Arterial stiffness is considered to be an intermediate marker with independent prognostic value. The objective of this study is to assess whether the estimation of arterial stiffness can improve CV risk stratification compared to SCORE in patients at community pharmacies.
MethodsObservational prospective epidemiological study in which consecutive individuals entering a participating Community Pharmacy are offered a voluntary measurement of blood pressure and estimation of pulse wave velocity by oscillometry (AGEDIO, IEM®) to stratify their CV risk according to SCORE compared to the use of arterial stiffness.
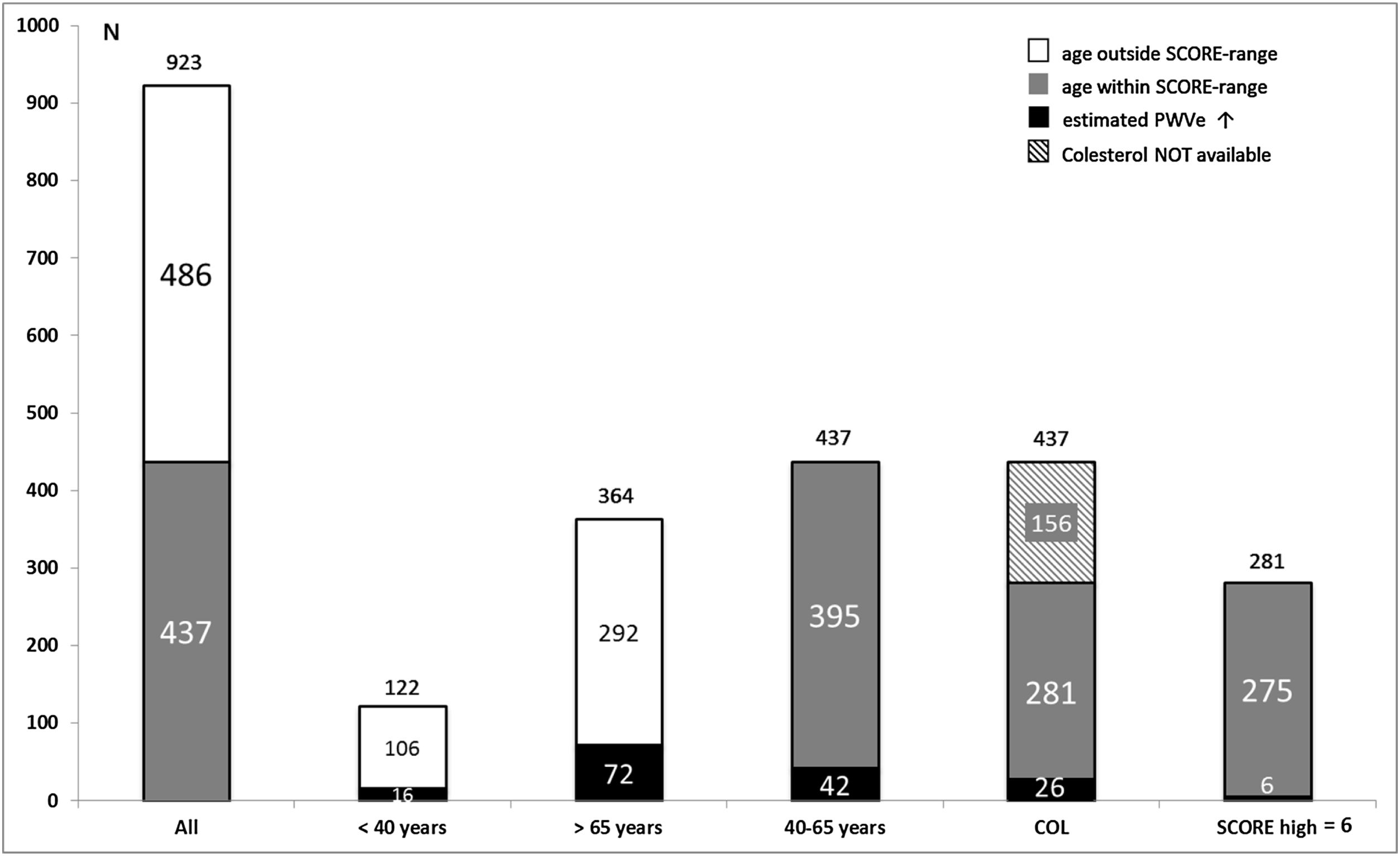
ResultsAfter nine months of recruitment, data from 923 patients (570 women, 353 men) were collected. 16/122 (13.1%) patients under 40 years and 72/364 (19.8%) over 65 years of age presented pathological stiffness and could be classified as high-risk, even though being out of the age-range of SCORE. Of the 437 (47.3%) patients who were susceptible to calculating SCORE, 42/437 patients (9.6%) presented pathological arterial stiffness. Cholesterol values were available in 281 patients (64.3%). Among them, according to SCORE, only 6 (2.1%) fell into the high-risk category.
ConclusionsMore than half of the subjects who randomly enter a community pharmacy had ages that make it impossible to calculate the CV risk by SCORE. Among them, arterial damage was detected in 18.1%. Of the other half, 9.6% presented arterial damage and, therefore, high CV risk, when SCORE only detected it in 2.1%. Therefore, estimating arterial stiffness in community pharmacies markedly improves detection of high CV risk compared to SCORE.
La rigidez arterial es un marcador intermedio con valor pronóstico independiente. Nuestro objetivo es valorar si la estimación de la rigidez arterial puede mejorar la estratificación del riesgo cardiovascular (CV) en comparación con SCORE.
MétodosEstudio epidemiológico observacional prospectivo en el que se ofrece a pacientes consecutivos que entran en una farmacia participante la medición voluntaria de la presión arterial y de la velocidad de onda de pulso estimada por oscilometría (AGEDIO, IEM®) para estratificar su riesgo CV según SCORE o según la presencia de rigidez arterial.
ResultadosTras 9 meses de reclutamiento, presentamos datos de 923 pacientes (570 mujeres, 353 hombres). Dieciséis/122 (13,1%) pacientes <40años y 72/364 (19,8%) >65años presentaron rigidez arterial patológica y fueron clasificados de alto riesgo, aun hallándose fuera del rango de edad de SCORE. De los 437 (47,3%) pacientes evaluables por SCORE, 42/437 pacientes (9,6%) mostraron rigidez elevada. Los valores de colesterol estaban disponibles en 281 de estos pacientes (64,3%). Entre ellos, según SCORE, solo 6 (2,1%) eran de la categoría de alto riesgo.
ConclusionesMás de la mitad de sujetos que entran aleatoriamente en una farmacia comunitaria tenían edades situadas fuera de los rangos de SCORE, imposibilitando el cálculo del riesgo CV con SCORE. En este grupo se constató daño arterial en el 18,1%. En la otra mitad, el 9,6% presentaron daño vascular y, consecuentemente, riesgo elevado, mientras que SCORE solo detectó riesgo elevado en el 2,1%. Por tanto, la estimación de la rigidez arterial en farmacias comunitarias mejora claramente la detección de riesgo CV elevado en comparación con SCORE.
The European Society of Hypertension (ESH)-Guidelines recommend formal cardiovascular (CV) risk assessment of patients using the SCORE system,1 unless the subject is already at high or very high risk due to established CV disease. However, SCORE has important shortcomings, such as limitations of age, absence of relevant risk factors like obesity or diabetes and lack of consideration of subclinical organ damage. Not surprisingly, although the SCORE system remains the valid paradigm of risk stratification, risk prediction is suboptimal, as several studies have shown that CV morbi/mortality in absolute numbers is higher in patients classified as low–moderate risk than in high risk patients,2 mainly because the quantities of the former group are much larger than the latter.3
As the presence of hypertension-mediated organ damage (HMOD) increases the risk of CV morbidity and mortality independently of blood pressure (BP),4 assessment of HMOD as a complementary examination is also suggested by ESH-Guidelines whenever feasible, especially in patients at low–moderate risk.5 Arterial stiffness, measured either as carotid–femoral pulse wave velocity (PWV) by applanation tonometry or calculated as elevated pulse pressure in older patients, belongs to the list of recognized HMOD for the evaluation of blood vessels since 2009.1 Several circumstances have nevertheless prevented arterial stiffness from becoming a broad tool for arterial examination. First, applanation tonometry is time-consuming and requires a certain expertise not available at most clinical settings. Second, together with other HMOD, arterial stiffness was equally downgraded in the most recent version of ESH-Guidelines due to questionable reproducibility and operator dependence as well as to a still unproven prognostic value of changes in PWV. And third, although intensive efforts in the research community are ongoing,6 a broad consensus about standardization of pulse wave assessment is lacking. While applanation tonometry remains the gold standard for measuring PWV, brachial oscillometry has emerged in the past decade as a validated method for estimating arterial stiffness,7–9 at least as a widely used screening technique, capable of identifying subjects with arterial damage as a first step in risk assessment in several settings.
Community pharmacies have been shown to be capable of reliably estimating PWV by brachial oscillometry in daily pharmaceutical practice in different European countries.10–13 Community pharmacies represent an important sanitary player in most health care systems due to the uncomplicated, simple accessibility for the general population and for the high quality education of many pharmaceutical, scientific associations. The ESH-Guidelines encourage the community of pharmacists to engage in the longer-term management of hypertension1 (HTN). Our objective is to determine to which extent estimation of PWV by brachial oscillometry, performed in daily pharmaceutical practice, can contribute to refine stratification of CV risk compared to the SCORE system as a strategy to improve the management of normotensive and hypertensive patients. Secondary objective is to assess the capability of community pharmacies to detect uncontrolled HTN in the study population and its relationship to increased stiffness.
Patients and methodsStudy design, pharmacies and populationOur study is based on the protocol COPHARTEN (primary prevention of essential hypertension shared by community pharmacies and primary care based on the determination of arterial stiffness), published earlier,13 focusing on the first, cross-sectional, observational step. In summary, the Spanish Association of Community and Family Pharmacies in the Valencia Region (SEFAC, Sociedad Española de Farmacia Familiar y Comunitaria), offered community pharmacies the possibility to take part in the study. The acceptance to participate necessarily implied a pre-study qualification by an online educational program (IMPACHTA, Training program in Arterial Hypertension), developed in conjunction between SEFAC and the Spanish Society of Hypertension (SEHLELHA). Paneling outside and inside the pharmacies provided accessible information about the project. Subjects consecutively entering the pharmacies for any reason and showing interest for the study were asked to participate by the personnel working in the pharmacies. Interested patients were given an explanation and more detailed information. Once agreed to participate, they were asked for their informed consent, and after signing, they got a copy. Exclusion criteria were refusal to participate, pregnancy or any disability that prevented or hindered understanding of the protocol.
Before accessing the data, all researchers were required to sign a document assuring confidentiality. All information collected was considered confidential and documented anonymously, according to the legal frame valid in the Valencia Region (Law 5/1999 and consecutive norms). This information cannot be used to identify the patients, being the only link between the data and the patient, a code used exclusively for this study. The study has been reviewed for approval by the Committee for Ethics of the University Cardenal Herrera-CEU. This cross-sectional, observational study does not imply the randomization of the sample or the application of additional interventions.
ProceduresIn all patients, personal history was assessed by means of a standard questionnaire.13 Body weight, height, and waist circumference were measured and body mass index (BMI) was calculated. Overweight was defined as BMI>25kg/m2, obesity was considered either as BMI>30kg/m2 or as waist circumference>88cm in women and >102cm in men. BP was measured using brachial oscillometry with Agedio® (IEM GmbH, Stolberg, Germany), a semi-automatic tensiometer with a software validated by the ESH, with the patient resting alone quietly. The Agedio® performs automatically a first measurement for the purpose of calibration. After an interval of respectively 1min, a second and a third measurement was realized for estimation of PWV, the mean value of the last two were used for calculating peripheral, central BP, PWV, augmentation pressure and augmentation index. HTN was defined as systolic BP≥140 or diastolic BP≥90mmHg or known anti-hypertensive treatment. BP was measured according to ESH recommendations as the mean value of the last two out of three consecutive measurements and taking into account published quality standards. Arterial stiffness was defined as estimated PWV (PWVe) exceeding the 90-percentile of the median value of the corresponding age group, according to seven 10-year intervals from 20 to over 70 years of age.14 We will use the emerging concept of “early vascular aging” (EVA), as a vascular age that exceeds the chronological age of a person, according to the pre-defined age groups.15
Statistical analysisContinuous variables were tested for normal distribution using Kolmogorov–Smirnov test. Results are shown as means (standard deviation, SD) or medians (25–75th percentile) for continuous variables and absolute values (%) for categorical variables. To compare baseline demographic and clinical characteristics among the different groups, we used analysis of variance (ANOVA) or the Kruskal–Wallis test for continuous variables. Differences in proportion were analyzed using the Chi-square test. We used a logistic regression to evaluate the relationship between significant variables found on the univariate analysis and all-cause mortality; variables with p<0.1 on the univariate analysis were included. All statistical analyses were performed using SPSS software (IBM SPSS Statistics for Windows, Version 26.0, Armonk, NY: IBM Corp). A two-tailed p value<.05 was considered statistically significant.
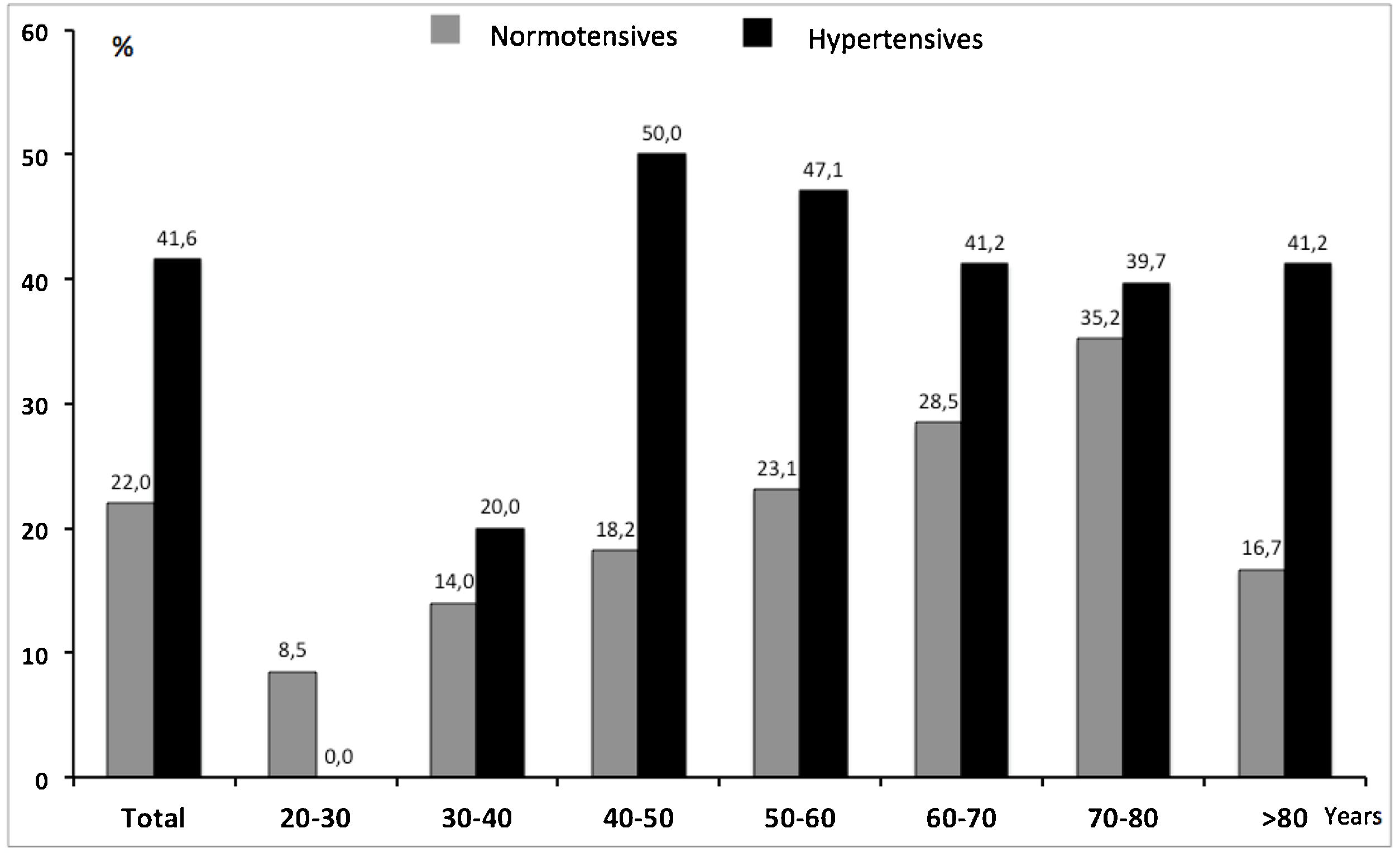
ResultsDemographic characteristics of the study populationA total of 963 patients were recruited between February and December 2021. Invalid measurements were recorded in 40 (4,3%) individuals, the final analysis comprised 923 subjects, with 570 (61.8%) females and 353 (38.2%) males, as shown in Table 1. Mean age in the whole group was 58.8 (±16.1, range 20–95) years, mean systolic and diastolic BP was 128/79 (±18.5/±11.3)mmHg, respectively. Abdominal obesity according to waist circumference was present in 37.1% of the population, 142 subjects (15.4%) were current smokers. Treated HTN was registered in 363 (39.3%) patients, 41.6% of which showed elevated BP. In the group of subjects without known HTN, 22.0% had elevated BP, the overall proportion of subjects with elevated BP was therefore 29.7% (n=274).
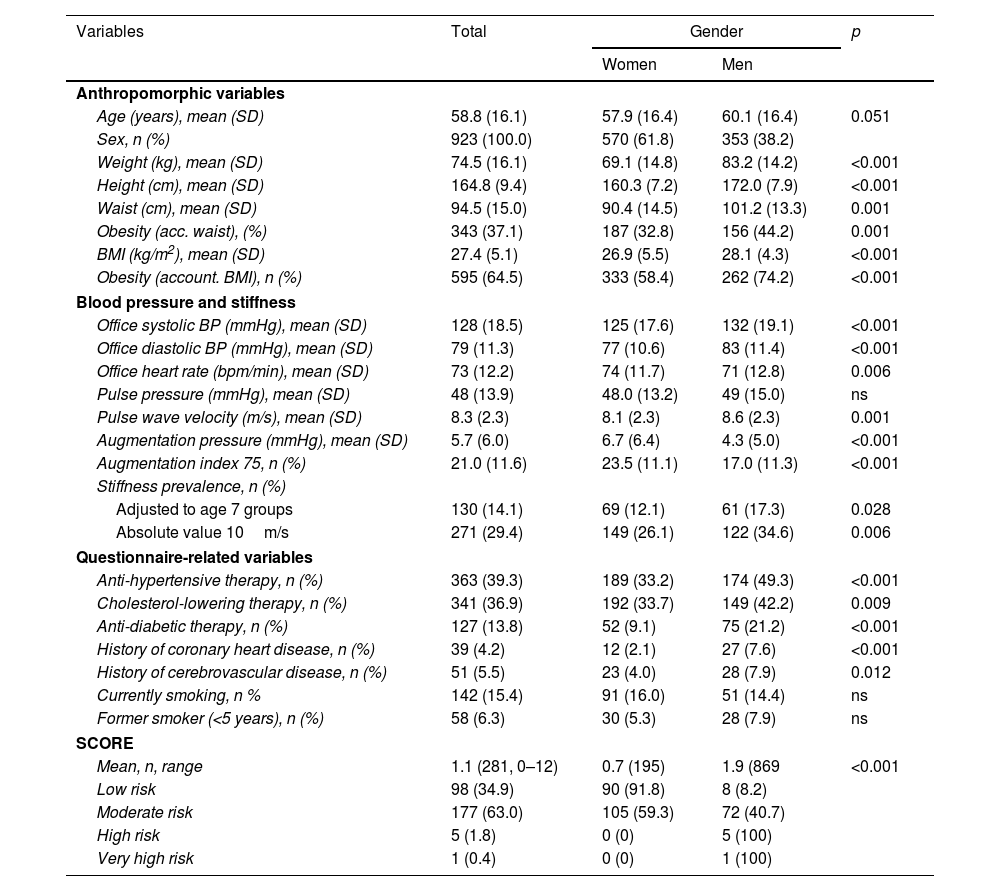
Characteristics of patients in the total cohort (n=923).
| Variables | Total | Gender | p | |
|---|---|---|---|---|
| Women | Men | |||
| Anthropomorphic variables | ||||
| Age (years), mean (SD) | 58.8 (16.1) | 57.9 (16.4) | 60.1 (16.4) | 0.051 |
| Sex, n (%) | 923 (100.0) | 570 (61.8) | 353 (38.2) | |
| Weight (kg), mean (SD) | 74.5 (16.1) | 69.1 (14.8) | 83.2 (14.2) | <0.001 |
| Height (cm), mean (SD) | 164.8 (9.4) | 160.3 (7.2) | 172.0 (7.9) | <0.001 |
| Waist (cm), mean (SD) | 94.5 (15.0) | 90.4 (14.5) | 101.2 (13.3) | 0.001 |
| Obesity (acc. waist), (%) | 343 (37.1) | 187 (32.8) | 156 (44.2) | 0.001 |
| BMI (kg/m2), mean (SD) | 27.4 (5.1) | 26.9 (5.5) | 28.1 (4.3) | <0.001 |
| Obesity (account. BMI), n (%) | 595 (64.5) | 333 (58.4) | 262 (74.2) | <0.001 |
| Blood pressure and stiffness | ||||
| Office systolic BP (mmHg), mean (SD) | 128 (18.5) | 125 (17.6) | 132 (19.1) | <0.001 |
| Office diastolic BP (mmHg), mean (SD) | 79 (11.3) | 77 (10.6) | 83 (11.4) | <0.001 |
| Office heart rate (bpm/min), mean (SD) | 73 (12.2) | 74 (11.7) | 71 (12.8) | 0.006 |
| Pulse pressure (mmHg), mean (SD) | 48 (13.9) | 48.0 (13.2) | 49 (15.0) | ns |
| Pulse wave velocity (m/s), mean (SD) | 8.3 (2.3) | 8.1 (2.3) | 8.6 (2.3) | 0.001 |
| Augmentation pressure (mmHg), mean (SD) | 5.7 (6.0) | 6.7 (6.4) | 4.3 (5.0) | <0.001 |
| Augmentation index 75, n (%) | 21.0 (11.6) | 23.5 (11.1) | 17.0 (11.3) | <0.001 |
| Stiffness prevalence, n (%) | ||||
| Adjusted to age 7 groups | 130 (14.1) | 69 (12.1) | 61 (17.3) | 0.028 |
| Absolute value 10m/s | 271 (29.4) | 149 (26.1) | 122 (34.6) | 0.006 |
| Questionnaire-related variables | ||||
| Anti-hypertensive therapy, n (%) | 363 (39.3) | 189 (33.2) | 174 (49.3) | <0.001 |
| Cholesterol-lowering therapy, n (%) | 341 (36.9) | 192 (33.7) | 149 (42.2) | 0.009 |
| Anti-diabetic therapy, n (%) | 127 (13.8) | 52 (9.1) | 75 (21.2) | <0.001 |
| History of coronary heart disease, n (%) | 39 (4.2) | 12 (2.1) | 27 (7.6) | <0.001 |
| History of cerebrovascular disease, n (%) | 51 (5.5) | 23 (4.0) | 28 (7.9) | 0.012 |
| Currently smoking, n % | 142 (15.4) | 91 (16.0) | 51 (14.4) | ns |
| Former smoker (<5 years), n (%) | 58 (6.3) | 30 (5.3) | 28 (7.9) | ns |
| SCORE | ||||
| Mean, n, range | 1.1 (281, 0–12) | 0.7 (195) | 1.9 (869 | <0.001 |
| Low risk | 98 (34.9) | 90 (91.8) | 8 (8.2) | |
| Moderate risk | 177 (63.0) | 105 (59.3) | 72 (40.7) | |
| High risk | 5 (1.8) | 0 (0) | 5 (100) | |
| Very high risk | 1 (0.4) | 0 (0) | 1 (100) | |
BMI: body mass index; BP: blood pressure.
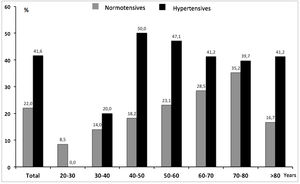
Uncontrolled BP was also analyzed according to age. We observed a different distribution depending on the a priori status of known or unknown (HTN). While in known hypertensives the proportion of uncontrolled BP was similar and not satisfactory in all age groups, in the normotensive group uncontrolled BP increased parallel to age (Fig. 1).
Diabetes was identified in 127 (13.8%) patients, 341 (36.9%) referred cholesterol-lowering therapy. Previous major CV events were reported in 82 patients, 39 (4.2%) cases of coronary heart disease, 51 (5.5%) of cerebrovascular disease, with 8 patients having suffered both. Only a small proportion of these patients were within the range of age to determine SCORE (17, 20.7%). As shown in Table 1, females were significantly younger, had lower BP and showed also a significantly lower proportion of obesity, anti-hypertensive, anti-diabetic and cholesterin-lowering therapy, and referred less frequently CV diseases. Current smoking was equally distributed in both sexes.
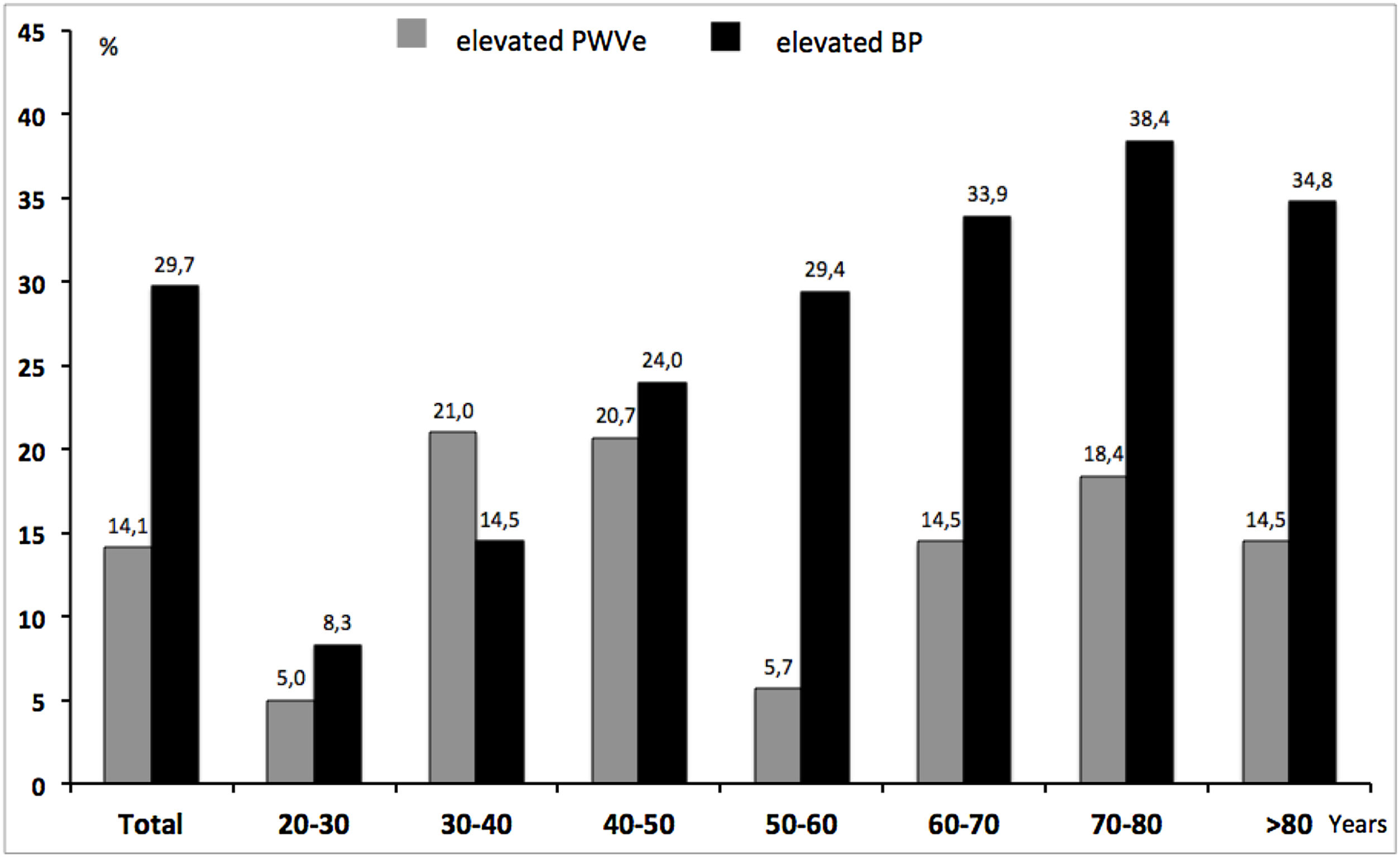
Mean PWVe was 8.3 (2.3)m/s, with significantly lower values in women 8.1 (2.3)m/s than in men 8.6 (2.3)m/s. As a whole, 14.1% of subjects were above the 90th reference percentile of PWVe, ranging from 5% in the youngest patients to 14.5% in older patients. The difference in EVA was clearly dependent on the status of HTN, as known hypertensives showed significantly higher PWVe than normotensives (7.4 (2.1)m/s vs. 9.7 (2.0)m/s, p<0.001).
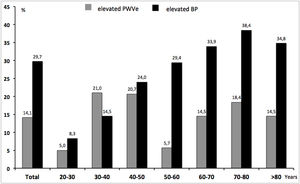
Fig. 2 shows the comparison between the percentage of EVA and the percentage of elevated/uncontrolled BP along age groups, independently of previous HTN-status. The proportion of elevated/uncontrolled BP (29.7%), in absolute terms, almost exactly doubled that of EVA (14.1%). Nevertheless, while the former increased constantly with rising age, EVA was higher than the prevalence of elevated/uncontrolled in younger ages and even surpassed it in the group of 30–40 years.
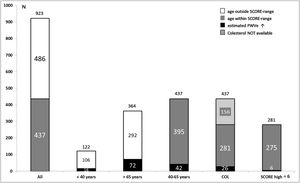
As shown in Fig. 3, out of globally 923 patients 486 (52.7%) were not susceptible to calculating SCORE because their ages laid outside the valid range. 122 (13.2%) subjects were younger than 40 years and 364 (39.4%) exceeded 65 years of age. Assessment of PWVe in these groups revealed values beyond the 90th percentile in 16 (13.1%) of the younger and 72 (19.8%) of the older participants, together 88 (18.1%) individuals.
Within the 437 (47.3%) participants older than 40 and younger than 65 years, the prevalence of EVA was 9.6% (26 subjects). Cholesterol values were available in 281 (64.3%) patients. The proportion of patients with EVA in this group remained almost identical (26 subjects, 9.2%). Table 1 contains the distribution of risk strata according to SCORE, showing 98 (34.9%) of patients with low CV risk, 177 (63.0%) with moderate risk, 5 (1.8%) with high risk and only one subject (0.4%) with very high risk, indicating that SCORE stratified almost only one fourth of participants at suspected high or very high risk compared to PWVe.
Considered as continuous variables, SCORE and PWVe were significantly associated (Pearson's correlation coefficient=0.614, p=0.01), in the 281 patients, in which SCORE could be determined. Dichotomic contingency tables and X2 could not be calculated because all 6 patients with high or very high risk showed PWVe below the 90th percentile of their age. The comparison between SCORE in patients without and with EVA was not significant (1.06 vs. 0.96, respectively, p=0.7).
Finally, we analyzed the association between history of prior CV events, either coronary heart or cerebrovascular disease, and the two estimators of risk, SCORE and PWVe in the small group of 17 patients with history of cardiac or cerebral disease, in which SCORE could be calculated. While all 17 patients were stratified according to SCORE in the group of moderate CV risk according to the five determinants of SCORE, the proportion of patients with prior CV event was significantly higher in the subjects with increased PWVe than in those with normal PWVe (4 of 26 subjects, 15.4% vs. 13/255 subjects, 5.1%, OR 3.4; CI 1.02–11.31, p=0.035, respectively).
DiscussionThree are the main findings of this cross-sectional study comparing the stratification of CV risk between the gold-standard algorithm used in Primary Care, SCORE, and an established intermediate marker of vascular damage, PWVe by brachial oscillometry, as assessed in community pharmacies in a real world setting.
First, risk stratification by PWVe almost doubles the number of subjects susceptible of CV risk evaluation, compared to SCORE, because 52.7% of the participating subjects were either younger than 40 or older than 65 years old.
Age is the most potent predictor of risk,16 it seems therefore reasonable that SCORE defined a threshold of use at 40 years, because CV events defined by SCORE as fatal CV events are very rare below the age of 40 years.17 Nevertheless, CV risk represents a continuum, and non-fatal CV events do occur before the age of 40, but remain ignored when only SCORE is used. Additionally, SCORE is limited to the age<65 years, assuming that beyond this limit of age CV risk should be high per definition, thereby not accounting for the fact that CV also differs in older ages.18
According to the Spanish Instituto Nacional de Estadística (INE),19 and restricting our analysis to the ages in which individuals are susceptible to be assessed by PWVe (>20 years of age, 34,854,927 subjects), the proportion of individuals between 40 and 65 years in Spain in 2021 was 41.8%, and that of subjects not susceptible to SCORE was 58.2% (31.5% of younger and 26.7% of older individuals). This percentage of participants comprising the ages of 20–40 as well as >65 years was slightly lower in our series (52.7%), the magnitude of the excluded population by SCORE is nevertheless very similar, comparable and remains impressive. Of note, taking both groups of individuals together, higher risk due to elevated PWVe was suspected in 18.1% (13.1% between 20 and 40 years and 19.8% in ages>65 years). Extrapolating these numbers into national categories would imply that a population-based screening campaign measuring PWVe in community pharmacies could identify approx. 3,650,195 subjects with suspected higher risk.
It is often argued that simple BP measurement should be performed firstly, more easily and yielding similar results as PWVe. Opponents of PWV underscore the strong association of BP with PWV in general, and with PWVe in particular, denying any added effect to the prognostic value of measuring BP.20–22 But PWVe represents a real novelty to the extent that stiffness, as subclinical target organ damage, is said to integrate not only BP but also other CV risk factors that transform it into an integrating variable of vascular age as well as an intermediate marker of CV factors over time, far beyond simple BP. In a previous pilot study we demonstrated in the same clinical setting the significant qualitative and quantitative differences of defining stiffness according to age groups in contrast to a threshold of 10m/s. The latter was mainly explained by age and BP, while stiffness according to age groups included pulse pressure, age, waist circumference and heart rate, that is, far more than only BP.12 Furthermore, two further arguments reinforce the unique role of PWVe compared to BP. First, frequency of elevated, uncontrolled BP was much higher than PWVe, as in other similar studies,10 even doubling its frequency, and second, the distribution among age groups differed clearly between BP and PWVe, indicating that equalizing BP and PWVe is absolutely not supported by our data. We therefore conclude that pathological stiffness and high BP are not superimposable concepts.
This is best explained by the second main finding of the study, the fact that within the ages of 40–65 years, PWVe identifies up to a four-fold higher number of patients with vascular damage and therefore, at assumed high risk. In fact, although SCORE includes BP, the number of identified patients at high risk is very low, even accounting for Spain as a low risk SCORE country. It should be clearly stated, that the high risk attributed to SCORE≥4 in our study is also assumed, as this is a cross-sectional study. In comparison, PWVe was pathological in almost 10% of patients between 40 and 65 years of age, underscoring that high risk cannot be easily detected by merely measuring BP.
And third, this study shows that risk stratification by SCORE is changing and variable, while PWVe appears to identify residual risk. 17 patients who had suffered a CV event and been consequently treated, when SCORE was performed, were stratified in the moderate risk group, indicating that SCORE is strongly dependent on the time-window, when it is calculated. In fact, it is not clear to which extent serial determinations of SCORE provide clinical benefit. Contrary to this finding, PWVe was significantly associated with prior CV events, even in a relatively small sample, pointing at the hypothesis that residual risk remains and that it is identified by PWVe even after optimal treatment of traditional risk factors, but not by SCORE.
Oscillometric assessment of EVA in pharmacies have also been published in Portugal11 with similar frequency of EVA (19.8%), and Austria,10 a CV high-risk country which could explain the higher prevalence of EVA (37.3%). It is noteworthy that in a different, population-based study, in a clinical setting in Portugal, determined with tonometry, Cunha et al. found an overall prevalence of EVA of 12.5%, very similar to our study.23 Of note, in Cunha's as well as in our study, EVA is more prevalent in younger than in older ages, reinforcing the concept that arterial stiffness is a condition that may precede the appearance of HTN rather than being its consequence.
This study has several limitations. Firstly, whatever risk is referred to is assumed risk, and this applies to SCORE, where the evidence is scarce, as well as to PWVe with even smaller evidence, since the design is cross-sectional. Planned, future steps in our COPHARTEN Project13 are already on the way to test our conclusions on a longitudinal level. Secondly, Guidelines include arterial stiffness either as pulse pressure>60mmHg in older patients or as carotid–femoral PWV over 10m/s,1 the emerging concept of EVA and its age-dependent definition have yet not been incorporated, although increasing evidence is accumulating.24,25 Thirdly, oscillometric measurements are still under debate under experts, although multiple validation studies have been published.7–9 It should be underlined that our proposal of oscillometric PWVe is strongly determined by the target of establishing large-scale screening programs of CV risk in pharmacies for the general population. Similarly, just like urine dipsticks cannot replace quantitative urine albumin excretion analysis and electrocardiography should not be postulated to replace echocardiography, oscillometry is not intended to replace tonometric measurements. On the other hand, tonometry can hardly serve as a tool of vast screening campaigns. Fourthly, our study was performed in a real world setting under daily pharmaceutical conditions. Cholesterol measurements were asked for every patient, but 36% did not provide it. Nevertheless, incomplete laboratory results do not alter the conclusions of the study, moreover, obtained results even under real, non-ideal conditions could be also interpreted as robust. Fifthly, Guidelines recommended in their 2018 edition the cooperation of pharmacists among other groups of health workers to manage HTN.1 Unfortunately, their presence in research is minimal if not completely absent, partly due to the high standards required for their participation, although their contribution necessarily should be under real world conditions, and not mimicking physicians only in randomized trials. Finally, SCORE2 has recently been published to improve risk prediction.26 Our study was designed long before SCORE2 emerged, besides, its knowledge among physicians will necessarily take years to take place, the present study reflects therefore better the present situation of risk management, although future work should compare our results with SCORE2 once known and implemented.
Strengths of our study are to be embedded in an ambitious long-term project,13 the motivating cooperation between physicians and pharmacists and the large numbers of patients that can be recruited in short time laps due to the participation of scientific societies of community pharmacies.
In summary, stratification of CV risk by PWVe using brachial oscillometry can be performed on a large-scale basis in the setting of pharmaceutical daily practice. The identification of subjects at high CV risk is up to a four-fold higher than using SCORE. Further, it almost doubles the number of subjects susceptible to be evaluated. Future longitudinal studies should provide evidence on the prognostic values of PWVe when measured in community pharmacies.
Ethical considerationsInformed consent was obtained according to the protocol.
FundingNone.
Conflicts of interestNone of the authors have conflicts of interest.
Manuel Adell, Vicente Baixauli, Otón Bellver, Lidón Castillo, Santiago Centelles, María Teresa Climent, José Antonio Costa, José Chordá, Edelmira Córcoles, Rosario Hernández, Sara Martínez, Zeneida Perseguer, Rosa Prats, Javier Reig, Enrique Rodilla, Desiré Ruiz, Fanny Ruiz Lozano, Luis Salar.