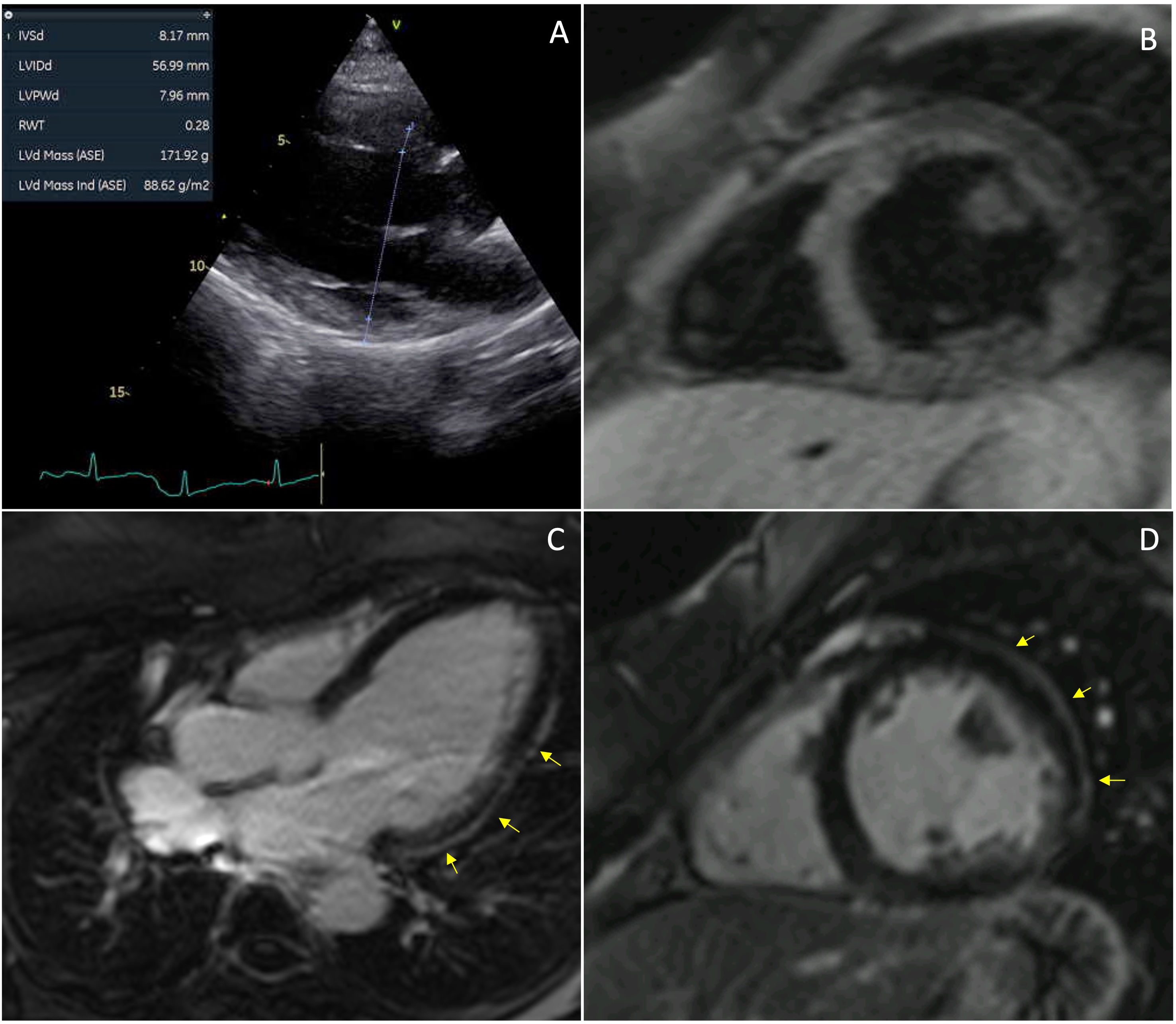
We report a 43-year-old woman admitted with dyspnea (NYHA III) and peripheral edema. Her medical history included metabolic syndrome, systemic lupus erythematosus and symptomatic ulcerative colitis, for which she started mesalazine 3 g daily three weeks prior. On admission, she exhibited acute heart failure (HF) signs. Vital signs: blood pressure of 135/66 mmHg, a heart rate of 110 bpm, and she was afebrile. Electrocardiography revealed sinus tachycardia and T-wave inversions in the lateral leads. Laboratory results: NT-proBNP 705 pg/mL (normal <133 pg/mL), eosinophils (6.4% of white blood cells, 470 cells/μL), and negative troponin. Echocardiography demonstrated mild left ventricular (LV) dilation, global hypokinesia, and reduced ejection fraction (38%) (Fig. 1, Video S1). Pulmonary embolism and coronary artery disease were excluded. Mesalazine was discontinued due to its temporal association with HF onset (Fig. S1), and guideline-directed HF therapy was initiated. One week later, cardiac magnetic resonance imaging showed dilated cardiomyopathy with subtle subepicardial late gadolinium enhancement, without active inflammation (Fig. 1). LV function normalized within three months (Video S2). This case highlights mesalazine-induced cardiomyopathy, a rare condition likely linked to hypersensitivity. As mesalazine is a first-line treatment for inflammatory bowel disease, prompt recognition of cardiotoxicity and discontinuation are critical to prevent adverse outcomes.
Dilated cardiomyopathy following mesalazine treatment. A, transthoracic echocardiography in parasternal long axis performed during acute presentation showing left ventricular (LV) dilation; B, T2-weighted cardiac magnetic resonance (CMR) imaging performed one-week after clinical presentation showing isointense myocardial signal, indicating the absence of edema; C and D, Subepicardial late gadolinium enhancement (LGE) in the basal-to-mid lateral wall of the LV (arrows).
None.
Ethical considerationsWe followed our institution's ethical protocols, obtained the patient's informed consent for publication, and ensured their privacy and non-identifiability.
The following are the supplementary data related to this article.