Editado por: Dr. Juan González Moreno - Hospital Universitario, Spain. Dra. Inés Losada López - Hospital Universitario, Spain
Más datosTransthyretin-related amyloidosis (ATTRv) is a progressive multisystem disorder, predominantly involving the peripheral nerve system (PNS) and heart. Quantification of small fiber damage may help guide treatment decisions, as amyloid deposits frequently affect those fibers early in disease course. Corneal confocal microscopy (CCM) is a promising method to monitor patients with ATTRv, due to similarities between corneal nerves and PNS, as the cornea is innervated by Aδ and C fibers.
MethodsWe compared CCM measures from ATTRv patients to a group of healthy individuals, matched by age and gender. We then investigated the correlations between small fiber tests (SFT): CCM, LDI-Flare and CDT, COMPASS-31 and disability scales (RODS and ONLS) in patients.
ResultsOf 20 patients (6 with V30M), mean age 50.3±15.3Y, 7 female (35%), six (30%) had polyneuropathy and 10 (50%) carpal tunnel syndrome. CDT was abnormal in 9 and LDI-flare in 6 patients. CCM was abnormal in 19 tested patients and significantly reduced when compared to controls (CNFL: 6.31±0.31 vs. 15.21±1.02mm/mm2, p<0.001). Mean COMPASS-31-scores were 22.27±22.84; RODS and ONLS were 38.15±12.33 and 2.05±2.3, with no significant differences between sub-group scores. Disease duration was significantly correlated with ONLS (0.43, p=0.05) and RODS (0.46, p=0.03). There were no significant correlations between measures of disability and SFT.
ConclusionsIn a diverse cohort of ATTRv patients, CCM was the most frequent abnormal measurement. CCM can be a useful test to triage patients in the early disease stages and with few or equivocal symptoms.
La amiloidosis por transtiretina (ATTRv) es un trastorno multisistémico progresivo que afecta predominantemente el sistema nervioso periférico (SNP) y el corazón. La cuantificación del daño de las fibras pequeñas puede guiar las decisiones de tratamiento, ya que los depósitos de amiloide frecuentemente afectan esas fibras en el comienzo de la enfermedad. La microscopia confocal corneal (MCC) es un método prometedor para monitorear la ATTRv, debido a las similitudes entre la estructura del nervio corneal y el SNP, ya que la córnea está inervada por fibras Aδ y C.
MétodosComparamos medidas de MCC de pacientes con ATTRv con un grupo de individuos sanos, emparejados por edad y sexo. Luego investigamos las correlaciones entre pruebas de fibras pequeñas (PFP): MCC, laser Doppler imager flare (LDI-flare) y cold detection threshold (CDT), así como Composite Autonomic Symptom Score-31 (COMPASS-31) y escalas de discapacidad (RODS y ONLS) en pacientes.
ResultadosDe 20 pacientes (seis con V30M) con una edad media de 50,3±15,3 años, y de los cuales siete eran mujeres (35%), seis (30%) tenían polineuropatía y 10 (50%) síndrome del túnel carpiano. La CDT fue anormal en nueve y la LDI-flare en seis pacientes. La MCC fue anormal en los 19 pacientes evaluados y se redujo significativamente en comparación con los controles (longitud de la fibra nerviosa corneal [CNFL]: 6,31±0,31 vs. 15,21±1,02mm/mm2, p <0,001). Las puntuaciones medias de COMPASS-31 fueron 22,27±22,84. Las escalas RODS y ONLS fueron 38,15±12,33 y 2,05±2,3, sin diferencias significativas entre V30M y no-V30M. La duración de la enfermedad se correlacionó significativamente, ONLS (0,43; p=0,05) y RODS (-0,46, p=0,03). No hubo correlaciones significativas entre las medidas de discapacidad y las PFP.
ConclusionesEn una cohorte diversa de pacientes con ATTRv, las medidas de MCC fueron las anormalidades más frecuentes. La MCC puede ser una prueba útil para clasificar a los pacientes en las primeras etapas de la enfermedad y con síntomas pocos o equívocos.
Transthyretin-related amyloidosis (ATTRv) is a progressive multisystem disorder, predominantly involving the peripheral nerve system (PNS) and heart. Symptoms of neuropathy and/or cardiomyopathy present either in young adults or later in life, but are relentlessly progressive, devastating and usually fatal, and cardiac involvement is frequently the most common cause of death.1 Small caliber fibers conveying sensory and autonomic information are commonly affected early in the disease course, being a classic presentation of the most common ATTRV30M variant, especially in endemic countries.2
To the present date, it is unclear whether the degree of small or large fiber involvement may predict disease progression in ATTRv, and several scales and electrophysiologic parameters have been proposed to quantify neuropathy. In a longitudinal analysis of the THAOS cohort, the amplitude and velocity of lower limb nerves were found to be a possible marker for disease progression.3 A comprehensive and updated scale, the modified NIS+7, designed to assess polyneuropathy impairment in ATTRv, was also the primary endpoint in phase III studies that led to approval of disease modifying therapies.4 Also, evidence from a systematic review and meta-analysis showed that the neuropathy progression in patients with ATTRv is faster than in other neuropathies, and an increase of 12 or more points in the neuropathy impairment scale per year is highly suggestive of ATTRv.5 While these comprehensive measurements may capture the different dimensions of the motor, sensory and autonomic domains, the required special equipment, and extensive training limit its utilization in clinical practice.
Corneal confocal microscopy (CCM) is a promising method to detect and to possibly monitor patients with diverse neuropathies, due to similarities between corneal nerve structure and peripheral nerve structures, as the cornea is innervated by Aδ and C fibers.6 CCM measurements, especially corneal nerve fiber length (CNFL) was proven useful to quantify the degree of nerve fiber loss in a large multicenter study of diabetes mellitus (DM) patients, with both manual and automated methods.7 Also, in a longitudinal study, rapid corneal fiber loss was demonstrated in a subset of patients with DM, which could potentially help to identify those with a higher risk of neuropathy progression.8 CCM abnormalities have also been detected in hereditary neuropathies such as Charcot–Marie–Tooth disease Type 1A, Friedreich's ataxia, and in Neurofibromatosis type 1, where CNFL was the most common abnormal parameter.9,10
Few studies aimed to investigate the usefulness of CCM measurements in ATTRv. In a pivotal cross-sectional study, CNFL was reduced in 15 patients with ATTRv when compared to controls and correlated with the severity of neuropathy and with the fiber loss detected in skin biopsies.11 In another recent cross-sectional study, corneal nerve fiber density and length were significantly lower in ATTRv patients with both cardiac and neuropathy phenotypes, and also in carriers, when compared to controls.12 In another study, an additional measurement obtained by CCM methods, the inferior whorl length, was helpful to detect preclinical small fiber changes in ATTRv and to differentiate patients from controls.13
Quantification of small fiber damage may help guide treatment decisions in subclinical or in early disease stages, when symptoms are scarce and non-specific, however there is limited availability of non-invasive and reproducible diagnostic methods. We aimed to investigate the value of CCM parameters in diagnosing small fiber neuropathy (SFN) in a genotypically and phenotypically diverse cohort of patients with ATTRv and to correlate those measures with autonomic symptoms, additional small fiber tests (SFT) and disability scales early in the disease course.
MethodsPatientsA retrospective chart review of patients with a confirmed TTR variant with cardiac and/or isolated neuropathic phenotypes (including small fiber, focal neuropathies, large fiber, and autonomic symptoms) presenting for the first time to the Prosserman Family Neuromuscular clinic, Toronto General Hospital-UHN, between June 2014 and May 2023, was done and those with baseline SFT were included. Common causes of neuropathy such as diabetes, thyroid disease, alcohol exposure, paraproteinemia, vitamin deficiencies and chemotherapy associated neuropathy were excluded. Cardiac involvement was confirmed with a full assessment by a specialist when symptoms and signs that could suggest amyloid cardiomyopathy were present, according to local protocols and international guidelines.14,15
All patients underwent neurologic examination, nerve conduction studies (NCS) and SFT including laser doppler imaging (LDI flare), cooling detection thresholds (CDT) and CCM measurements. Information about disability scales (RODS and ONLS) and COMPASS-3116 was extracted from disability scales and patient charts. NCS and SFT were done either in the same clinical visit or in separate visits in proximity (within less than 2 months). A historical control group of 20 age-and sex-matched healthy individuals was used for CCM measurement comparison. The control group consisted of individuals without the diagnosis of neuropathy, selected from a local population. The study protocol was approved by the Research Ethics Board of the University Health Network.
Electrophysiologic studiesNCS were performed using the Sierra Wave instrument (Cadwell Laboratories Inc., Kennewick, WA). Age and height-adjusted reference values were used, according to the standards of our local electrophysiology laboratory. A minimal set of sensory and motor nerve conductions from upper and lower limb motor and sensory nerves were performed at the clinician discretion, according to symptoms and objective neurologic signs, to investigate the possibility of large fiber neuropathy or entrapments. All NCS included in this study were done prior to TTR stabilizers, small interfering RNAs or antisense oligonucleotide therapies, except for 2 patients who had been on patisiran (less than a year), one patient on inotersen (around 6m) and one on diflunisal (3m).
LDI flare imaging and CDT testingLDI flare measurement was conducted with the MoorLDI2 Laser Doppler blood perfusion imager (Moor Instruments, UK) in the skin between the 1st and 2nd metatarsal heads on the dorsum of the foot, after the skin was heated with the probe for 20min. The heated area was scanned with the LDI instrument and the area of the hyperemic flare in response to heating was measured and processed offline. The area of the flare was calculated with the Moor LDI version 3.11 software. CDT were performed with the Medoc TSA-II Neurosensory Analyzer (Ramat-Yishai, Israel) using the method of limits as described elsewhere.17
CCM testingStudy participants underwent examination of small nerve fibers adjacent to Bowman's layer of the cornea in both eyes using the Rostock Cornea Module of the Heidelberg Tomograph III (Heidelberg Engineering, Smithfield RI, USA), after application of a topical anesthetic and a viscous gel medium. In our laboratory, 40 images, taken over a depth of 50μm in 1.3μm incremental steps are obtained in a 6-s automated protocol. Several sets of 40 images are captured, and the best images from these sets are analyzed, including 3 per each eye. From each individual, corneal nerve fiber length (CNFL measured in mm/mm2), corneal nerve fiber density (CNFD in fibers/mm2) and corneal nerve branch density (CNBD in branches/mm2) were calculated following semi-automated image analysis using the ACCMetrics Image Analysis Software v1.1 (University of Manchester, UK). Our main surrogate measure was the CNFL, which corresponds to the total length of all nerve fibers and branches visualized in a CCM image and was the most reproducible measure in our laboratory.7
Statistical analysisAll analysis were performed with STATA 16.1 version or later. Clinical and demographic data were described with means/standard deviation or counts and proportions whenever appropriate. Unpaired t-tests or Mann–Whitney tests were used to compare groups according to variant (V30M vs non-V30M) for the continuous variables: measures of disability, neurophysiologic parameters, and small fiber tests and for CCM parameters between patients and controls. Fisher exact test was used to assess differences in proportions between variant groups. Correlations between disability scales, duration of disease and CNFL were calculated using Spearman's rank correlation coefficient or Kendall's tau accordingly. p values<0.05 were considered statistically significant.
ResultsOf 20 patients from 17 families, 7 were female (35%) and mean age was 50.3±15.3 years. The most frequent variant was early-onset V30M (6 patients). Twelve patients presented with a predominantly neuropathic phenotype (small and/or large-fiber symptoms or signs), and 6 with a predominantly cardiomyopathy phenotype. Family history was positive in 4 (66%) patients with the V30M variant and in 11 (68%) patients with non-V30M variants. Most patients had low levels of disability, with 15 (75%) presenting with Polyneuropathy Disability Score system (PNDs) stage I, and only one patient needed bilateral support for walking (stage IIIb) at the time of presentation. There was no difference in age of onset, positive family history, duration of disease or measures of disability between groups of V30M and non-V30M variants. Clinical characteristics and manifestations by variant type are detailed in Table 1.
Clinical characteristics and phenotypes.
| Clinical characteristics and measures of disability by group | |||
|---|---|---|---|
| V30M | Non V30M | p-Value | |
| N (female) | 6 (1) | 14 (6) | 0.35 |
| Age of onset (y, mean±SD) | 42.5±16.28 | 47.78±12.09 | 0.42 |
| Duration of disease (y, mean±SD) | 5.07±5.12 | 2±2.09 | 0.17 |
| PND score N (%) | I (4) II (2) III (0) IV (0) | I (11) II (2) III (1) IV (0) | 0.69 |
| FAP stage N (%) | 1 (6) 2 (0) 3 (0) | 1 (12) 2 (2) 3 (0) | 0.48 |
| RODS (mean±SD) | 36.83±12.65 | 38.71±12.63 | 0.76 |
| ONLS (mean±SD) | 2.16±2.13 | 2.0±2.44 | 0.88 |
| Clinical phenotypes by variant | ||||||
|---|---|---|---|---|---|---|
| Polyneuropathy | Cardiomyopathy | Small fiber sensory symptomsa | Autonomic symptomsb | CTS | Other | |
| Variant (N of patients) | ||||||
| T80A (2) | 2 | 1 | 2 | 2 | 2 | 1 (spinal stenosis) |
| I127V (1) | 1 | – | 1 | – | 1 | – |
| V30M (6) | 3 | 1 | 4 | 3 | 2 | 0 |
| I127M (1) | – | – | 1 | 1 | – | Cataracts |
| I84S (1) | 1 | 1 | 1 | – | 1 | – |
| A120S (1) | – | – | 1 | – | 1 | – |
| V30L (1) | – | – | 1 | – | 1 | – |
| P64S (2) | 0 | 0 | 2 | 0 | 0 | 0 |
| G62A (1) | 1 | – | – | – | 1 | – |
| A117S (1) | – | – | – | – | 1 | – |
| P84L (3) | 1 | 0 | 1 | 2 | 0 | 0 |
Eight patients had polyneuropathy (4 with non-recordable sural nerves), 3 had demyelinating features and about half of them presented with carpal tunnel syndrome. Large and small fiber tests are shown in Table 2. CCM parameters were the most common abnormal tests, showing reduced values in all 19 patients who had those tests (one patient declined). CNFL in the whole group was significantly reduced when compared to controls (6.31±1.36 vs. 15.21±4.56mm/mm2, p<0.001) and to our reference values.7 There were no differences in CNFL, CNFD and CNBD between patients with V30M and non-V30M patients. CDT was abnormal in about half of our sample and LDI flare in less than half, and also did not differ between V30M and non-V30M patients (Table 2).
Large and small fiber tests by group.
| Nerve conduction studies | |||
|---|---|---|---|
| V30M | Non V30M | p-Value | |
| Polyneuropathy N (%) | 2 (33) | 6(42) | 0.56 |
| Demyelinating features N (%) | 2 (33) | 1 (6) | 0.28 |
| CTS N (%) | 3 (50) | 7(43) | 0.68 |
| Sural nerve amplitude (μV, mean±SD) | 13.70±12.89 | 11.01±11.71 | 0.65 |
| Small fiber testsa | |||
|---|---|---|---|
| V30M | Non V30M | p-Value | |
| Cooling detection thresholds | |||
| N abnormal (%) | 3 (100) | 6 (42) | Upper limbs (0.55) |
| Mean±SD (upper limb/lower limb) | 30.76±1.37/31.86±1.12 | 29.28±2.34/24.86±9.76 | Lower limbs (0.28) |
| LDI flare | 0 (0) | 6 (42) | |
| N abnormal (%), mean±SD | 2.96±0.72 | 2.34±0.77 | 0.22 |
| CCM | |||
| N abnormal (%) | 5 (100) | 14(100) | |
| CCM parametersa | |||
|---|---|---|---|
| V30M | Non V30M | p-Value | |
| Patients | |||
| CNFL (mm/mm2) (mean±SD) | 7.23±0.95 | 6.79±1.50 | 0.55 |
| CNFD (fibers/mm2) (mean±SD) | 11.27±5.82 | 12.64±3.81 | 0.53 |
| CNBD (branches/mm2) (mean±SD) | 13.32±7.15 | 9.99±6.60 | 0.70 |
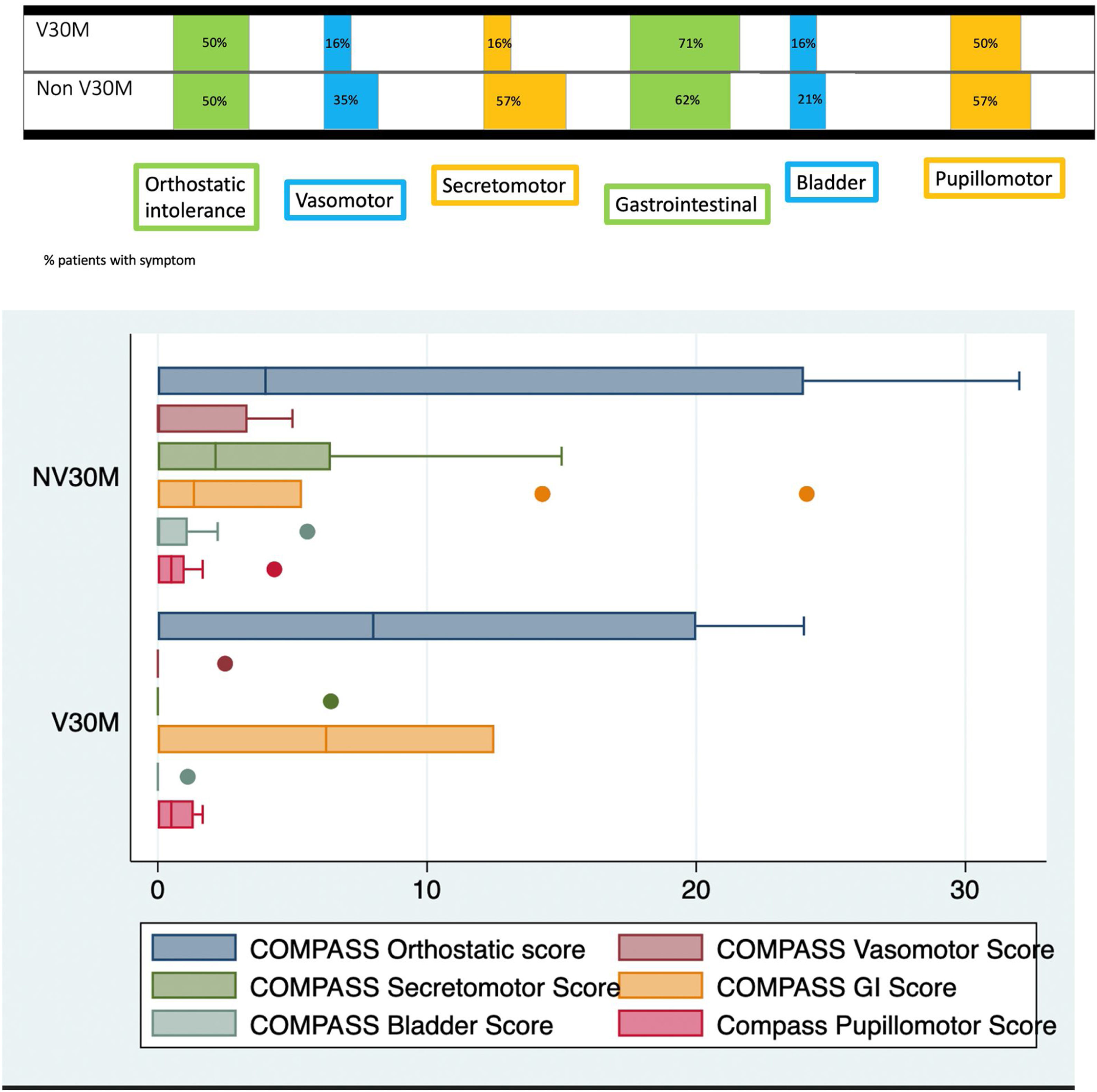
Mean COMPASS-31-scores were 22.27±22.84 in the whole group and values for the different domains according to subgroups are displayed in Fig. 1. There was no difference in mean scores between V30M and non-V30M groups (p=0.64). The most common autonomic symptoms were orthostatic intolerance, gastrointestinal symptoms and pupillomotor symptoms (mainly sensitivity to bright lights). Measures of disability were significantly correlated with duration of disease (ONLS: 0.43, p-value 0.05; RODS: −0.46, p-value 0.03). On the other hand, there were no significant correlations between CNFL, duration of disease, measures of disability and SFT parameters (Supplementary Table 1).
DiscussionIn a diverse cohort of patients with ATTRv, with common (V30M) and less common variants, we found that CNFL was abnormal in all patients, including those with either a predominantly neuropathy or a predominantly cardiomyopathy phenotype. CNFL was the most sensitive parameter of all SFT in our patients, who were recently diagnosed and had low levels of disability, which suggests that this surrogate measure has significant utility in the early symptomatic or pre-symptomatic stages. In comparison with a previous report, our group was assessed in an earlier phase of disease, before starting or within less than one year of being treated with specific medications, and also had lower disability levels.11
Our findings corroborate the results of another recent study that investigated patients with low levels of disability, diverse gene variants and similar levels of autonomic symptoms, showing significantly reduced CNFL values in comparison to controls.12 In their group, CNFL values were higher than in our patient cohort, which could be explained by other factors such as a different population and method of image selection. Additionally, most patients in their group were being treated with stabilizers, small interfering RNAs or antisense oligonucleotide therapies, which could have partially impacted their results. This prior work also showed that while CNFD and CNFL were significantly lower, CNBD did not differ significantly between patients and controls. Considering the high reproducibility of CNFL measurements and well-defined normative values in our laboratory, we did not include additional CCM parameters, which could limit the generalizability of our results.
The utility of CCM measurements in the investigation of suspected incipient neuropathy, when other tests are normal, cannot be underestimated, as it may help select patients for both interventional studies and for potentially disease modifying approved therapies. However, given that there is an independent production of TTR by the retinal pigment and ciliary pigment epithelial cells, follow-up studies may be necessary to assess the relationship between the neuropathy progression and this surrogate measure in patients undergoing treatment.18,19
Furthermore, current gene silencing therapies do not cross the blood–brain, but there is some evidence that tafamidis, a TTR stabilizer, can cross the CSF-blood and eye–blood barriers and, therefore, potentially affect those measurements.18
The inclusion of pre-symptomatic carriers in future ATTRv studies, as done in a prior study including 5 such carriers may lead to more insights concerning subclinical changes.12 Furthermore, exploring additional measures such as the sub-basal whorl-like nerve plexus may add precision in the staging and prognosis of ATTRv patients, although limited literature exists.13
Our findings suggest that CNFL measurements do not reflect the level of disability in the early phases of disease in ATTRv patients and may not be related to changes in other parameters such as LDI-flare and thermal thresholds, which may show abnormalities only in a more advanced phase. Also, where SFT are not available, NCS are usually the most used surrogate measurement, but may suffer from floor effects, as was the case in our group, where 4 individuals had absent sural nerve sensory potentials.20 Although some expert groups suggest following patients clinically on an annual basis several years prior to the predicted age of disease onset (PADO), the arbitrary 10-year mark may not be applicable to all variants and to non-endemic countries.21,22
Early small fiber function impairments may be very disabling and misinterpreted at the same time, due to the non-specific nature of the symptoms. Additionally, broad genotypic diversity, noted in different studies (with V30M being reported in the majority of patients from endemic countries), may impact the diagnostic yield in non-endemic areas, as most patients may harbor different and rare variants, as was the case in our series.23 Furthermore, the lack of family history and incomplete penetrance impose an additional complexity in the diagnosis of ATTRv in patients from non-endemic countries. An important aspect of our study was the demonstration of the usefulness of CCM tests in all patients, regardless of variant type, and the lack of differences between V30M and non-V30M patients. The ability to detect small fiber damage may help promote increased awareness of the disease in non-endemic countries, thus contributing to a higher likelihood of requesting genetic tests for at-risk individuals and potentially reducing disease burden.24
Some limitations of our study are the small sample and the retrospective design. As ATTRv is a rare disease, recruitment for prospective observational studies is limited, which makes data from interventional studies frequently more valuable and informative. Furthermore, our patients come from a single tertiary center, specialized in neuromuscular diseases, which could potentially bring some degree of referral bias, further limiting its generalizability.
ConclusionsIn a diverse cohort of ATTRv patients with low levels of disability, CNFL was the most frequent abnormal measurement. CCM can be a useful test to triage patients in the early stages of disease, especially those with few or equivocal symptoms. The heterogeneity of our population, as in other non-endemic countries, is a pressing argument for the introduction of non-invasive SFT as a first tier in the diagnostic algorithm of suspected ATTRv neuropathy.
Ethical considerationsWritten informed consent was obtained from patients.
FundingThere are no funding sources to declare.
Conflict of interestMonica Alcantara, Shabber Mannan and James de la Cruz have no conflicts of interest to declare. Vera Bril has worked as a consultant for: Grifols, CSL, UCB, Argenx, Takeda, Alnylam Octapharma, Pfizer, Powell Mansfield Inc, Akcea, Ionis, Immunovant, Sanofi, Momenta (J&J), Roche, Janssen, AZ-Alexion, NovoNordisk, Japan tobacco. Vera Bril has received research support from: AZ-Alexion, Grifols, CSL, UCB, Argenx, Takeda, Octapharma, Akcea, Momenta (J&J), Immunovant, Ionis.