sagittal and coronal planes.
The CT-scan images were classified according to the 2015 Fleischner consensus statement on the classification of interstitial lung abnormalities (ILA) as having or not having fibrotic ILA.
Pulmonary function testsThe PFT were done according to the American Thoracic Society and European Respiratory Society guidelines10. The parameters measured included the forced expiratory flow between 25% and 75% of forced vital capacity (FVC), forced expiratory volume (FEV1), FVC/FEV1, vital capacity, total lung capacity, residual volume and the DLCO.
Ethical considerationsThe Local Ethics Committee approved the present study (247/2020), and the 1964 Helsinki Declaration and its later amendments were followed. Written informed consent was obtained for every patient.
Statistical analysisCategorical variables are presented as frequencies and percentages, continuous variables as means and standard deviations (normal distribution) or, in the case of variables with skewed distributions, with medians and interquartile ranges. Normal distribution was verified using the visual distribution of the variable (histogram). All participants for whom the variables of interest were available were included in the final analysis. For missing data, no assumptions were made. Chi-squared test was performed for categorical variables, the Mann-Whitney U test for non-parametric data, and the T test for independent variables for continuous variables.
We used logistic regression with backward stepwise regression to examine the relationship between predictor variables and the presence of interstitial lung abnormalities (ILA), as indicated by evidence of fibrosis on thoracic computed tomography (CT) scan, impaired diffusing capacity of the lung for carbon monoxide (DLCO) on pulmonary function testing (PFT), or both. Predictor variables were selected based on a combination of statistical significance in univariate analysis (p < 0.25) and biologic plausibility. Backward stepwise regression was employed to identify the most significant predictors of the outcome variable. Starting with the full model, variables were removed one by one until all remaining variables were statistically significant.
We performed separate analyses in two groups of patients: one group including all patients, and one group including only mechanically ventilated patients. The latter analysis was conducted to assess the impact of respiratory mechanics of ventilation, such as driving pressure and plateau pressure, on the outcome.
The reported p-values are two-tailed, with a p-value less than 0.05 indicating statistical significance. Analyses and graphs were performed using SPSS (version 27, IBM).
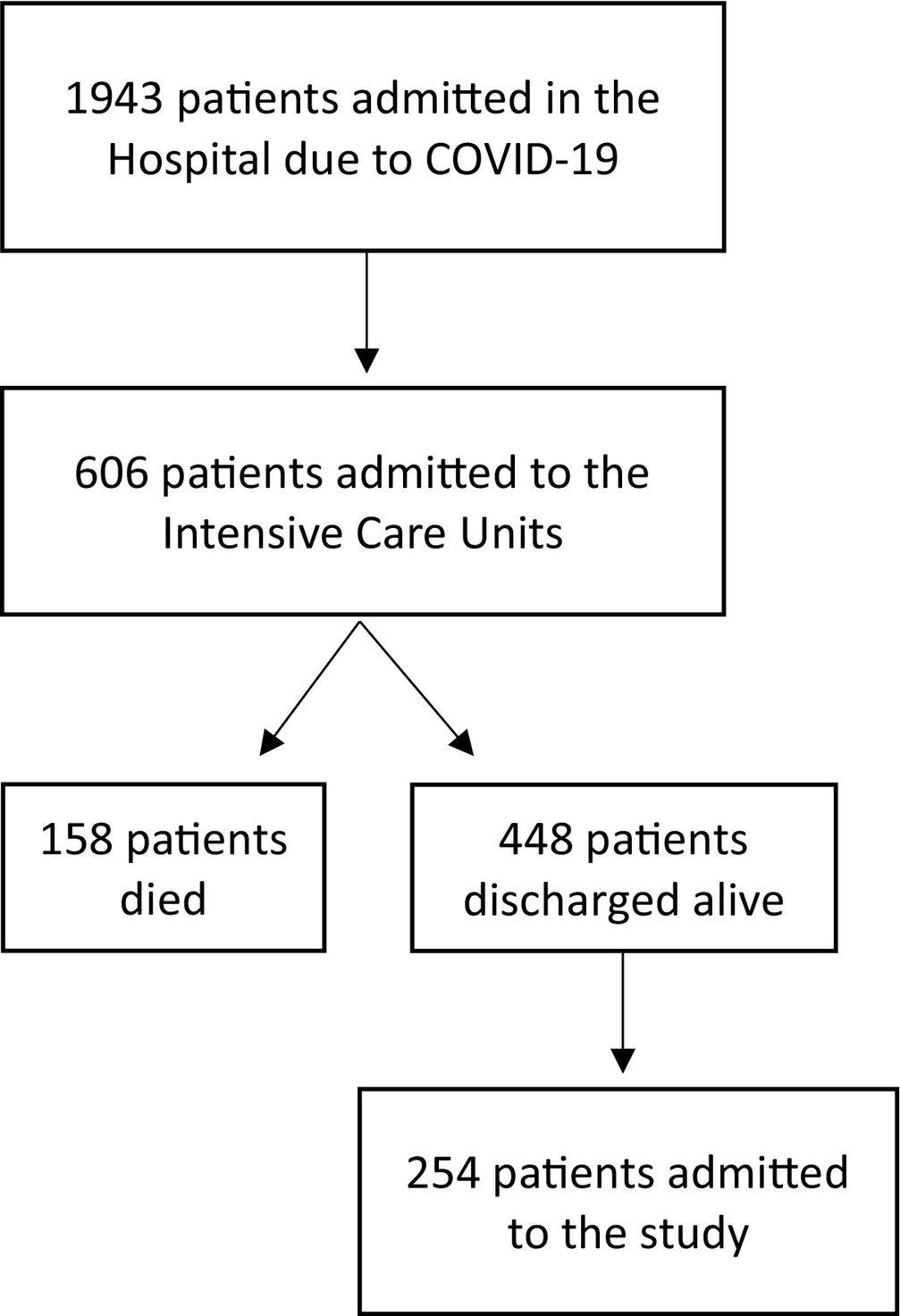
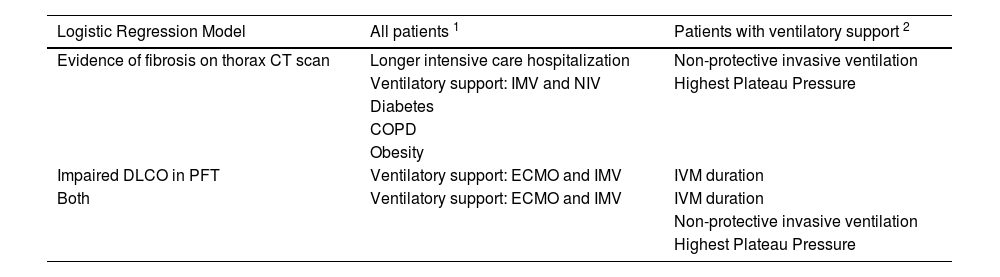
ResultsCharacteristics of study sampleIn the period between March 2020 to March 2021, 1943 patients were admitted to our hospital (Centro Hospitalar Universitário São João, Porto, Portugal) with a SARS-CoV-2 infection confirmed by RT-PCR assay of nasal/pharyngeal swabs. Six hundred and six patients were admitted to intensive care. Of these, 448 patients (74%) were discharged alive from the ICU. Out of them, 254 (42%) accepted to participate in this study. No patients were lost to follow-up. Fig. 1 shows the flowchart of the study.
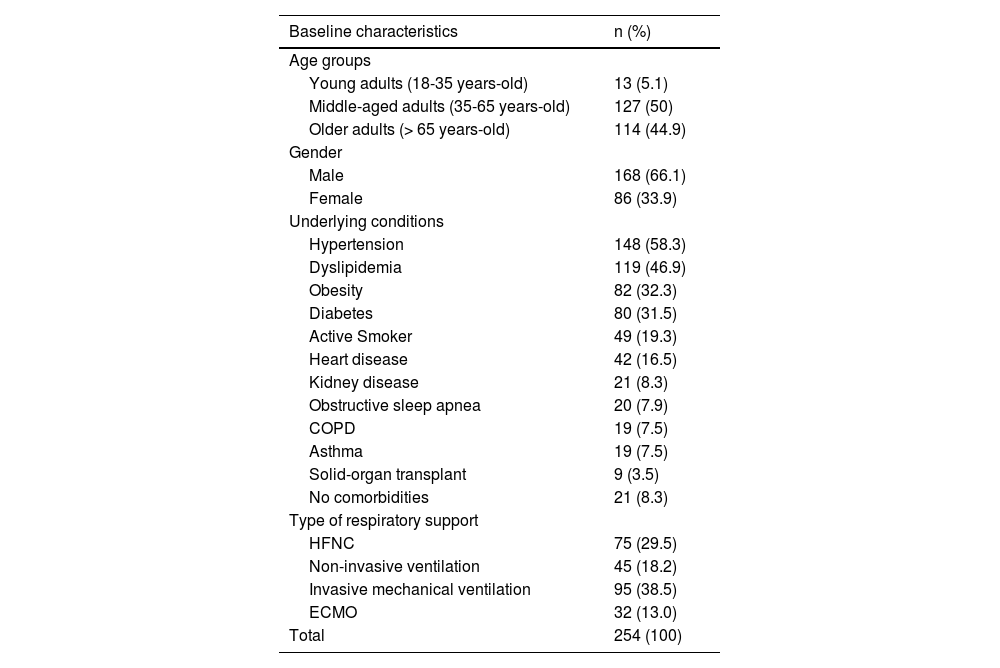
The median age was 64 (interquartile range 56 - 73), and most patients were male (66%). Most patients had comorbidities, the most common being hypertension, dyslipidaemia, obesity, and diabetes mellitus. The mean SAPS II score was 35.0 (standard deviation 14), and the mean APACHE II score was 16.3 (standard deviation 6). Patients’ basal characteristics are outlined in Table 1.
Characteristics of severe COVID-19 patients included in this study. COPD: chronic obstructive pulmonary disease, ECMO: extracorporeal membrane oxygenation, HFNC: high-flow nasal cannula.
| Baseline characteristics | n (%) |
|---|---|
| Age groups | |
| Young adults (18-35 years-old) | 13 (5.1) |
| Middle-aged adults (35-65 years-old) | 127 (50) |
| Older adults (> 65 years-old) | 114 (44.9) |
| Gender | |
| Male | 168 (66.1) |
| Female | 86 (33.9) |
| Underlying conditions | |
| Hypertension | 148 (58.3) |
| Dyslipidemia | 119 (46.9) |
| Obesity | 82 (32.3) |
| Diabetes | 80 (31.5) |
| Active Smoker | 49 (19.3) |
| Heart disease | 42 (16.5) |
| Kidney disease | 21 (8.3) |
| Obstructive sleep apnea | 20 (7.9) |
| COPD | 19 (7.5) |
| Asthma | 19 (7.5) |
| Solid-organ transplant | 9 (3.5) |
| No comorbidities | 21 (8.3) |
| Type of respiratory support | |
| HFNC | 75 (29.5) |
| Non-invasive ventilation | 45 (18.2) |
| Invasive mechanical ventilation | 95 (38.5) |
| ECMO | 32 (13.0) |
| Total | 254 (100) |
Median ICU and hospital length of stay were ten days (interquartile range 5-26) and 26 days (interquartile range 13-27). At admission, all patients presented with respiratory dysfunction. Other dysfunctions were also present at admission in some patients, namely renal (79, 31.1%), hepatic (44, 19.9%), hematologic, cardiovascular (each 41, 16.1%) and neurologic dysfunction (19, 8.4%).
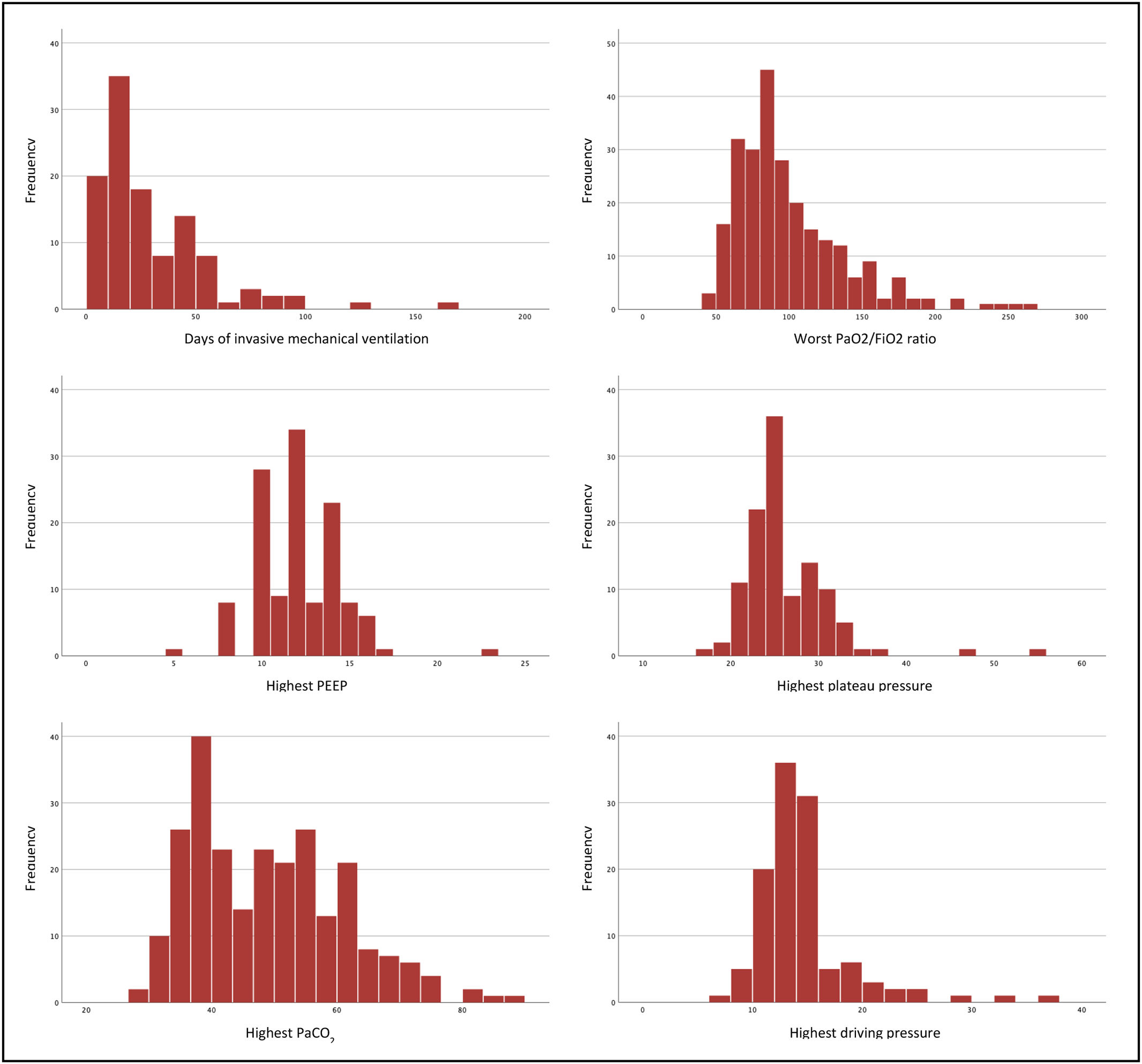
One hundred and twenty-five patients (49.2%) were intubated and mechanically ventilated, including 30 patients out of 32 that were on ECMO. Of those that were not put on invasive ventilation, 75 (29.5%) were on high-flow nasal oxygen, 45 (18.2%) were on non-invasive ventilation, and 9 (3.1%) were on conventional oxygen therapy. In most (76.8%) of mechanically ventilated patients, protective ventilation was achieved (plateau pressure inferior to 30 cmH2O and driving pressure lower than 15 cmH2O). The median time of ventilation was 20.5 days (IQR 29). A more detailed overview of these patients is presented in Fig. 2.
In 191 patients both thorax CT scan and PFT were performed, in 48 only the CT-scan was performed and in 15 only the PFT.
Radiological assessmentA thorax CT scan was performed at 3 to 6 months in 239 patients (94%). All patients revealed improvement from the acute to the convalescent phases of COVID-19 infection. At the follow-up CT scan, 172 patients (72%) still presented with imagiological changes. One-hundred and thirty-two patients (55.7%) had ground glass opacities, and 98 patients (43.2%) had some evidence of fibrosis in the CT scan. The fibrosis was apparent with four different patterns, namely usual interstitial pneumonia-pattern fibrosis in 75 patients (32.3%), traction bronchiectasis in 49 patients (20.8%), bronchiolectasis in 44 patients (18.6%) and fibrotic atelectasis in 35 patients (14.9%).
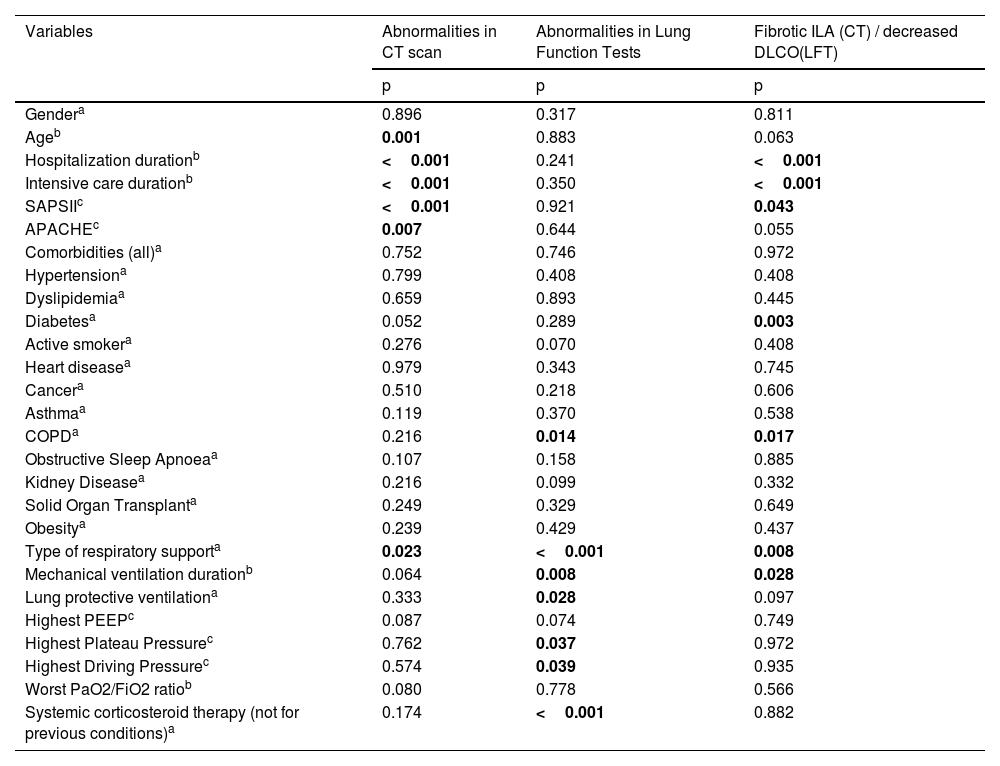
Univariate analysis and multivariate logistic regression models were performed to explore the risk factors associated with imagiological changes. The univariate analysis is presented in Table 2. There was a statistically significant association between the persistence of abnormalities in the convalescent phase CT-scan and older age (p < 0.01), more extended hospital and ICU stay (p < 0.001), higher SAPS II (p < 0.001) and APACHE scores (p<0.01) and having been invasively ventilated (p < 0.05). No statistically significant association was observed between abnormalities in the CT scan and the presence of OSA, COPD or asthma.
Univariate analysis: The relationship between clinical variables in 254 patients with severe COVID-19 at three to six months follow-up chest computed tomography and pulmonary function tests. COPD: chronic obstructive pulmonary disease, CT: computed tomography, DLCO: diffusion capacity of carbon monoxide, ILA: intersticial lung abnormalities, LFT: Lung function tests, PEEP: positive end-expiratory pressure.
| Variables | Abnormalities in CT scan | Abnormalities in Lung Function Tests | Fibrotic ILA (CT) / decreased DLCO(LFT) |
|---|---|---|---|
| p | p | p | |
| Gendera | 0.896 | 0.317 | 0.811 |
| Ageb | 0.001 | 0.883 | 0.063 |
| Hospitalization durationb | <0.001 | 0.241 | <0.001 |
| Intensive care durationb | <0.001 | 0.350 | <0.001 |
| SAPSIIc | <0.001 | 0.921 | 0.043 |
| APACHEc | 0.007 | 0.644 | 0.055 |
| Comorbidities (all)a | 0.752 | 0.746 | 0.972 |
| Hypertensiona | 0.799 | 0.408 | 0.408 |
| Dyslipidemiaa | 0.659 | 0.893 | 0.445 |
| Diabetesa | 0.052 | 0.289 | 0.003 |
| Active smokera | 0.276 | 0.070 | 0.408 |
| Heart diseasea | 0.979 | 0.343 | 0.745 |
| Cancera | 0.510 | 0.218 | 0.606 |
| Asthmaa | 0.119 | 0.370 | 0.538 |
| COPDa | 0.216 | 0.014 | 0.017 |
| Obstructive Sleep Apnoeaa | 0.107 | 0.158 | 0.885 |
| Kidney Diseasea | 0.216 | 0.099 | 0.332 |
| Solid Organ Transplanta | 0.249 | 0.329 | 0.649 |
| Obesitya | 0.239 | 0.429 | 0.437 |
| Type of respiratory supporta | 0.023 | <0.001 | 0.008 |
| Mechanical ventilation durationb | 0.064 | 0.008 | 0.028 |
| Lung protective ventilationa | 0.333 | 0.028 | 0.097 |
| Highest PEEPc | 0.087 | 0.074 | 0.749 |
| Highest Plateau Pressurec | 0.762 | 0.037 | 0.972 |
| Highest Driving Pressurec | 0.574 | 0.039 | 0.935 |
| Worst PaO2/FiO2 ratiob | 0.080 | 0.778 | 0.566 |
| Systemic corticosteroid therapy (not for previous conditions)a | 0.174 | <0.001 | 0.882 |
A multivariate logistic regression model was performed, as presented in Table 3. Generally, there was an association with statistical significance between abnormalities in CT scans and more extended intensive care hospitalisation, invasive or non-invasive mechanical ventilation, respiratory support with ECMO, diabetes mellitus, COPD and obesity. When the analysis was performed solely in patients placed on invasive ventilation, a statistically significant association was observed between abnormalities in CT scan and non-protective ventilation, higher plateau pressures and longer duration of invasive mechanical ventilation.
Multivariable logistic regression analysis: The relationship between clinical variables in 254 patients with severe COVID-19 at three to six months follow-up chest computed tomography and pulmonary function tests. The presented variables are those with p<0.05. COPD: chronic obstructive pulmonary disease, CT: computed tomography, diffusion capacity of carbon monoxide, ECMO: extracorporeal membrane oxygenation, NIV: non-invasive mechanical ventilation, PEEP: positive end-expiratory pressure.
| Logistic Regression Model | All patients 1 | Patients with ventilatory support 2 |
|---|---|---|
| Evidence of fibrosis on thorax CT scan | Longer intensive care hospitalization | Non-protective invasive ventilation |
| Ventilatory support: IMV and NIV | Highest Plateau Pressure | |
| Diabetes | ||
| COPD | ||
| Obesity | ||
| Impaired DLCO in PFT | Ventilatory support: ECMO and IMV | IVM duration |
| Both | Ventilatory support: ECMO and IMV | IVM duration |
| Non-protective invasive ventilation | ||
| Highest Plateau Pressure |
PFT were available in 206 out of 254 patients (81.1%). At 3 to 6 months, no alterations were present in 112 patients (54.4%). Ninety-four patients (45.6%) had at least one abnormality. The most frequent impaired parameter was the DLCO in 58 patients (29.7%), followed by a restrictive pattern in 35 patients (17.7%) and an obstructive pattern in 13 patients (6.6%).
Univariate analysis and multivariate logistic regression models were performed to explore the risk factors associated with impaired lung function. The univariate analysis is presented in Table 2. Using the univariate analysis, an association was seen between the use of mechanical ventilation and abnormalities in the PFT (p < 0.001). This association was mainly related to the higher duration of mechanical ventilation (p <0.01), non-attainment of protective ventilation (p <0.05), higher plateau pressure (p < 0.05) and higher driving pressure (p < 0.05). The multivariate logistic regression model, described in Table 3, showed a statistically significant association between PFT abnormalities and the need for respiratory support, namely with invasive mechanical ventilation and ECMO. As observed with the abnormalities in the CT scan, when the analysis was performed in ventilated patients, a statistically significant association was observed between impaired lung function and non-protective ventilation, higher plateau pressures and longer duration of invasive mechanical ventilation.
DiscussionIn our study, we found that most patients still presented with imaging changes at the 3 to 6 months follow-up. Ground glass opacities and evidence of fibrosis were the most common findings, affecting about one-half of patients each. This finding is concurrent with recent studies7. Furthermore, imagiological changes seem to fade over time, as shown by Bellan M et al. (2021), where at the CT scan at one-year follow-up, 76.8% of severe COVID-19 patients presented with none to mild involvement11. Xiaojun Wu et al. (2021) also showed persistent radiological changes at 12 months after discharge in only 20 patients (24%)8. The magnitude of lung damage in these patients has also been described in previous coronavirus outbreaks, namely with Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS)12,13.
Since the outbreak of SARS-CoV-2, many efforts have been made to understand the clinical course of COVID-19 and the factors associated with the worst prognosis. More recent studies have focused on the long-term consequences of COVID-19. However, more information is needed on the long-term impact and reversibility of sequelae caused by this infection, namely in severe patients.
Our study also demonstrated alteration in the lung function, not previously known, in 94 (45.6%) patients, with the most frequent impaired parameter being the DLCO, observed in 29.7% of the patients. Restrictive and obstructive patterns were seen in 35 and 13 patients, respectively. Previous studies have shown similar results7,9,11,14. An impairment of DLCO was also seen in patients with SARS in around 15 to 25% of cases, especially in patients admitted to intensive care units15. The PFT abnormalities correlated to mechanical ventilation use, mostly if in more extended periods with no protective ventilation achieved.
Analysing the CT scan results and PFT, we found that more than half of the patients had alterations compatible with some degree of pulmonary fibrosis.
The results of the multivariate logistic regression analysis suggest a significant association between interstitial lung abnormalities detected on CT scans and several clinical factors, including extended hospitalization in the ICU, invasive or non-invasive mechanical ventilation, respiratory support with ECMO, diabetes mellitus, COPD, and obesity. These findings are consistent with previous studies linking these factors to the development or exacerbation of interstitial lung abnormalities. In addition, the analysis conducted solely in patients placed on invasive ventilation revealed a significant association between abnormalities in CT scans and non-protective ventilation, higher plateau pressures, and longer duration of invasive mechanical ventilation. This is an important finding, as it suggests that mechanical ventilation strategies may play a role in the development or worsening of interstitial lung abnormalities in critically ill patients. Similarly, the multivariate logistic regression analysis showed a significant association between abnormalities in PFTs and the need for respiratory support, particularly invasive mechanical ventilation and ECMO. When the analysis was restricted to ventilated patients, impaired lung function was significantly associated with non-protective ventilation, higher plateau pressures, and longer duration of invasive mechanical ventilation. These results suggest that careful monitoring and management of lung function may be crucial in determining the need for and duration of respiratory support in critically ill patients. Overall, these findings underscore the importance of identifying and managing risk factors for interstitial lung abnormalities in critically ill patients, particularly those requiring mechanical ventilation or ECMO. Further research is needed to understand better the underlying mechanisms linking these clinical factors to the development and progression of interstitial lung abnormalities and to identify effective interventions to prevent or mitigate their impact on patient outcomes.
There were some limitations to our study. The main limitation was the unavailability of CT scans and PFT before diagnosing SARS-CoV-2 infection, which prevented us from knowing the true extent of alterations given solely by the SARS-CoV-2 disease. Secondly, the study was performed in only one hospital. A larger cohort from different hospitals would consider a more diverse population. Thirdly, a longer follow-up would be needed to assess persistent lung damage. Fourthly we did not consider the occurrence of nosocomial pneumonia, which could have had an important impact on our findings.
ConclusionsIn our sample, most patients with severe COVID-19 infection still exhibit abnormalities in CT scans or lung function tests up to six months after hospital discharge. These results support other papers, suggesting that long-COVID should be considered as a differential diagnosis when assessing patients with chest CT-scan or PFT changes months after recovery from acute COVID-19.
FundingNo funding
CRediT authorship contribution statementLúcia Ribeiro-Dias: Investigation, Writing – original draft. Joana Fernandes: Investigation, Writing – review & editing. António Braga: Investigation, Writing – review & editing. Tatiana Vieira: Investigation, Writing – review & editing. António Madureira: Writing – review & editing. Vencelau Hespanhol: Writing – review & editing. Isabel Coimbra: Supervision, Writing – review & editing. José Artur Paiva: Conceptualization, Writing – review & editing. Lurdes Santos: Conceptualization, Writing – review & editing. André Silva-Pinto: Investigation, Supervision, Formal analysis, Writing – review & editing.
No