Inclusion of direct-acting antivirals into clinical practice in patients with chronic HCV (CHC) has been a milestone in medicine.
Patients and methodsAnalytical, prospective study, involving 126 patients with chronic HCV treated with direct-acting antivirals. Efficacy and safety of treatment and factors associated with failure treatment were evaluated.
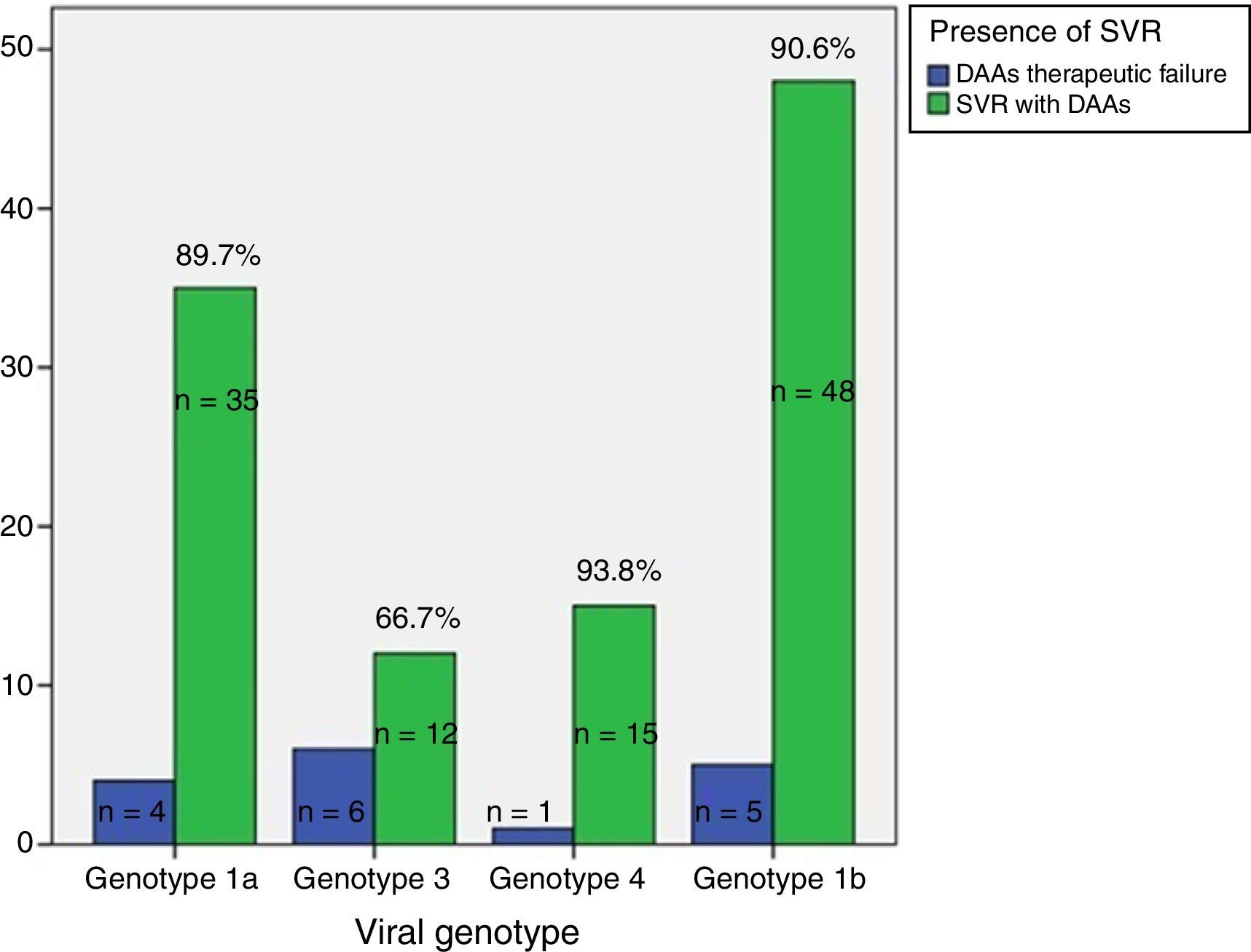
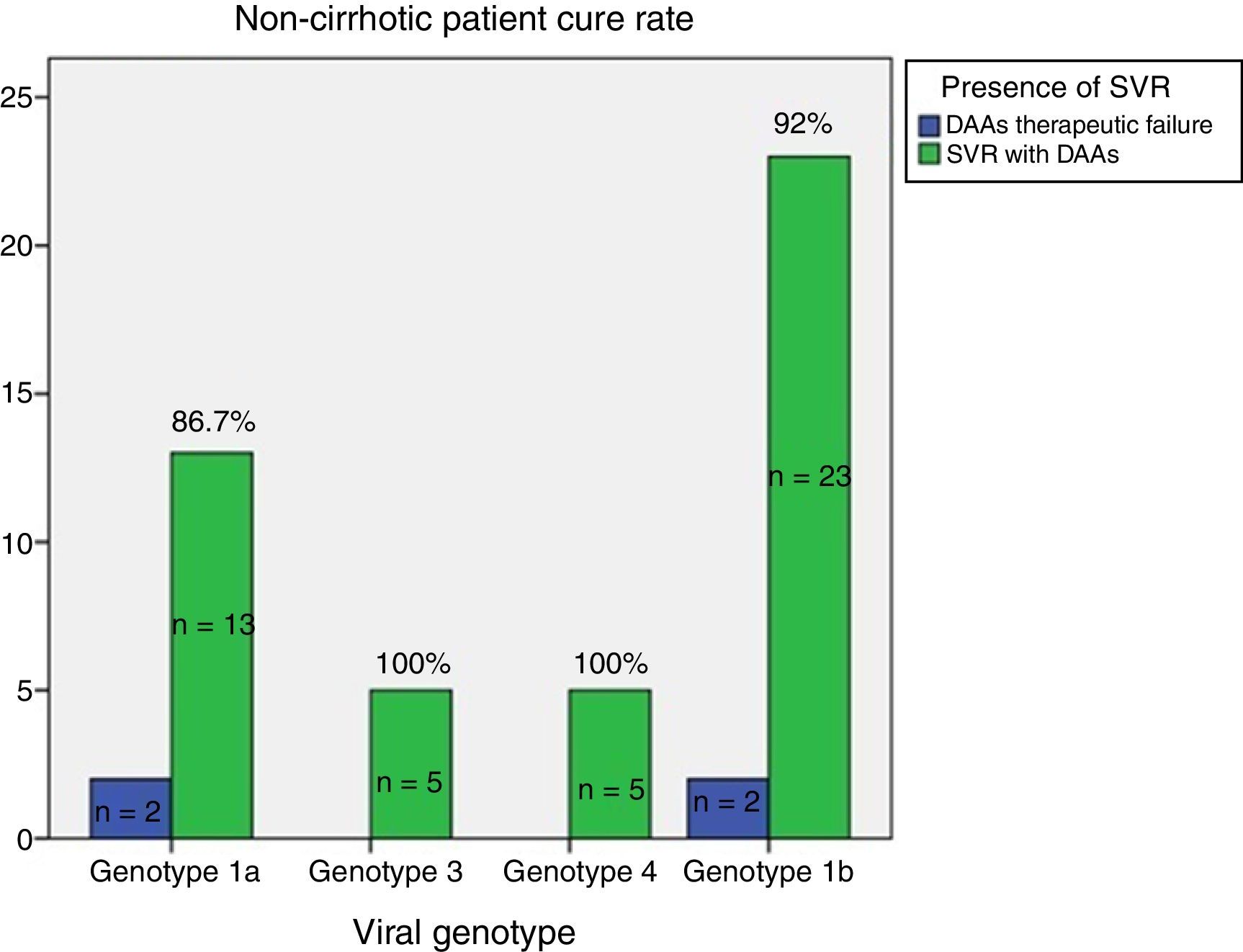
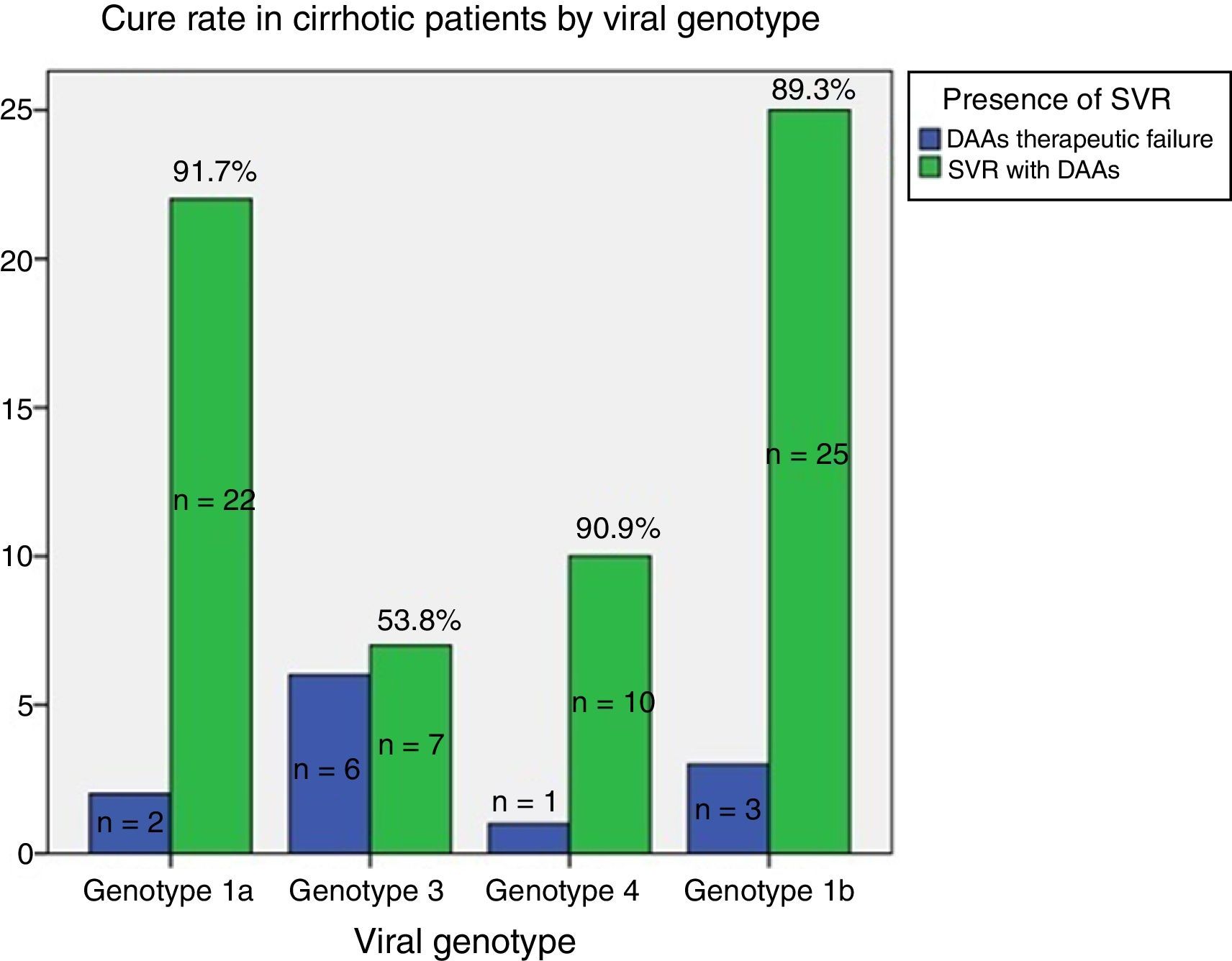
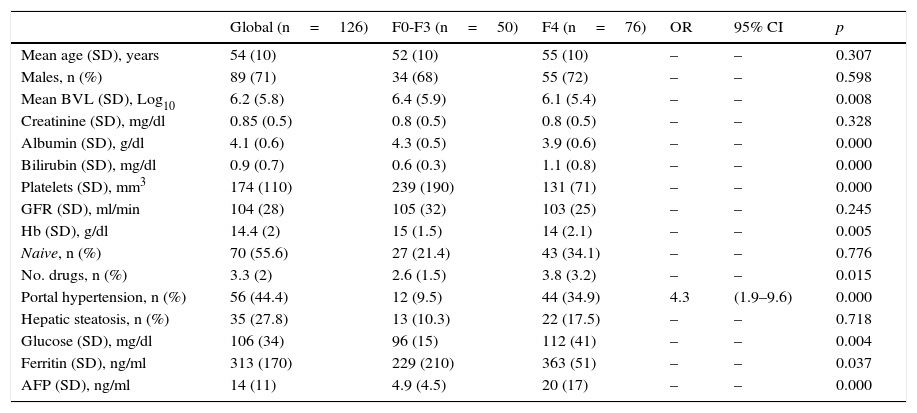
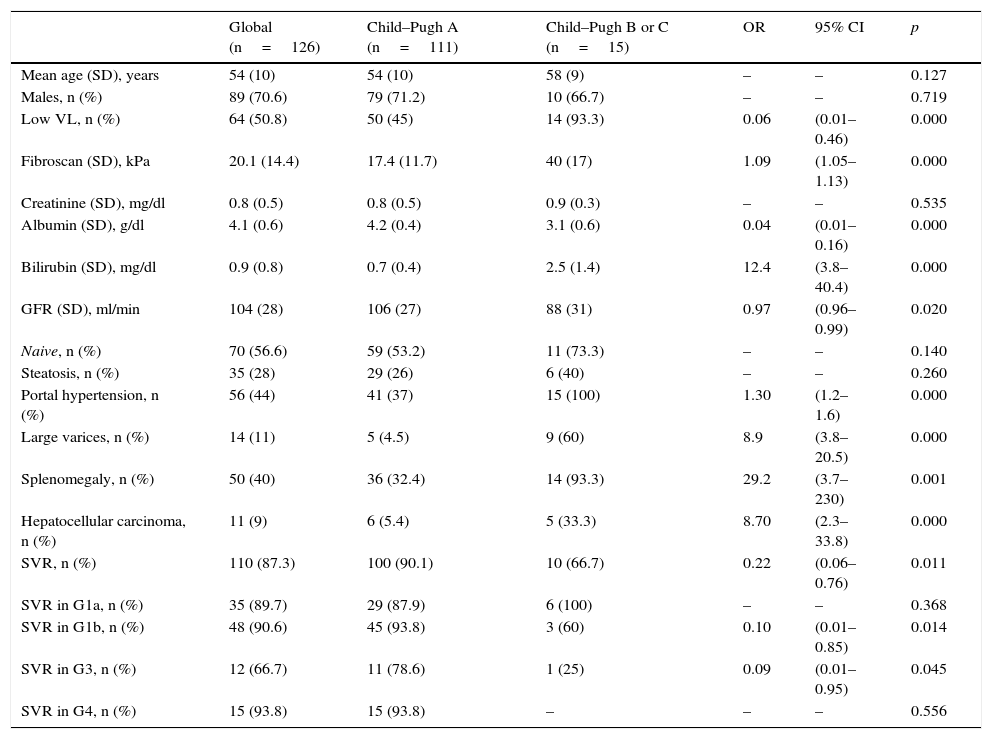
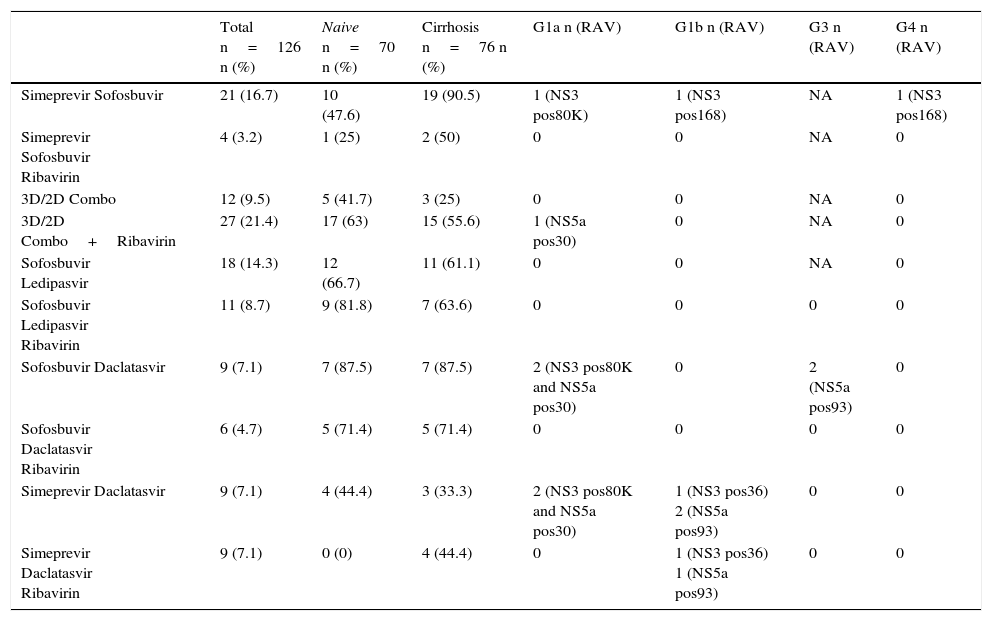
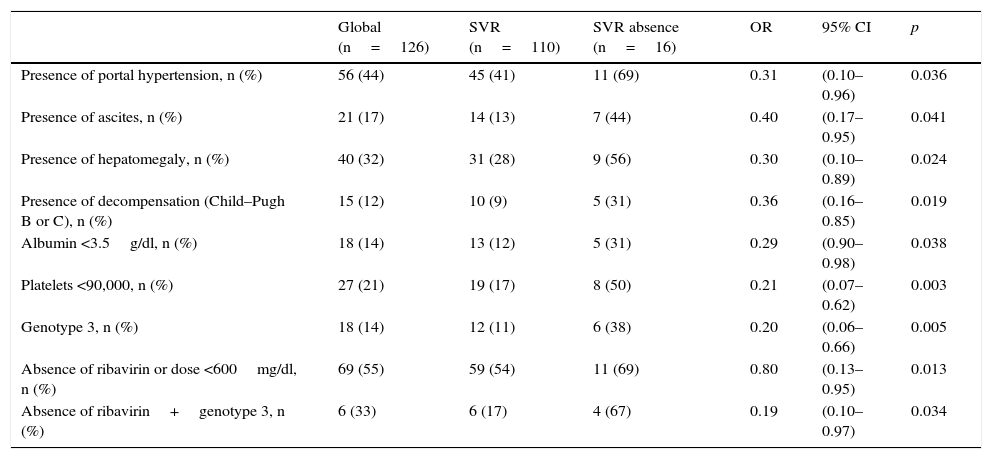
ResultsAge 54±10. Male (70%). Cirrhosis (60%). Distribution according to genotypes: G1a (31%), G1b (42%); G3 (14%); G4 (13%). Child–Pugh B and C (n=15). Naïve (56%). SVR rate was (87.3%): Child-A (91%), Child-B (75%) and Child-C (60%). The best cure rates were achieved with a 3D/2D±ribavirin (SVR=97.4%; n=39) and sofosbuvir/ledipasvir±ribavirin (RVS=93.1%; n=29) combination. An SVR rate of <90% was achieved with sofosbuvir+simeprevir±ribavirin (SVR=88%, n=25), simeprevir+daclatasvir±ribavirin 73%, n=15). The association of ribavirin to these last three therapeutic options (n=19) improved cure rates (SVR=94.7%, 18/19) compared to its absence (n=39; SVR=77%). Improvement in MELD (40%). Output transplant list (20%). Substitutions associated with resistors NS3: G1a (positions 80K; n=5); G1b and G4 (position 168 and 36; n=4), while for NS5a: G1a (position 30; n=2) and G1b and G3 (position 93; n=3). Variables associated with failure in multivariate analysis (p<0.05): presence of ascites, G3 and ribavirin dosage <600mg/day.
DiscussionThe presence of genotype 3, ascites or dosage of ribavirin <600mg/day were associated with higher failure rates. The use of ribavirin >600mg/day in cirrhotic G1 or G3, who will be treated with sofosbuvir+simeprevir or daclatasvir is recommended where no baseline resistance test is available.
La inclusión en práctica real de los antivirales de acción directa en pacientes con hepatitis crónica por VHC ha supuesto un hito histórico en Medicina.
Pacientes y métodosEstudio analítico, prospectivo que incluyó 126 pacientes con hepatitis crónica por VHC tratados con antivirales de acción directa. Evaluamos la eficacia y seguridad del tratamiento y factores asociados a fracaso terapéutico.
ResultadosEdad 54±10 años. Varón (70%). Cirrosis (60%). Distribución según genotipos: G1a (31%), G1b (42%); G3 (14%); G4 (13%). Child-Pugh B y C (n=15). Naïve (56%). Tasa RVS fue (87,3%): Child-A (91%), Child-B (75%) y Child-C (60%). Las mejores tasas de curación se alcanzaron con las combinaciones Combo 3D/2D±ribavirina (RVS=97,4%; n=39) y sofosbuvir/ledipasvir±ribavirina (RVS=93,1%; n=29). Tasas<90% se registraron con: sofosbuvir+simeprevir±ribavirina (RVS=88%; n=25), simeprevir+daclatasvir±ribavirina (RVS=78%; n=18) y sofosbuvir+daclatasvir±ribavirina (RVS=73,3%; n=15). La adicción de ribavirina a estas 3 últimas opciones terapéuticas (n=19) mejoraba las tasas de curación (RVS=94,7%; 18/19) frente a su ausencia (n=39; RVS=77%). Mejoría MELD (40%). Salida lista trasplante (20%). Sustituciones asociadas a resistencias NS3: G1a (posiciones 80K; n=5); G1b y G4 (posición 168 y 36; n=4), mientras para NS5a: G1a (posición 30; n=2) y G1b y G3 (posición 93; n=3). Variables asociadas al fracaso en análisis multivariante (p<0,05): presencia de ascitis, G3 y dosis de ribavirina<600mg/día.
DiscusiónLa presencia de genotipo 3, ascitis o dosis de ribavirina<600mg/día se asoció a mayores tasas de fracasos. Sería recomendable el uso de ribavirina≥600mg/día en cirróticos G1 o G3, que vayan a ser tratados con sofosbuvir+simeprevir o daclatasvir, si no hubiese disponibilidad de un test de resistencia basal.