Background/Objective: The purpose of this study was to examine quality of life (QoL) in breast cancer patients from Colombia and to explore the relationship between QoL, habitual optimism, and social support. Method: A sample of 95 breast cancer patients treated in a hospital in Bogotá were administered the QoL instrument EORTC QLQ-C30 and the Life Orientation Test LOT-R. Additionally, they were asked to indicate from whom (physicians, friends, nurses, etc.) they wished and received social support. Reference data for the EORTC QLQ-C30 and the LOT-R were taken from a representative sample of the general Colombian population. Results: The breast cancer patients showed detriments to their QoL on most functioning scales and symptom scales of the EORTC QLQ-C30, while their general assessments of health and QoL were not worse than those of the controls. Optimism was positively correlated with QoL. Most patients wanted and received social support from their physicians and friends/family. Conclusions: The results suggest that optimism helps patients better cope with disease. A general assessment of global QoL cannot replace the more specific assessments of the functioning domains and symptoms.
Antecedentes/Objetivo: El objetivo del estudio fue examinar la calidad de vida (QoL, por sus siglas en inglés) en pacientes con cáncer de mama colombianas, y explorar la asociación entre calidad de vida, optimismo disposicional y apoyo social. Método: Se entrevistó una muestra de 95 pacientes con cáncer de mama tratadas en un hospital de Bogotá y les fue aplicado el instrumento de medición de calidad de vida EORTC QLQ-C30 y el test de orientación ante la vida (LOT-R). Adicionalmente, se preguntó acerca de su apoyo social usando varias preguntas. Resultados: En la mayoría de las escalas de funcionamiento del EORTC QLQ-C30 y de las escalas de síntomas, las pacientes con cáncer mostraron detrimentos en su QoL, mientras en la evaluación general de calidad de vida y salud las medias de las pacientes no fueron más bajas que las de los controles. El optimismo estuvo positivamente correlacionado con la calidad de vida. La mayoría de las pacientes desearon y recibieron apoyo social de su médico y de sus amigos/familiares. Conclusiones: Los resultados sugieren que el optimismo ayuda a las pacientes a afrontar mejor la enfermedad. Una evaluación general de la QoL no parece poder sustituir la evaluación más específica de los síntomas y dominios de funcionamiento.
Quality of Life (QoL) is an important outcome criterion in oncology (De la Torre-Luque, Gambara, López, & Cruzado, 2016). Several studies have been performed to assess quality of life in breast cancer patients and survivors (Chu et al., 2016; Ghislain et al., 2016; Lemieux, Goodwin, Bordeleau, Lauzier, & Theberge, 2011; Mols, Vingerhoets, Coebergh, & van de Poll-Franse, 2005). However, most of them have been conducted in Western countries, and there are relatively few examinations from other parts of the world. In Latin America, breast cancer incidence has increased, but the age-standardized incidence rate there is still only about 50% of that in Western Europe (Justo, Wilking, Jonsson, Luciani, & Cazap, 2013). At the same time, in comparison to Europe, survival rates are lower in Latin America, where approximately 30-40% of the diagnoses are metastatic, due to late stages of diagnoses and poorer access to treatment (Justo et al., 2013). Efforts are currently being made to implement policies that address the growing incidence of breast cancer in Latin America (Nigenda, Gonzalez-Robledo, Gonzalez-Robledo, & Bejarano-Arias, 2016).
There are multiple instruments for measuring QoL in breast cancer patients (Maratia, Cedillo, & Rejas, 2016). One of the most often used questionnaires is the EORTC QLQ-C30 (Aaronson et al., 1993). It has been translated into many languages, and normative values are available for several European countries (Hinz, Singer, & Brähler, 2014), South Korea (Yun, Kim, Lee, Park, & Kim, 2007), and Colombia (Finck, Barradas, Singer, Zenger, & Hinz, 2012). This questionnaire covers multiple functioning domains and symptoms, and it also comprises a 2-item scale for making a global assessment of general health/QoL. Several studies have found that breast cancer patients’ and survivors’ general assessment of their global health/QoL was only marginally lower than that of the general population, despite the fact that the patients reported detriments in many specific domains (Arndt, Merx, Stegmaier, Ziegler, & Brenner, 2005; Hinz, Mehnert et al., 2017).
While psycho-oncological research has historically been mainly deficit-oriented, focusing on depression, anxiety, and loss of functioning, in recent years, a resource-oriented perspective has been gaining increasing attention. Factors such as habitual optimism (Colby & Shifren, 2013; Ha & Cho, 2014; Saboonchi, Petersson, Alexanderson, Branstrom, & Wennman-Larsen, 2016), self-efficacy (Shelby et al., 2014), sense of coherence (Rohani, Abedi, Sundberg, & Langius-Eklof, 2015), and social support (Spatuzzi et al., 2016) have been studied as protective or buffering factors in breast cancer patients. Habitual optimism is defined as a general tendency to expect positive outcomes (Carver & Scheier, 2014). It is associated with physical and mental health, quality of life, and even mortality (Anthony, Kritz-Silverstein, & Barrett-Connor, 2016). Social support includes emotional and instrumental support. Several questionnaires have been developed for assessing the generalized degree of social support a person receives. In the field of oncology it is of interest from whom the patients want to get and from whom they actually receive social support. A German study found that cancer patients prefer to get social support from physicians and from family/friends, while there was much less interest in other sources of social support such as psychologists, social workers, and clergy (Zenger, Ernst, Götze, Stolzenburg, & Hinz, 2010). In this study, we intend to test whether this pattern is also found in Colombia, and whether the need for social support is associated with QoL.
In summary, the purpose of this study was (a) to examine the QoL of Colombian breast cancer patients in comparison with the general population, including a comparison between general QoL assessments and specific functioning domains and symptoms, (b) to explore the relationship between clinical treatment variables and QoL, (c) to determine the degree of habitual optimism and its relationship to QoL, and (d) to explore the patients’ desire for social support and the effectiveness of social support in Colombian breast cancer patients.
MethodParticipantsPatients were recruited through the oncology department and the breast cancer unit (“Clínica del seno”) of a large hospital in Bogotá, Colombia. To meet the inclusion criteria, patients needed a formal diagnosis of breast cancer and to be undergoing oncology treatment at the time of the study. The ethics committee of the clinic and also the ethics committee for research of the Universidad de los Andes approved the study, and informed consent was obtained from all participants. All eligible patients were approached by their practitioner and informed about the study. If they agreed to participate the research team provided them the questionnaires and an envelope they could seal to maintain confidentiality. If patients asked for assistance in filling out the questionnaires they were given face-to-face interviews. Of the 127 women originally selected to be a part of the study, 95 agreed to participate (response rate 75%).
To compare the patients’ data with normative scores, we used the mean scores obtained in a study examining a representative sample of the Colombian general population, including the questionnaires EORTC QLQ-C30 (Aaronson et al., 1993; Finck et al., 2012) and LOT-R (Scheier, Carver, & Bridges, 1994). Details of the sampling method have been published elsewhere (Finck et al., 2012; Zenger et al., 2013). Out of the 1,500 individuals from that study, we selected a random subgroup of women with a mean age identical to that of the patients’ sample. This resulted in a subsample of n = 367 women with a mean age of 55.7 years of age, range: 25-86 years.
InstrumentsEORTC QLQ-C30. The quality of life questionnaire EORTC QLQ-C30 (Aaronson et al., 1993) was specifically designed for cancer patients. It consists of 30 items, which belong to five functioning scales, three symptom scales, six single symptoms, and a 2-item general health/QoL scale. A summary score of the EORTC QLQ-C30 can be calculated (Giesinger et al., 2016) that averages across all functioning and symptom scores except financial difficulties and the global health/QoL subscale. This sum score and the 2-item global health/QoL score are used as the main QoL outcome measures. All scores are transformed to the range 0-100. High scores on the functioning scales and on the global health/QoL scale indicate good QoL, while high scores on the symptom scales indicate reduced QoL. One item example of the Emotional functioning scale is: “Did you worry?”, the answer options are 0 (not at all), 1 (a little bit), 2 (quite a bit), and 3 (very much). The Colombian Spanish version was used for this study. Cronbach's alpha coefficient for the functioning scales ranged between .65 and .88 in a Colombian general population sample (Finck et al., 2012).
LOT-R. Habitual optimism was tested with the Life Orientation Test-Revised (LOT-R) (Scheier et al., 1994). It consists of two subscales, optimism and pessimism, with three items each, along with four filler items. An item example is: “In uncertain times, I usually expect the best.” On a five-point Likert scale, answer options range from 0 (strongly disagree) to 4 (strongly agree). The scale range is 0-12 for the subscales. A study with the general Colombian population yielded the following reliability coefficients: alpha= .72 (Optimism), alpha= .57 (Pessimism), and alpha = .58 (total score) (Zenger et al., 2013). Originally, the test was designed as a unidimensional instrument. Though confirmatory factorial analyses found better fit indices for a two-factorial model (optimism and pessimism) (Cano-García et al., 2015; Hinz, Sander et al., 2017), we also consider the original unidimensional sum score, composed of the optimism and the inverted pessimism subscale.
Social support. The patients were asked to indicate from whom they wished to receive social support in coping with the disease, and from whom they actually received such support. Six possible sources of support were named: physician, psychologist, social worker, clergy (spiritual advisor), self-help group, and friends/family. The patients who reported that they had received support were also asked to evaluate whether the support was helpful, using a five-point Likert scale (1 = not at all, …, 5 = very much). Dichotomous variables were calculated based on the responses, indicating whether the support was helpful (categories much and very much) or not (categories not at all, little, and partly).
Statistical analysesMean score differences between subgroups of patients were performed with t-tests. Cohen's d was used to express the effect size (mean score differences in relation to the pooled standard deviation). Associations between LOT-R scales and scales of the EORTC QLQ-C30 were calculated with Pearson correlations. All statistics were performed with SPSS version 24.
ResultsStudy sampleIn total, 95 women were willing to take part in the study. The mean age was M = 55.7 years, SD = 11.5 years, range: 23-89 years. The distribution of marital status was as follows: single (16%), married or living with a partner (61%), divorced (14%), and widowed (9%). 95% of the women reported a religious affiliation. Concerning occupational status, the percentages were employed (27%), freelancers (14%), unemployed (2%), housewife (33%), student (1%), informal work (2%), and retired (21%). The frequencies of cancer treatments were: surgery (80%), radiation (45%), chemotherapy (87%), and hormone therapy (41%).
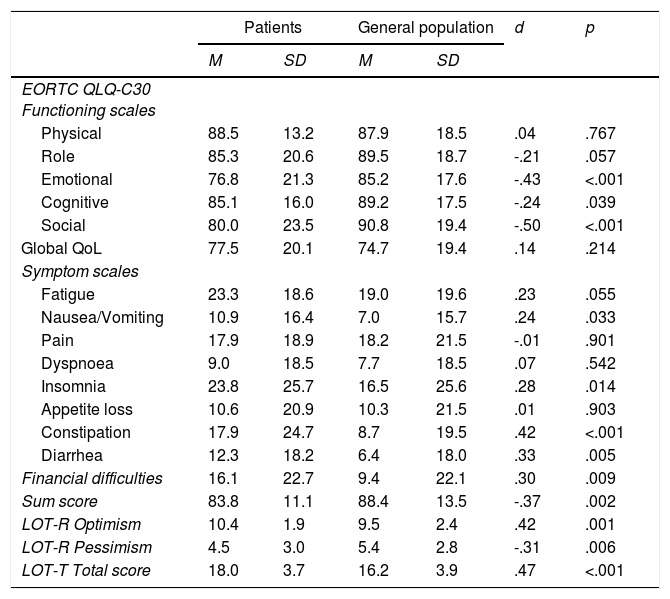
Comparison with the general populationTable 1 shows the mean scores of the EORTC QLQ-C30 and the LOT-R. With one exception (Physical functioning), all functioning mean scores of the patients were lower (worse QoL) than those of the general population, and all symptom scales and symptom items (except pain) showed higher mean scores in the patients’ sample. However, for the 2-item general health/QoL scale the mean scores of the patients were even higher (better general QoL) than those of the general population. The breast cancer patients were significantly more optimistic (LOT-R) than the general population (Table 1).
EORTC QLQ-C30 and LOT-R mean scores of the patients and the general population.
| Patients | General population | d | p | |||
|---|---|---|---|---|---|---|
| M | SD | M | SD | |||
| EORTC QLQ-C30 Functioning scales | ||||||
| Physical | 88.5 | 13.2 | 87.9 | 18.5 | .04 | .767 |
| Role | 85.3 | 20.6 | 89.5 | 18.7 | -.21 | .057 |
| Emotional | 76.8 | 21.3 | 85.2 | 17.6 | -.43 | <.001 |
| Cognitive | 85.1 | 16.0 | 89.2 | 17.5 | -.24 | .039 |
| Social | 80.0 | 23.5 | 90.8 | 19.4 | -.50 | <.001 |
| Global QoL | 77.5 | 20.1 | 74.7 | 19.4 | .14 | .214 |
| Symptom scales | ||||||
| Fatigue | 23.3 | 18.6 | 19.0 | 19.6 | .23 | .055 |
| Nausea/Vomiting | 10.9 | 16.4 | 7.0 | 15.7 | .24 | .033 |
| Pain | 17.9 | 18.9 | 18.2 | 21.5 | -.01 | .901 |
| Dyspnoea | 9.0 | 18.5 | 7.7 | 18.5 | .07 | .542 |
| Insomnia | 23.8 | 25.7 | 16.5 | 25.6 | .28 | .014 |
| Appetite loss | 10.6 | 20.9 | 10.3 | 21.5 | .01 | .903 |
| Constipation | 17.9 | 24.7 | 8.7 | 19.5 | .42 | <.001 |
| Diarrhea | 12.3 | 18.2 | 6.4 | 18.0 | .33 | .005 |
| Financial difficulties | 16.1 | 22.7 | 9.4 | 22.1 | .30 | .009 |
| Sum score | 83.8 | 11.1 | 88.4 | 13.5 | -.37 | .002 |
| LOT-R Optimism | 10.4 | 1.9 | 9.5 | 2.4 | .42 | .001 |
| LOT-R Pessimism | 4.5 | 3.0 | 5.4 | 2.8 | -.31 | .006 |
| LOT-T Total score | 18.0 | 3.7 | 16.2 | 3.9 | .47 | <.001 |
Note. M: Mean; SD: Standard deviation; d: effect size; p: significance.
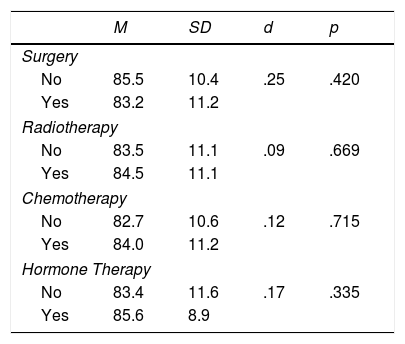
Mean scores of the EORTC QLQ-C30 sum score are given in Table 2, broken down by treatment conditions. There were no statistically significant differences between the treatment groups.
Differences in the EORTC QLQ-C30 sum score.
| M | SD | d | p | |
|---|---|---|---|---|
| Surgery | ||||
| No | 85.5 | 10.4 | .25 | .420 |
| Yes | 83.2 | 11.2 | ||
| Radiotherapy | ||||
| No | 83.5 | 11.1 | .09 | .669 |
| Yes | 84.5 | 11.1 | ||
| Chemotherapy | ||||
| No | 82.7 | 10.6 | .12 | .715 |
| Yes | 84.0 | 11.2 | ||
| Hormone Therapy | ||||
| No | 83.4 | 11.6 | .17 | .335 |
| Yes | 85.6 | 8.9 | ||
Note. M: Mean; SD: Standard deviation; d: effect size; p: significance.
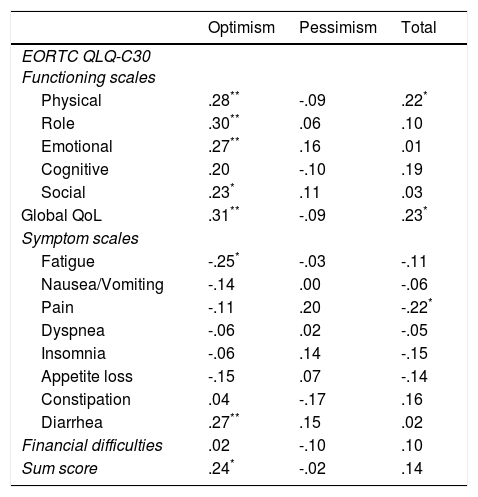
Table 3 lists the correlations between the LOT-R scales and the EORTC QLQ-C30 scales. Optimism was significantly correlated with four of the five functioning scales, while there was no significant association between pessimism and the EORTC QLQ-C30 scales and symptoms.
Correlations between the LOT-R scores and QoL.
| Optimism | Pessimism | Total | |
|---|---|---|---|
| EORTC QLQ-C30 Functioning scales | |||
| Physical | .28** | -.09 | .22* |
| Role | .30** | .06 | .10 |
| Emotional | .27** | .16 | .01 |
| Cognitive | .20 | -.10 | .19 |
| Social | .23* | .11 | .03 |
| Global QoL | .31** | -.09 | .23* |
| Symptom scales | |||
| Fatigue | -.25* | -.03 | -.11 |
| Nausea/Vomiting | -.14 | .00 | -.06 |
| Pain | -.11 | .20 | -.22* |
| Dyspnea | -.06 | .02 | -.05 |
| Insomnia | -.06 | .14 | -.15 |
| Appetite loss | -.15 | .07 | -.14 |
| Constipation | .04 | -.17 | .16 |
| Diarrhea | .27** | .15 | .02 |
| Financial difficulties | .02 | -.10 | .10 |
| Sum score | .24* | -.02 | .14 |
Note
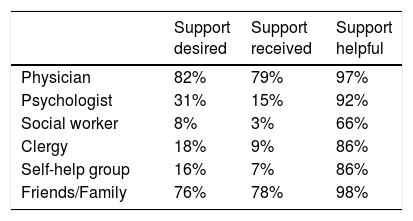
Sources of social support are reported in Table 4. Most patients (≥ 75%) wanted and received social support from physicians and their friends/family. The other sources of social support were less desired and more seldom received. All of the listed types of social support were perceived as being helpful by most of the women who received them. The most effective sources were physicians and friends/family. The right column of Table 4 refers only to those women who received that kind of support. There were no statistically significant relationships between these social support variables and the EORTC QLQ-C30 sum score (data not shown).
DiscussionThe first aim of this study was to assess the quality of life of the breast cancer patients in comparison with normative data from Colombia. With one exception (Physical functioning), the patients’ sample had lower mean scores for all of the functioning scales. The most significant differences were found for Emotional functioning and Social functioning. Again, with one exception (pain), the symptom burden was higher in the breast cancer patients’ sample. On the general health/QoL subscale, however, the patients reported health states that were even somewhat better than those of the general population. This seemingly paradoxical result has also been found in other studies. German breast cancer patients (Arndt et al., 2005; Hinz, Mehnert et al., 2017; Waldmann, Pritzkuleit, Raspe, & Katalinic, 2007), assessed with the same questionnaire, also reported higher degrees of symptoms and lower levels of functioning than people from the general population but only small differences on this global scale. This effect might be due to response shift, a change in the meaning of a person's self-evaluation of a target construct (Sprangers & Schwartz, 1999). It is conceivable that such response shift effects are more pronounced when general life satisfaction or global features of QoL are assessed in comparison with more specific aspects. The relatively low level of pain in the breast cancer patients’ sample may be due to differences in the respondents’ internal frame of reference. While the patients will probably relate this item to disease-specific pain, the general population might be counting all kinds of everyday pain such as headaches or back pain.
The manual of EORTC QLQ-C30 reference values (Scott et al., 2008) includes a list of cancer patients’ mean scores, including those of 2,782 breast cancer patients. Their EORTC QLQ-C30 functioning mean scores were lower (worse QoL) than those of our Colombian breast cancer patients’ sample, and in most dimensions the symptom scores of the manual's sample were higher. However, in that sample, a high number (58% of those with known status) suffered from recurrent/metastatic cancer. Other European studies done in Italy (Spatuzzi et al., 2016), Greece (Kontodimopoulos, Ntinoulis, & Niakas, 2011), Spain (Arraras et al., 2016), and Turkey (Demirci et al., 2011) have also found worse QoL levels in breast cancer patients, while in a study done in the Netherlands (Bantema-Joppe et al., 2015), the breast cancer patients reported better QoL. The mean scores of a study from Brazil (Evangelista et al., 2016) were similar to our Colombian mean scores. One possible reason why only moderate detriments in QoL were reported may be related to a selection bias. Women of relatively high socioeconomic status were more likely to be included because they were also more likely to be receiving treatment at the hospital that was participating in the study. This may in turn have contributed to higher assessments of QoL. Another Colombian study of breast cancer patients (Salas Zapata & Grisales Romero, 2010) found that higher levels of education were associated with higher levels of QoL.
The modes of therapy had no statistically significant influence on QoL. Another large study with more than 1,000 breast cancer patients (Waldmann et al., 2007) also failed to detect significant differences in QoL based on whether the participants were receiving radiotherapy, chemotherapy, or hormone therapy. However, this does not necessarily mean that these therapy modalities had no impact on QoL. The patients cannot be randomly assigned different types of treatment, and the sample sizes were too small to derive conclusions definitive enough to enable comparing between the subgroups of our sample. Additionally, there was some overlap between the therapy options.
Optimism was associated with QoL. All functioning scales were positively correlated with the optimism subscale of the LOT-R, and six out of the nine symptom scales and items were negatively correlated. The highest correlation was found for the general assessment of health/QoL (r = .31). Such positive associations between optimism and QoL are also found in the general population (Schou-Bredal et al., 2017). Religious beliefs and personality traits proved to be mediators for the relationship between optimism and well-being (Matthews & Cook, 2009); we were unable to test the role of such mediators in our study. Beyond the correlations with QoL, it is remarkable that the patients’ mean optimism score was not lower, but in fact, actually higher than that of the general population. Other studies have reported this finding as well. LOT-R mean scores in breast cancer samples were between 16.2 and 16.9 in other studies (Garner et al., 2015; Saboonchi et al., 2016; Thieme, Einenkel, Zenger, & Hinz, 2017), which is even higher than the mean score of the general population in Europe. Though a cancer diagnosis and treatment often evokes anxiety, the general expectation that things will develop in a positive way is not lowered, at least in terms of the mean scores. This may, at least in part, be due to advances in treating cancer. Encouraging patients’ optimism about the course their diseases might take may help them to both better cope with being ill and more quickly regain quality of life.
We did not use a standardized questionnaire to assess social support because we were interested in exploring the sources of such support. A German study found that cancer patients want and indeed get social support from their physicians and families/friends, while other professionals (psychologists, social workers, pastors) are less involved (Zenger et al., 2010). This was also found in the Colombian breast cancer sample of this study. Physicians should be aware that breast cancer patients do not only perceive them as experts in the medical domain, but that the patients also hope for psychosocial support that cannot be delegated to other professional groups. The patients gave a subjective and summarizing statement whether they had received social support; in this study we cannot distinguish between emotional and instrumental support, and we cannot compare the subjective assessment with objective criteria. The large majority was pleased with the efforts made by the physicians to be psychosocially supportive. However, only about half of the patients who wished to receive additional support (psychological, spiritual) did indeed receive it. Barriers to additional care options need to be assessed and evaluated. Integrating a brief form of quality of life monitoring into daily clinical routine may be an effective tool for detecting physical and mental problems and for tracking their course over time. Computer-based assessment methods can further facilitate (Giesinger et al., 2009) that kind of monitoring.
Some limitations of the study should be mentioned. The patients were treated in a large hospital in the capital of Colombia. The generalizability to other clinics and regions of the country cannot be assessed. Individuals with higher socioeconomic status might have been overrepresented, a factor which might contribute to the relatively good QoL assessments. The sample size was too small to draw sound conclusions concerning the comparison of subgroups. The subjective assessments of social support may be biased by social desirability. Since there are cultural differences in reporting QoL problems and supportive care needs (Shim et al., 2006) it is difficult to assess the cross-cultural generalizability of the findings.
Taken together, the study did reveal detriments to breast cancer patients’ QoL in several specific dimensions. Solely evaluating global QoL can hide this negative impact. Habitual optimism is not reduced in cancer patients, and strengthening a patient's optimistic outlook might help her cope with the diseases. Physicians have a special responsibility to provide psychosocial support. Barriers to additional psychosocial care should receive further attention.
We acknowledge support from the German Research Foundation (DFG) and Universität Leipzig within the program of Open Access Publishing.