Recovery from COVID-19 can be accompanied by persistent symptoms and complications, collectively termed post-COVID syndrome or ‘long COVID’. This study explores the prevalence and nature of long-term physical and psychological complications among recovered COVID-19 patients in Dhaka, six months post-discharge.
MethodsA cross-sectional study was conducted with 384 patients from COVID-dedicated hospitals in Dhaka. Data on psychological and physical outcomes, including fatigue, insomnia, and dementia, were collected using validated tools. Confidence intervals (CIs) were used to examine associations and determine statistical significance.
ResultsAmong the participants, the most common symptoms at hospital admission were cough (93.9%), fever (87.2%), and dyspnea (66.9%). Post-discharge, 74% of respondents reported health issues, with general weakness (58.5%) being the most common. Older participants (≥50) had a higher likelihood of longer hospital stays, with only 35.9% hospitalized for ≤7 days (CI: 45%–55% in <50). They also exhibited higher comorbidity rates, including hypertension (57.1%; CI: 38%–45%) and diabetes (53.7%; CI: 22%–28%). Older participants were more likely to experience complications, with 93.1% reporting at least one (CI: 65%–75%). Insomnia was prevalent in both age groups (82.0%; CI: 78%–85%), with dementia more common in older participants (34.6%; CI: 25%–35%).
ConclusionsWhile older adults exhibited higher rates of dementia and longer hospital stays, the high prevalence of psychological complications across all groups emphasizes the need for comprehensive post-COVID care strategies, particularly for older patients.
La recuperación de la COVID-19 puede ir acompañada de síntomas y complicaciones persistentes, denominados colectivamente síndrome post-COVID o “COVID prolongado”. Este estudio explora la prevalencia y la naturaleza de las complicaciones físicas y psicológicas a largo plazo entre los pacientes recuperados de COVID-19 en Dhaka, seis meses después del alta.
Métodosse realizó un estudio transversal con 384 pacientes de hospitales dedicados a la COVID en Dhaka. Los datos sobre resultados psicológicos y físicos, incluida la fatiga, el insomnio y la demencia, se recopilaron mediante herramientas validadas. Se utilizaron intervalos de confianza (IC) para examinar las asociaciones y determinar la significación estadística.
ResultadosEntre los participantes, los síntomas más frecuentes al ingreso hospitalario fueron tos (93,9%), fiebre (87,2%) y disnea (66,9%). Después del alta, el 74% de los encuestados informaron problemas de salud, siendo la debilidad general (58,5%) la más común. Los participantes de mayor edad (≥50) tuvieron una mayor probabilidad de estancias hospitalarias más prolongadas, con solo el 35,9% hospitalizados durante ≤7 días (IC: 45%–55% en <50). También mostraron tasas de comorbilidad más altas, incluida hipertensión (57,1%; IC: 38%–45%) y diabetes (53,7%; IC: 22%–28%). Los participantes de mayor edad tenían más probabilidades de experimentar complicaciones: el 93,1% informó al menos una (IC: 65%–75%). El insomnio fue prevalente en ambos grupos de edad (82,0%; IC: 78%–85%), y la demencia fue más común en los participantes de mayor edad (34,6%; IC: 25%–35%).
ConclusionesSi bien los adultos mayores mostraron tasas más altas de demencia y estancias hospitalarias más prolongadas, la alta prevalencia de complicaciones psicológicas en todos los grupos enfatiza la necesidad de estrategias integrales de atención post-COVID, particularmente para los pacientes mayores.
Recovery from COVID-19 infection varies. A majority of those infected will have minimal health problems after recovery; however, some individuals experience long-term symptoms and complications1, referred as ‘post-acute sequelae of SARS-CoV-2 infection (PASC)’ or ‘long COVID’2 when signs and symptoms last longer than 12 weeks3. Eighty-seven percent (87%) of COVID-19 patients had at least one symptom two months after their initial diagnosis4. Sudre et al., (2021) reported that those within the age range ≥70years, of female gender, and had the presence of five or more symptoms during the acute phase of COVID-19 infection were associated with an increased risk of developing long COVID5 and individuals with pre-existing medical conditions such as hypertension, diabetes, and chronic lung disease, were also at increased risk of developing long COVID6. Long COVID, also referred to as post-pandemic syndrome, can have a significant impact on the physical, mental, and social well-being of affected individuals and hence significantly affect their ability to carry out daily activities7. Persistent symptoms included fatigue, dyspnea, chest pain, and cognitive impairment4,8.
Understanding the incidence, nature, and severity of post-COVID complications is crucial for healthcare professionals to provide optimal care, and also for public health policymakers to plan effective strategies for managing the long-term health consequences of the pandemic. In light of these considerations, this research on post-COVID complications among recovered patients has significant implications for care, clinical practice, and public health.
Materials and methodsStudy design and settingThis cross-sectional study was conducted among COVID-19 patients treated according to the World Health Organization (WHO) criteria in selected COVID-dedicated hospitals in Dhaka city. The study aimed to assess the long-term psychological and physical outcomes in patients who had recovered from COVID-19 infection.
Sample size and participantsThe sample size was calculated using the formula: n = z2pq/d2, where z corresponds to the z-score for a 95% confidence level (1.96), p represents the estimated prevalence of the outcome (50%), and d is the margin of error (5%). To insure a representative estimate of post-COVID complications and reliable findings, data were collected from the resulted sample size of 384 recovered patients who had been discharged from the hospital at least six months prior to the study.
Data collection procedureData were collected from individuals who had recovered from COVID-19 at least six months prior to the study. Patients were initially contacted by phone, and the nature of the study was explained. After obtaining verbal consent, they were invited to a designated outpatient department. Written consent was subsequently obtained before administering a pre-tested, Bangla-translated version of the Depression, Anxiety, and Stress Scale (DASS-21) through face-to-face interviews.
Quality controlThe data collection team received rigorous training on administering the assessment tools to insure consistency and minimize bias. The Bangla-translated versions of the scales were pre-tested on a small group of participants to confirm clarity and cultural appropriateness. Data were cross-checked for completeness and accuracy before analysis.
Inclusion and exclusion criteriaThe study included patients who met specific inclusion criteria to insure the relevance and reliability of the findings. Participants had to be 18 years of age or older and have recovered from COVID-19 at least six months prior to data collection. Additionally, only those who were willing to provide informed consent were eligible to participate in the study. Patients were excluded if they had pre-existing severe psychiatric or neurological conditions, as these could confound the assessment of post-COVID-19 outcomes. Individuals who were unwilling to participate or unable to provide informed consent were also excluded from the study. These criteria were designed to insure a homogeneous study population and enhance the validity of the results.
Assessment toolsThe Depression, Anxiety, and Stress Scale (DASS-21) was used to evaluate emotional well-being. This scale comprises 21 items divided into three subscales, each with seven items focusing on depression, anxiety, and stress. The depression subscale assessed feelings of dysphoria, hopelessness, lack of interest, and anhedonia, while the anxiety subscale evaluated autonomic arousal, situational anxiety, and anxious affect. The stress subscale measured difficulty relaxing, nervous arousal, irritability, and over-reactivity. Participants responded to each item on a 4-point Likert scale, and their scores were categorized as indicating depression (above 9), anxiety (above 7), or stress (above 14)9. Insomnia was assessed using a dedicated 7-item scale, with each item scored from 0 to 4; a total score above 7 was indicative of insomnia10.
To measure physical and cognitive health, the study employed the Fatigue Assessment Scale (FAS) and the Self-Administered Gerocognitive Exam (SAGE). The FAS consisted of 10 items scored on a 5-point scale, with scores above 18 indicating varying levels of fatigue11. The SAGE test, designed to evaluate cognitive functions, included 12 items covering memory, judgment, problem-solving, language, and visual–spatial abilities. Together, these tools provided a comprehensive understanding of the participants' mental, emotional, and cognitive health status, facilitating a multidimensional analysis of post-COVID-19 outcomes.
Data analysisCollected data were analyzed using the latest version of SPSS for Windows. Descriptive statistics were used to summarize the data, while inferential statistics were applied to explore associations between variables with confidence intervals (CIs) used to assess statistical significance.
ResultsThe majority of respondents were either employed in the private sector or were housewives (Table 1). Most participants were aged between 26–45 years (41.8%) and 46–65 years (38.4%). Nearly half had completed higher education, while more than one-third reported a monthly income of 25,001–35,000 BDT, which is considered below average12. The most common clinical symptoms at the time of hospital admission were cough (93.9%), fever (87.2%), and dyspnea (66.9%). Other symptoms, though less frequently reported, included chest pain, joint pain, loss of appetite, myalgia, diarrhea, headache, anosmia, vertigo, red eyes, and sore throat.
Socio demographic characteristics and clinical manifestations of respondents.
| Demographic Characteristics | Frequency | Percentage | |
|---|---|---|---|
| Age in years | Up to 25 | 48 | 12.5 |
| 26–45 | 160 | 41.8 | |
| 46–65 | 148 | 38.4 | |
| >65 | 28 | 7.2 | |
| Religion | Islam | 315 | 82.1 |
| Hindu | 69 | 17.9 | |
| Education | Up to Primary | 28 | 7.2 |
| Secondary | 86 | 22.4 | |
| Higher Grade | 186 | 48.5 | |
| Above Grade | 84 | 21.9 | |
| Occupation | Government Service | 23 | 6.1 |
| Private Service | 112 | 29.1 | |
| Business | 60 | 15.5 | |
| Housewife | 113 | 29.3 | |
| Labour | 72 | 18.7 | |
| Others | 04 | 1.3 | |
| Family Income | Upto 15,000 | 68 | 17.5 |
| 15,001–25,000 | 99 | 25.8 | |
| 25,001–35,000 | 116 | 30.3 | |
| >35000 | 101 | 26.4 | |
| Common clinical manifestations | Cough | 360 | 93.9 |
| Fever | 335 | 876.2 | |
| Dyspnea | 257 | 66.9 | |
Approximately, 74% of respondents reported health problems after hospital discharge (Table 2). The most commonly reported symptoms were general weakness (58.5%), joint pain (24.1%), headache (18.4%), and chronic cough (15.7%). At least 80% of respondents indicated adherence to their doctors' advice post-discharge.
Distribution of respondents by Health Problems after release from Hospital (n = 384).
| Health Problems after release from Hospital | Yes (%) | No (%) |
|---|---|---|
| Have health problem | 283 (73.7) | 101 (26.3) |
| General Weakness | 224 (58.5) | 160 (41.5) |
| Chronic Cough | 60 (15.7) | 324 (84.3) |
| Difficulty in respiration | 25 (6.7) | 359 (93.3) |
| Anorexia | 56 (14.7) | 328 (85.3) |
| Vertigo | 56 (14.7) | 328 (85.3) |
| Joint pain | 92 (24.1) | 292 (75.9) |
| Headache | 70 (18.4) | 314 (81.6) |
| Bodyache | 56 (14.7) | 328 (85.3) |
| Chest pain | 21 (5.7) | 363 (94.3) |
| Muscle pain/spasm | 20 (5.4) | 364 (94.6) |
| Fatigue | 16 (4.3) | 368 (95.7) |
| Insomnia | 57 (15.0) | 327 (95.0) |
| Hair Loss | 10 (2.7) | 374 (97.3) |
| Other problems | 26 (7.0) | 358 (93.0) |
| Follow doctor's advice | 304 (79.2) | 80 (20.8) |
Participants aged ≥50 had a higher likelihood of longer hospital stays compared to those aged <50. The Confidence Intervals (CIs) indicate that 45% to 55% of participants aged <50 were hospitalized for ≤7 days, while for participants aged ≥50, this proportion was only 35.9%. This trend persists for the 15–21 day range, where older participants were disproportionately represented (19.2%; CI: 10%–15%) (Table 3).
Relationship Between Age and Length of Hospital Stay (n = 384).
| Hospital Stay (Days) | Age < 50 Frequency (%) | Age ≥ 50 Frequency (%) | Total Frequency (%) | 95% CI |
|---|---|---|---|---|
| ≤7 | 119 (50.2%) | 53 (35.9%) | 172 (44.8%) | 0.45–0.55 |
| 8–14 | 91 (38.4%) | 63 (42.9%) | 154 (40.1%) | 0.35–0.50 |
| 15–21 | 23 (9.7%) | 28 (19.2%) | 51 (13.3%) | 0.10–0.15 |
| >21 | 4 (1.9%) | 3 (1.9%) | 7 (1.8%) | 0.01–0.05 |
| Total | 237 (61.7%) | 147 (38.2%) | 384 (100%) | – |
| Mean ± SD | 9.1 ± 4.739 | 10.3 ± 4.564 | 9.6 ± 4.704 | – |
Older participants (≥50) exhibited a higher prevalence of comorbidities, such as hypertension (57.1%; CI: 38%–45%), diabetes mellitus (53.7%; CI: 22%–28%), and cardiovascular disease (14.2%; CI: 8%–10%). The younger group (<50) showed fewer comorbidities overall, with dyslipidemia (1.2%; CI: 3%–5%) and kidney disease (1.7%; CI: 1%–3%) being the least common (Table 4).
Distribution by Age and Co-Morbidity (n = 384).
| Co-Morbidities | Age < 50 Frequency (%) | Age ≥ 50 Frequency (%) | Total Frequency (%) | 95% CI |
|---|---|---|---|---|
| Suffering | 115 (48.5%) | 140 (95.2%) | 255 (66.4%) | 0.60–0.70 |
| No Suffering | 122 (51.5%) | 7 (4.8%) | 129 (33.6%) | 0.30–0.40 |
| Hypertension | 75 (31.6%) | 84 (57.1%) | 159 (41.4%) | 0.38–0.45 |
| Diabetes Mellitus | 22 (9.2%) | 79 (53.7%) | 101 (26.3%) | 0.22–0.28 |
| Cardiovascular Disease | 14 (5.9%) | 21 (14.2%) | 35 (9.1%) | 0.08–0.10 |
| Asthma | 35 (14.7%) | 7 (4.7%) | 42 (16.5%) | 0.14–0.18 |
| Dyslipidemia | 3 (1.2%) | 12 (8.1%) | 15 (3.9%) | 0.03–0.05 |
| Hypothyroidism | 4 (1.7%) | 9 (6.1%) | 13 (3.4%) | 0.02–0.04 |
| Kidney Disease | 4 (1.7%) | 4 (2.0%) | 8 (2.1%) | 0.01–0.03 |
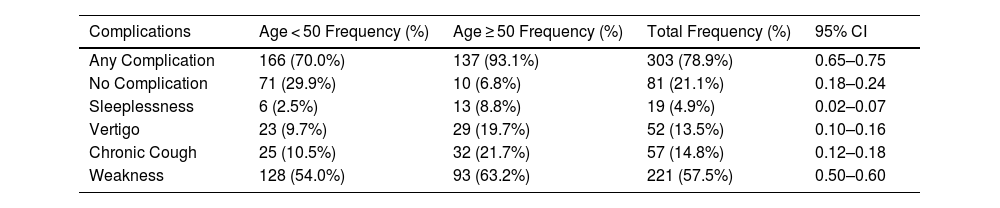
In Table 5, complications were more common among participants aged ≥50, with 93.1% reporting at least one complication (CI: 65%–75%), compared to 70% in the <50 age group. Specific complications, such as sleeplessness (8.8%; CI: 2%–7%), vertigo (19.7%; CI: 10%–16%), and chronic cough (21.7%; CI: 12%–18%), were disproportionately higher in older participants.
Age and COVID-19 Complications After Treatment (n = 384).
| Complications | Age < 50 Frequency (%) | Age ≥ 50 Frequency (%) | Total Frequency (%) | 95% CI |
|---|---|---|---|---|
| Any Complication | 166 (70.0%) | 137 (93.1%) | 303 (78.9%) | 0.65–0.75 |
| No Complication | 71 (29.9%) | 10 (6.8%) | 81 (21.1%) | 0.18–0.24 |
| Sleeplessness | 6 (2.5%) | 13 (8.8%) | 19 (4.9%) | 0.02–0.07 |
| Vertigo | 23 (9.7%) | 29 (19.7%) | 52 (13.5%) | 0.10–0.16 |
| Chronic Cough | 25 (10.5%) | 32 (21.7%) | 57 (14.8%) | 0.12–0.18 |
| Weakness | 128 (54.0%) | 93 (63.2%) | 221 (57.5%) | 0.50–0.60 |
The results from Table 6 focus on psychological complications such as suffering, anxiety, and insomnia were prevalent across both age groups, with insomnia being the most common issue (82.0%; CI: 78%–85%). Notably, dementia was more prevalent among older participants (34.6%; CI: 25%–35%) compared to younger participants (24.1%; CI: 20%–30%), reflecting the potential neurological impact of COVID-19 on older individuals. Although the prevalence of PTSS (14.3%; CI: 10%–18%) and depression (10.9%; CI: 8%–15%) was slightly higher in older participants, these differences were not as pronounced. These results highlight the need for mental health support as a critical component of post-COVID care, particularly for older adults.
Age and Psychological Problems After Release from Hospital (n = 384).
| Psychological Problems | Age < 50 Frequency (%) | Age ≥ 50 Frequency (%) | Total Frequency (%) | 95% CI |
|---|---|---|---|---|
| Suffering | 187 (78.9%) | 131 (89.1%) | 318 (82.8%) | 0.75–0.85 |
| PTSS | 29 (12.2%) | 26 (17.7%) | 55 (14.3%) | 0.10–0.18 |
| Depression | 31 (13.1%) | 11 (7.4%) | 42 (10.9%) | 0.08–0.15 |
| Anxiety | 79 (33.3%) | 53 (36.1%) | 132 (34.3%) | 0.30–0.40 |
| Chronic Fatigue | 47 (19.9%) | 21 (14.2%) | 68 (17.7%) | 0.15–0.20 |
| Insomnia | 193 (81.4%) | 122 (82.9%) | 315 (82.0%) | 0.78–0.85 |
| Dementia | 57 (24.1%) | 51 (34.6%) | 108 (28.1%) | 0.25–0.35 |
This study focused on individuals who were hospitalized due to COVID-19 and released at least six months prior to the commencement of the study. Statistiscally noteworthy differences were observed for the proportion of respondents, particularly within the 26–35 years age range, who reported ongoing health issues post-recovery, aligning with previous studies that suggest COVID-19 survivors may experience lingering effects13. According to the WHO's 2023 report, 10–20% of individuals experience a range of mid- and long-term symptoms following COVID-19 recovery14. In this study, however, the percentage of respondents suffering from post-COVID complications was much higher, with 79% reporting persistent symptoms, a finding far greater than what was reported by the WHO. This disparity may be attributed to the fact that the study sample largely consisted of individuals who had been hospitalized for severe cases of COVID-19, many of whom had pre-existing co-morbidities. The prevalence of co-morbidities in this study was reported at 66%, which is concerning as co-morbid conditions can contribute to prolonged recovery and worsen health outcomes. Previous studies, such as those by Paul et al.15 and Khandker et al.16, have reported a lower prevalence of co-morbidities, which further supports the notion that the severity of COVID-19 illness and the presence of underlying conditions influence recovery trajectories.
The length of hospital stay for patients with COVID-19 has been a critical factor in recovery. In this study, patients aged ≥50 experienced longer hospital stays compared to those under 50, aligning with findings by Yusef et al.17, who also reported extended hospital stays in patients older than 60. Age and underlying comorbidities, including diabetes, chronic kidney diseases, and chronic obstructive pulmonary disease, were associated with prolonged hospital stays and increased illness severity18. Similarly, a systematic review by Justino et al.19 found hypertension, diabetes, and respiratory diseases to be the most common co-morbidities among COVID-19 patients, particularly in older age groups. These findings highlight the influence of age and co-morbidities on the progression and severity of COVID-19.
Regarding clinical improvements, the study found that individuals under 50 years old demonstrated better recovery outcomes compared to their older counterparts. This observation is consistent with broader research indicating that older adults and individuals with comorbid conditions face a higher risk of severe COVID-19 outcomes, including prolonged symptoms and complications20. Additionally, a systematic review and meta-analysis underscored that individuals aged 50 years or older have an elevated risk of mortality compared to those younger than 5021. Co-morbidities such as kidney diseases, cardiovascular disease, and respiratory conditions further compound the risk of severe outcomes, highlighting the importance of managing these conditions in older patients to improve their prognosis.
The study also examined the psychological effects of COVID-19, revealing that a notable proportion of respondents experienced mental health challenges, including post-traumatic stress syndrome (14.3%), depression (10.9%), and anxiety (34.3%). However, this study did not find a clear relationship between these psychological issues and age, which differs from findings by Hoang et al.22,23 in Vietnam, who reported that age was a risk factor for PTSD, anxiety, and depression. The high prevalence of psychological problems observed in this study aligns with other research suggesting that the COVID-19 pandemic has exacerbated mental health issues such as anxiety, depression, and PTSD24. Furthermore, studies conducted in Bangladesh have also documented an increase in psychological problems due to the pandemic, which may contribute to the long-term mental health burden in individuals recovering from COVID-1925.
This study found that dementia was more prevalent in individuals aged ≥50, with the older group exhibiting a notably higher rate of dementia compared to younger individuals. This finding aligns with existing literature indicating that aging increases the risk of developing dementia, a condition potentially exacerbated by the neurological effects of COVID-1926. Several studies have documented that COVID-19 can cause neurological complications such as delirium, stroke, and encephalitis, which may contribute to the development of dementia in the long term27. These results underscore the importance of prioritizing the mental health and neurological well-being of COVID-19 survivors, particularly among older individuals.
ConclusionThe study revealed that a considerable proportion of recovered COVID-19 patients experienced post-COVID complications, with fatigue, shortness of breath, and joint pain being the most common. The majority of respondents had pre-existing comorbidities, and patients aged ≥50 years had a longer average hospital stay compared to those under 50 years. These findings underscore the need to understand the prevalence and nature of post-COVID complications among recovered patients to enhance care and develop effective strategies for managing the long-term health impacts of the pandemic. However, the study's relatively small sample size from a single city limits its generalizability to broader populations of post-COVID patients worldwide.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical considerationEthical approval for the study was obtained from the Research Ethics Committee of the Faculty of Health and Life Sciences, Daffodil International University, Dhaka, Bangladesh. The approval reference number was [FAHSREC/DIU/2023/SMIG-61]. In addition, permission to conduct the study was granted by the hospital authorities. Before beginning the data collection, participants were informed about the objectives, methods, and potential benefits of the study through an information sheet provided in Bengali. They were assured that their participation was voluntary and that they could withdraw at any time without any consequences. Informed verbal consent was obtained from each participant before the interview. The study adhered to the ethical principles outlined in the Declaration of Helsinki and insured that no harm came to participants as a result of their involvement.
CRediT authorship contribution statementMd. Monir Hossain Shimul: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. Salamat Khandker: Supervision, Writing – review & editing. Salim Khan: Methodology, Writing – review & editing.
The authors are grateful to all the dear patients and respective hospital authorities who participated in this project.
| Item No | Recommendation | Page No | |
|---|---|---|---|
| Title and abstract | 1 | (a) Indicate the study's design with a commonly used term in the title or the abstract | 1–2 |
| (b) Provide in the abstract an informative and balanced summary of what was done and what was found | 1–2 | ||
| Introduction | |||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported | 4 |
| Objectives | 3 | State-specific objectives, including any prespecified hypotheses | 4 |
| Methods | |||
| Study design | 4 | Present key elements of study design early in the paper | 5 |
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection | 5 |
| Participants | 6 | (a) Give the eligibility criteria, and the sources and methods of selection of participants | 5 |
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable | 5 |
| Data sources/measurement | 8⁎ | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group | 5–6 |
| Bias | 9 | Describe any efforts to address potential sources of bias | 5–6 |
| Study size | 10 | Explain how the study size was arrived at | 5 |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why | 5 |
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding | 6 |
| (b) Describe any methods used to examine subgroups and interactions | |||
| (c) Explain how missing data were addressed | 5–6 | ||
| (d) If applicable, describe analytical methods taking account of sampling strategy | 5 | ||
| (e) Describe any sensitivity analyses | |||
| Results | |||
| Participants | 13⁎ | (a) Report numbers of individuals at each stage of study—eg numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analyzed | 6 |
| (b) Give reasons for non-participation at each stage | |||
| (c) Consider use of a flow diagram | |||
| Descriptive data | 14⁎ | (a) Give characteristics of study participants (eg demographic, clinical, social) and information on exposures and potential confounders | 6–7 |
| (b) Indicate number of participants with missing data for each variable of interest | |||
| Outcome data | 15⁎ | Report numbers of outcome events or summary measures | 6–7 |
| Main results | 16 | (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (eg, 95% confidence interval). Make clear which confounders were adjusted for and why they were included | 6–7 |
| (b) Report category boundaries when continuous variables were categorized | |||
| (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period | |||
| Other analyses | 17 | Report other analyses done—eg analyses of subgroups and interactions, and sensitivity analyses | |
| Discussion | |||
| Key results | 18 | Summarize key results with reference to study objectives | 7–8 |
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both the direction and magnitude of any potential bias | 8 |
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence | 7–8 |
| Generalisability | 21 | Discuss the generalizability (external validity) of the study results | 7–8 |
| Other information | |||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based | 1 |