Conditioned fear learning is crucial for survival, and failure of fear extinction is closely related to the development of anxiety disorders. This study explores how different executive function (EF) subcomponents—shifting, updating, and inhibition—modulate conditioned fear extinction and reinstatement at both behavioral and neural levels. A total of 88 participants (age range: 17–23 years) completed the Stroop Task, Digit Size-Parity Switching Task, and Memory Updating Task to assess their executive function abilities. Participants underwent a classical fear extinction paradigm while their shock expectancy ratings and prefrontal cortical activity were recorded using functional near-infrared spectroscopy (fNIRS). Results indicated that individuals with higher shifting ability exhibited greater fear expectancy during fear acquisition (β = -0.406, p = 0.032) and reinstatement (β = -0.834, p = 0.004), along with higher prefrontal cortex activity (p < 0.05), suggesting heightened responses to threatening stimuli. Individuals with higher updating ability showed a slower decrease in fear expectancy during the early extinction phase (β = 0.038, p = 0.002), but maintained lower expectancy during the extinction recall phase (β = -0.769, p = 0.006), indicating poorer extinction learning but better extinction memory retention. Individuals with higher updating ability also exhibited more extinction recall in the prefrontal cortex regions (ps < 0.045). Individuals with higher inhibitory ability showed higher expectancy for CS+ and lower expectancy for CS− during extinction learning (β = -0.409, p = 0.008), along with a slower decrease in fear expectancy (β = -0.022, p = 0.055). Furthermore, individuals with higher inhibition ability showed lower PFC activity in immediate extinction (ps < 0.0421), suggesting slower extinction learning but better regulation of safety cues. By clarifying the roles of these executive function components, our study highlights the cognitive mechanisms that could inform interventions aimed at improving fear extinction, offering potential strategies for mitigating anxiety-related disorders.
According to the World Health Organization (WHO) data, anxiety disorders ranked eighth in the global disease burden in 2019, significantly impacting societal health (Antony & Swinson, 1996; Javaid et al., 2023; World Health Organization, 2023). This indicates that understanding and addressing anxiety disorders is crucial for public health. Exposure therapy, based on the principle of fear extinction, is widely regarded as an effective treatment for anxiety disorders (Rauch et al., 2006; Vervliet et al., 2013). However, more than 50 % of patients do not benefit from this treatment (Craske et al., 2008; Craske et al., 2014). One reason for this poor efficacy is that many patients face difficulties during the process of fear extinction. Fear extinction refers to the process by which a conditioned fear response gradually weakens when a previously feared stimulus is repeatedly presented without any negative consequences (Bouton, 2002; Milad & Quirk, 2012; Pavlov, 1927). Therefore, studying the factors that lead to difficulties in fear extinction is important for understanding the etiology of anxiety disorders and may provide new insights and methods for improving clinical intervention strategies.
Fear extinction is a core process in the fear conditioning paradigm, a classical model for studying fear learning and its neural mechanisms (Pavlov, 1927). The fear conditioning paradigm consists of three key stages: fear acquisition, fear extinction, and fear reinstatement (Bouton & King, 1983; Milad & Quirk, 2012). During the fear acquisition phase, individuals repeatedly pair a neutral cue (conditioned stimulus, CS, e.g. geometric figure) with an unconditioned stimulus (US, e.g. shock), leading to the formation of a conditioned fear response. When the CS is later presented alone without the US, the conditioned fear response gradually diminishes, a process known as fear extinction (Bouton & Moody, 2004; LeDoux, 2000; Milad & Quirk, 2012; Pavlov, 1927). Importantly, fear extinction does not erase the original fear memory, but rather forms a new extinction memory, whereby the individual learns through a new process that the CS no longer predicts threat (Bouton, 1993; Bouton & King, 1983; Craske et al., 2014). In fear extinction, three important concepts need to be clarified: extinction learning, which refers to the immediate reduction in fear responses during extinction training; inhibitory learning, where individuals form a new safety association instead of forgetting or erasing the original CS-US association—this means that the old fear memory persists, but the individual can inhibit the fear response through cognitive regulation (Craske et al., 2014; Sewart & Craske, 2020); and extinction memory, which reflects the ability to retrieve and maintain the learned safety information in the future, preventing the return of fear responses (Vervliet et al., 2013). Although fear extinction occurs in most situations, extinction memory retrieval may fail in some specific contexts, leading to the reappearance of fear responses, a phenomenon known as fear reinstatement. When individuals experience traumatic stimuli or similar environments, the original fear memory may be reactivated, causing them to exhibit fear responses again (Bouton & King, 1983; Craske et al., 2008). Previous research indicates that individuals with anxiety disorders exhibit significant difficulties in fear extinction. The patients with anxiety disorders exhibited stronger conditioned fear responses and higher fear reinstatement compared with healthy controls, reflecting impairments in cognitive regulation and emotional control Craske et al., 2014; Dunsmoor & Paz, 2015; Johnson & Casey, 2015; Lissek & van Meurs, 2015; Milad & Quirk, 2012; Vervliet et al., 2013).
Fear extinction learning relies not only on emotional processing but also on cognitive function, particularly executive functions (EF) (Buhle et al., 2014; Hartley & Phelps, 2010; Milad & Quirk, 2012). EF is a set of high-order cognitive abilities that help individuals effectively plan, control, and regulate behavior in complex or novel situations (Burgess & Simons, 2005; Espy, 2004; Miller & Cohen, 2001; Vandierendonck, 2016). EF is essential for emotion regulation (Etkin et al., 2015; Zelazo & Cunningham, 2007) and is typically divided into three core components: shifting, updating, and inhibition (Anderson et al., 2001; Lehto et al., 2003; Miyake et al., 2000). Shifting, also known as task-switching, involves the ability to flexibly shift attention or switch between different tasks or mental sets. Impairment in shifting has been linked to difficulties in adjusting emotional responses, making it challenging for individuals to use adaptive emotion regulation strategies (Marceau et al., 2018; Mennies et al., 2021; Yang et al., 2017). Updating refers to the process of continuously monitoring and updating working memory with new information. It is essential to remove outdated or irrelevant information, particularly negative emotional content, which could otherwise interfere with fear extinction learning (Gustavson & Miyake, 2016; Roberts et al., 2021). Inhibition is the ability to suppress automatic or dominant responses, playing a critical role in preventing the intrusion of negative emotions and memories (Tang & Schmeichel, 2014; von Hippel & Gonsalkorale, 2005). These EF components are typically assessed using cognitive tasks such as the Stroop task (inhibition), the N-back task (updating), and task-switching paradigms (shifting) (Friedman & Miyake, 2017; Miyake et al., 2000). Based on these studies, executive function may facilitate fear extinction learning by enhancing individuals' selective attention to task-relevant information and inhibiting irrelevant emotional interference (Shields et al., 2016). Specifically, stronger shifting and inhibitory abilities may help individuals more effectively differentiate between safety and fear cues and inhibit automatic fear responses, while updating helps maintain and apply inhibitory memories, thereby enhancing the effect of fear extinction (Schweizer et al., 2011; Stout et al., 2018). Furthermore, inhibition plays a crucial role in this process by modifying memory representations to further facilitate the differentiation between safety and threat cues (Niederstrasser et al., 2017; Plas et al., 2024). In fear extinction learning, memories compete within an individual, with the new memory (CS-noUS, where there is no longer an association between the conditioned stimulus and the unconditioned stimulus) competing against the old memory (CS-US, where the original association between the conditioned stimulus and the unconditioned stimulus persists). Higher executive functions, particularly strong updating and switching abilities, enable individuals to more easily update and switch to the new memory, while inhibiting the old memory, thereby facilitating the success of fear extinction.
Understanding the neural basis linking executive function (EF) and fear extinction (FE) is crucial, as it can reveal how cognitive processes influence emotional regulation and inform targeted interventions for anxiety disorders. From a neural perspective, fear extinction learning primarily involves the amygdala, prefrontal cortex (PFC), and hippocampus (Maren et al., 2013; Quirk & Mueller, 2008). Animal studies have shown that the infralimbic region (IL) of the medial prefrontal cortex (mPFC), analogous to the ventromedial prefrontal cortex (vmPFC) in humans, is a key region for fear extinction (Lingawi et al., 2019). In human studies, activation of the dorsolateral prefrontal cortex (DLPFC) and vmPFC during fear extinction is closely related to the reduction of fear responses (Fullana et al., 2018). During the extinction recall phase, higher levels of vmPFC activation in response to extinction stimuli are positively correlated with better retention of extinction memory (Milad et al., 2007). In contrast, individuals with extinction recall failure often show insufficient activation of the medial prefrontal cortex (Marin et al., 2017; Suarez-Jimenez et al., 2020). The medial prefrontal cortex reduces fear responses by inhibiting amygdala activity (Marek et al., 2019). In individuals with anxiety disorders, extinction recall is often accompanied by weaker functional connectivity between the prefrontal cortex and amygdala (Gold et al., 2020). Additionally, studies have found that during fear extinction, functional coupling between the DLPFC and vmPFC jointly regulates amygdala activity, contributing to the reduction of fear responses (Hartley & Phelps, 2010). Neuromodulation of the DLPFC can further enhance the persistence of fear extinction (Deng et al., 2021).
Executive functions (EF) involve the activation of the prefrontal cortex (PFC) (Collette et al., 2006; Feng et al., 2022). The PFC is broadly associated with multiple EF components, such as shifting, updating, and inhibition. For example, during shifting tasks, both the prefrontal and parietal cortices show increased activation. Damage to the prefrontal cortex can result in higher shifting costs, highlighting the role of these regions in flexible cognitive control (Aron et al., 2004; Loose et al., 2017). Different subregions of the PFC may be functionally specialized. Specifically, increased shifting costs are linked to greater activation in the dorsolateral prefrontal cortex (DLPFC) and medial prefrontal cortex (mPFC), whereas ventromedial prefrontal cortex (vmPFC) activation tends to be lower (Wager et al., 2005).In tasks measuring updating function, such as the N-back task, lateral PFC activation increases in response to higher cognitive load (Mencarelli et al., 2019; Yaple et al., 2019). Material updating tasks that involve intuitive changes particularly engage the mPFC (Stöttinger et al., 2018). Inhibitory function is also closely tied to prefrontal cortex activity (Zheng et al., 2008; Zhang et al., 2017), as shown in the Go/No-Go task. For instance, individuals with obsessive-compulsive disorder (OCD) exhibit significantly reduced activation in the DLPFC compared to healthy controls (Page et al., 2009). A key area of interest is the interaction between the vmPFC and DLPFC, which is essential not only for general executive function tasks but also for processes such as fear extinction. This interaction suggests that the vmPFC and DLPFC may share common neural circuits, potentially influencing each other during cognitive tasks. Understanding these interactions could provide valuable insights into how individual differences in EF impact emotional learning, such as fear acquisition and extinction learning. However, while the role of EF in general cognitive tasks is well-established, its impact on fear acquisition—a key component of conditioned fear responses—remains less clear. Furthermore, it is important to distinguish between fear extinction (the reduction of a conditioned fear response over time) and extinction learning (the process by which an individual learns that a previously feared stimulus is now safe) (Furini et al., 2014; Myers & Davis, 2007; Wen et al., 2021). Both processes are influenced by the complex interplay of the vmPFC and DLPFC(Herry et al., 2010; Milad & Quirk, 2002; Sotres-Bayon et al., 2006), but individual differences in EF may alter the effectiveness of fear acquisition and extinction learning. This knowledge gap highlights the need for further research to clarify these relationships.
Therefore, this study aims to investigate the impact of individual executive functions (shifting, updating, and inhibition) on conditioned fear extinction and fear reinstatement, as well as their potential neural correlates. To further explore potential neural correlates, this study utilizes functional near-infrared spectroscopy (fNIRS) to monitor hemodynamic changes in the prefrontal cortex (PFC) (Hoshi et al., 2001), revealing how executive function regulates neural activity during fear extinction. In this study, participants completed the number size-parity shifting task, the active digit memory task, and the Stroop color-word interference task to assess their shifting, updating, and inhibition abilities. Subsequently, participants underwent the classical conditioned fear extinction paradigm (Milad & Quirk, 2012; Pare & Duvarci, 2012), during which their behavioral responses and PFC activity were recorded. Based on previous research (Milad & Quirk, 2012; Pezze & Feldon, 2004; Stout et al., 2018), we hypothesize that the executive function levels including shifting, updating, and inhibition will modulate the shock expectancy ratings and the oxygenated hemoglobin (Hbo) concentration in the prefrontal cortex (mPFC and DLPFC).
MethodParticipantsWe conducted a power analysis using the web app for "Effect Size Analysis for Mixed Effects Models Based on Summary Statistics" by Murayama et al. (2022). This post-hoc analysis utilized multilevel data from this study (N = 86) to examine the effects of the subcomponents of executive function (shifting, updating, inhibition) on conditional fear extinction and extinction reconsolidation (t = 3.037). Under the conditions of α = 0.05 and power = 0.80, the results indicated that 77 samples are needed to detect the effect with an 80 % probability. Therefore, to account for attrition and dropout between the study phases, the research plans to recruit 94 participants, with ages ranging from 17 to 23 years (M = 18.98, SD = 1.19). All participants were right-handed, had normal or corrected-to-normal vision, were free from color blindness or color weakness, and had no history of physical or psychiatric disorders. Before the experiment, participants read and signed informed consent forms. The experimental procedures were approved by the Ethics Committee of Sichuan Normal University (NO. SICNU-220118). Due to five participants failing to successfully acquire the conditioned fear response (Their CS+ shock expectancy rating is lower than or equal to the CS- rating), the final valid sample consisted of 88 participants (33 males). All participants received monetary compensation after completing the experiment. Before the experiment, participants completed the State-Trait Anxiety Inventory (STAI) and the Beck Depression Inventory-II (BDI-II), which were used to assess state anxiety, trait anxiety (Spielberger, 2010), and depressive symptoms (Beck et al., 1996). Table 1 presents the means (M) and standard deviations (SD) of age, BDI-II scores, STAI-State anxiety, and STAI-Trait anxiety for males, females, and overall samples in the experiment.
Demographic characteristics and psychometric results of participants.
The conditioned stimuli (CS) used in the experiment consisted of two circles of different sizes: a large circle with a diameter of 11 cm and a small circle with a diameter of 5 cm. To avoid potential material effects in the experiment, all participants were randomly assigned to ensure that each participant had different circles for CS+ and CS-. During the experiment, CS+ was paired with the US, while CS- was not paired with any shock.
The US was a 200-millisecond wrist shock, controlled by a constant-pressure electrical stimulation device (model: SXC-4A, Sanxia Technique Inc., China). The shock intensity was determined through individual adjustments before the experiment. Participants first received a series of electrical stimuli and rated the intensity of the shock using a visual analog scale from 1 to 10, where 1 indicated “extremely slight” and 10 indicated “very uncomfortable.” The final shock intensity was set at a self-rated score of 7, corresponding to “highly uncomfortable but not painful” (Dou et al., 2020, 2023; Haaker et al., 2013).
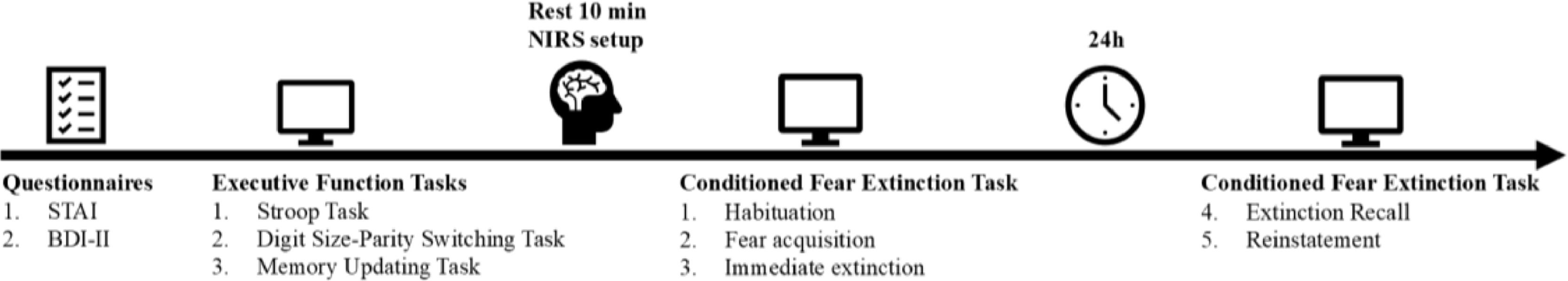
Experimental procedureAll participants arrived at the laboratory to complete a two-day experimental task(see Fig. 1). On the first day, participants first completed questionnaires, including the STAI and BDI-II. Then, they completed the executive function tasks in sequence (which included three sub-tasks, with task order counterbalanced across participants), lasting approximately 20 min. After completing the tasks, participants took a ten-minute break while the experimenter prepared and fitted them with the NIRS device cap. Once the NIRS device was set up and data recording began, all participants sequentially completed the habituation, fear acquisition, and extinction tasks. On the second day (24-hour interval), all participants returned to the laboratory to complete the extinction recall and reinstatement tasks. All experimental tasks were programmed using E-Prime 2.0 software, the specific details of each task are as follows.
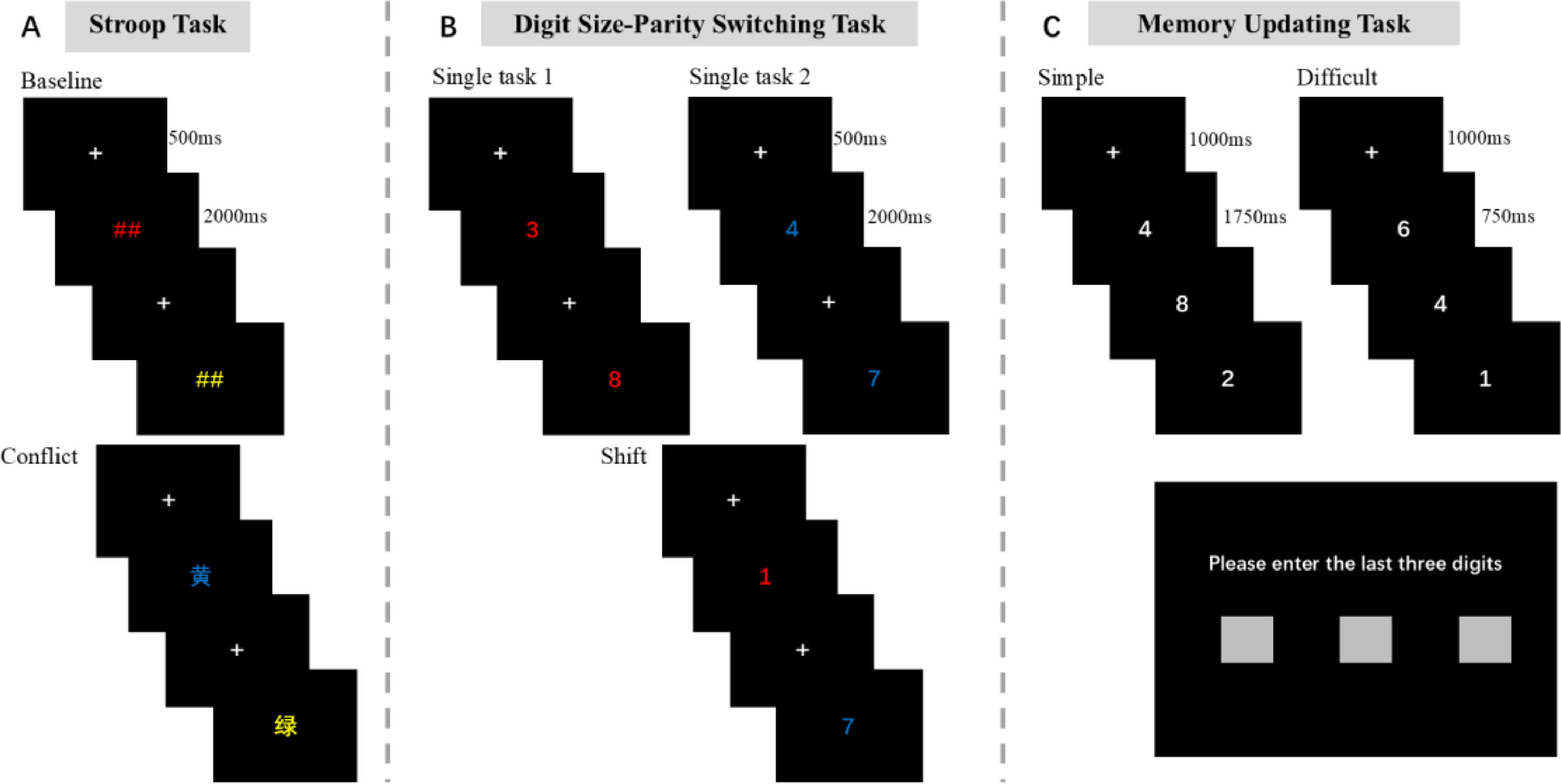
In this study, three subcomponents of executive function—shifting, updating, and inhibition—were measured using the digit size-parity switching task, the memory updating task, and the Stroop task (Chen & Li, 2005; Friedman et al., 2008; Xing et al., 2017) (see Fig. 2).
Executive Function Tasks. A Stroop Task: Inhibitory control is measured by the reaction time difference between the conflict task and the baseline task. A larger reaction time difference indicates weaker inhibitory control. The formal experiment consisted of 96 trials, divided into two baseline task groups and two conflict task groups. B Shifting ability is measured by the reaction time difference between the switch task and the single task (switch cost). Smaller reaction time differences indicate better-shifting ability. The formal experiment consists of 96 trials, including two single-task groups and two switch-task groups. C Active Digit Memory Task: The dependent variable is the number of correctly recalled digits; the more digits correctly recalled, the stronger the updating ability. The formal experiment includes two conditions (simple and difficult), with 16 trials for each condition.
Stroop Task (Stroop, 1935; van Veen & Carter, 2005): This task is used to measure inhibitory control, including baseline and conflict tasks. In the baseline task, the screen displays the “##” symbol in red, yellow, blue, or green, and participants are required to press the corresponding key based on the color (“D,” “F,” “J,” “K”). In the conflict task, inconsistent color-word pairs are displayed (e.g., the word "red" in blue font), and participants need to ignore the meaning of the word and only judge the font color. (see Fig. 2A).
Digit Size-Parity Switching Task (Bi et al., 2022; Gollan et al., 2014; Monsell, 2003): This task is used to measure shifting ability, with two conditions: single-task and switch-task. In the single-task condition, red digits require size judgment (greater than 5, press "A"; less than 5, press "L"), and blue digits require parity judgment (odd, press "A"; even, press "L"). In the switch-task condition, red and blue digits appear randomly, and participants need to switch between size and parity judgments based on color. (see Fig. 2B).
Memory Updating Task (Chen et al., 2002): This task is used to assess updating ability, with simple and difficult conditions. In the simple condition, each digit is displayed for 1.75 seconds; in the difficult condition, it is displayed for 0.75 seconds. Participants need to remember the last three digits in a series of randomly presented digits and recall them in order after the sequence ends. The digit sequences are 5, 7, 9, and 11 digits long, with each length presented four times. (see Fig. 2C).
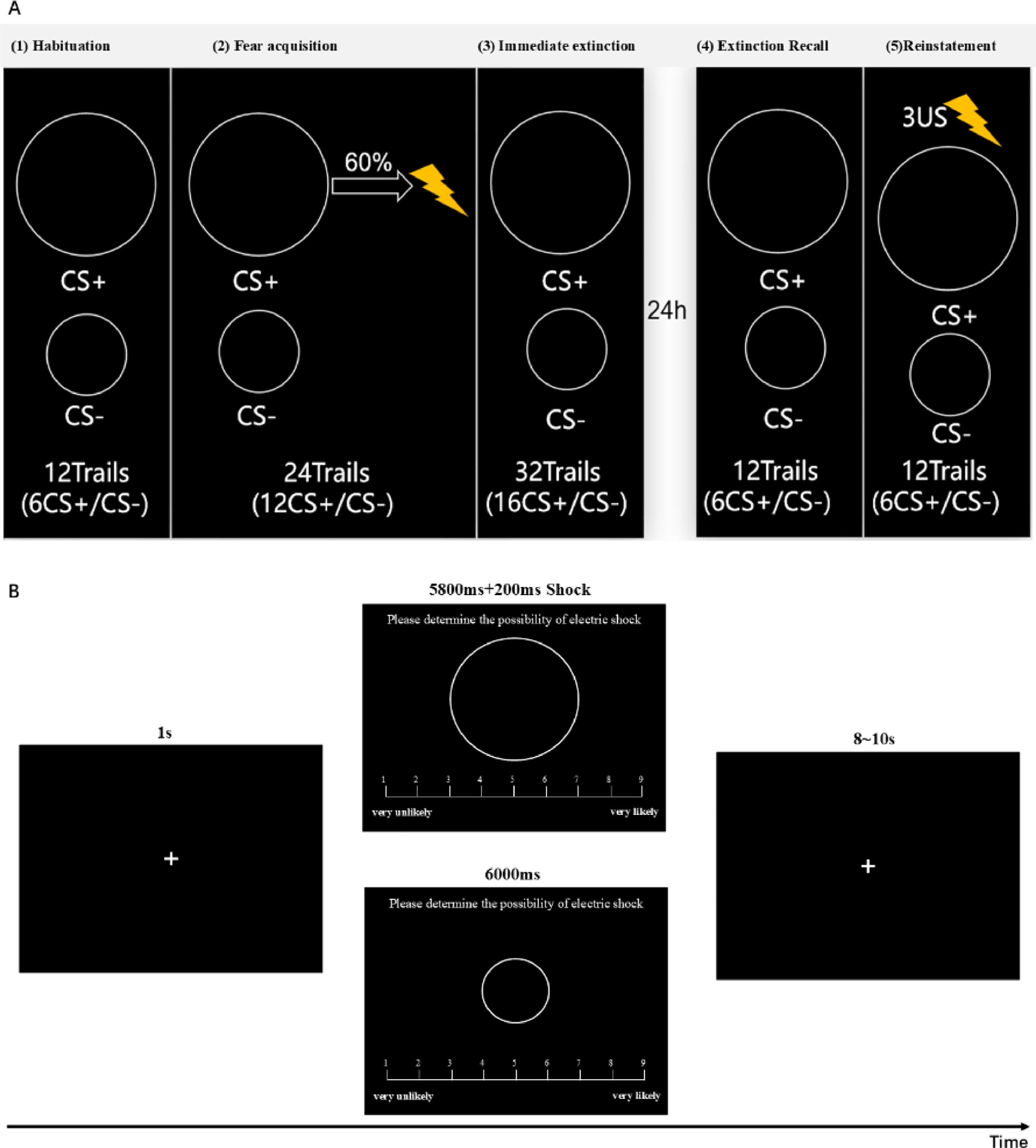
Conditioned fear extinction taskThe study employed a classic conditioned fear acquisition and extinction learning paradigm (Lonsdorf et al., 2017; Milad & Quirk, 2012; Phelps et al., 2004), completed over two days with a 24-hour interval. The first day included shock threshold testing, a Habituation phase, Fear Acquisition, and Immediate Extinction. On the second day, the experiment included a Delayed Extinction Recall test and a Reinstatement task. In the Habituation phase, CS+ and CS- were each presented six times, lasting 6 seconds each, with an interstimulus interval of 9 to 12 s, without any shock. During the subsequent Fear Acquisition phase, CS+ and CS- were each presented 12 times, with 60 % of CS+ paired with a shock that occurred within 200 ms before the end of the stimulus, while CS- was never paired with a shock. Following Fear Acquisition, participants entered the Immediate Extinction phase, where CS+ and CS- were each presented 16 times without any shock. In the Delayed Fear Recall test, CS+ and CS- were each presented six times without any shock, aimed at assessing participants' retention of extinction memory. In the Reinstatement task, after participants received three unexpected shocks, CS+ and CS- were each presented six times to observe the reinstatement of fear responses (see Fig. 3A). The experiment assessed individuals’ responses to fear acquisition and extinction using shock expectancy ratings. During the Acquisition, Immediate Extinction, Delayed Fear Recall, and Reinstatement phases, participants rated the likelihood of receiving a shock during each CS presentation on a scale from 1 to 9, where 1 indicated "very unlikely to receive a shock" and 9 indicated "very likely to receive a shock" (see Fig. 3B).
Conditioned Fear Extinction Task. A Five-phase conditioned fear paradigm with a 24-hour interval: (1) Habituation – 12 trials (6 CS+/6 CS-), no shock; (2) Fear Acquisition – 24 trials (12 CS+/12 CS-), 60 % CS+ paired with shock; (3) Immediate Extinction – 32 trials (16 CS+/16 CS-), no shock; (4) Extinction Recall – 12 trials (6 CS+/6 CS-), no shock; (5) Reinstatement – 3 unsignaled shocks followed by 12 trials (6 CS+/6 CS-), no shock. B Shock expectancy rating procedure: 1-second fixation, 6-second CS display with rating scale (1-9), followed by 8–10 s interval.
In this study, we constructed linear mixed-effects models (LMM) (Vanbrabant et al., 2015) for the dependent variable of unconditioned stimulus (US) expectancy ratings with a random intercept for participants across four experimental phases (acquisition, extinction, extinction recall, and reinstatement) and three executive function subcomponents (Shifting, updating, and inhibition). For example, in the acquisition stage, stimulus(i.e. CS+, CS-), trial, executive function as a continuous variable(z-score transformation for each subcomponent), and stimulus × trial × executive function interaction were included in the model and the dependent variable was expectancy ratings. The models in the rest stages were the same as those in the acquisition stage.
All analyses were conducted in RStudio (RStudio Team, 2016). Data processing utilized the tidyr (Wickham & Girlich, 2022) and tidyverse (Wickham et al., 2019) packages, statistical analysis employed lme4 (Bates et al., 2015) and lmerTest (Kuznetsova et al., 2017), and visualization was performed using ggplot2 (Wickham, 2016).
During data preprocessing, one participant from the updating group (Z=−4.66952) and one from the inhibition group (Z = 3.33503) were excluded as outliers based on Rosner’s test. In the acquisition phase, 88 participants were included (5 excluded due to failed acquisition), while 86 participants were retained for the extinction, extinction recall, and reinstatement phases (2 excluded for atypical extinction performance).
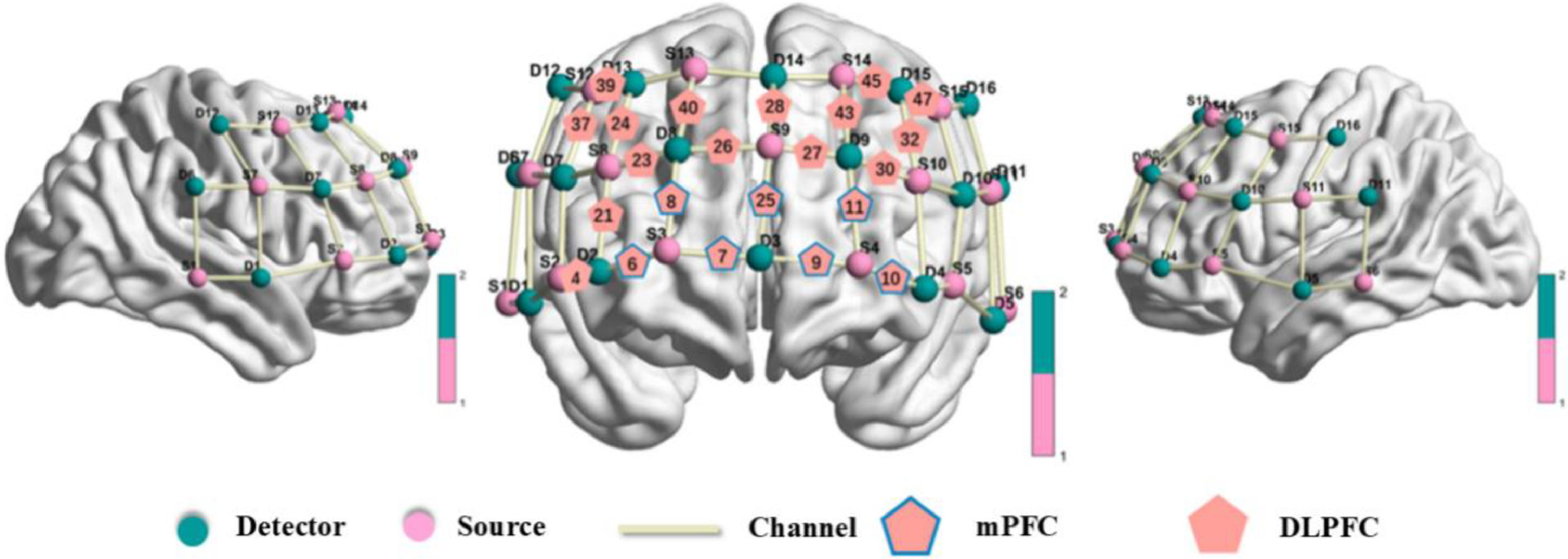
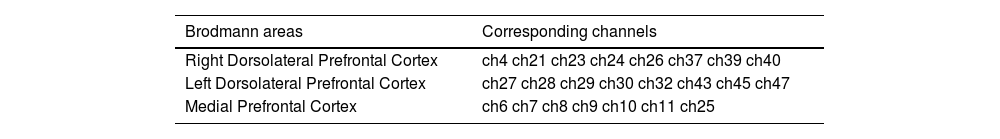
fNIRS recording and preprocessingThe study used a multi-channel functional near-infrared spectroscopy (fNIRS) system (NirScan, HuiChuang, China) to record brain activity. The optical probes were positioned according to the international 10-20 system, focusing on monitoring the bilateral dorsolateral prefrontal cortex (DLPFC) and medial prefrontal cortex (mPFC). The instrument configuration included 15 emitters and 16 detectors, forming 48 effective observation channels, with an emitter-detector distance of approximately 3 cm. The wavelengths of the light were 760 nm and 830 nm, and the sampling rate was 11 Hz. The brain regions corresponding to the channels were selected based on the Brodmann area reference table provided by the HuiChuang system (see Table 2 and Fig. 4). The observed brain regions were those with an area proportion greater than 40 % or the largest proportion.
Region of interest (ROI) in the prefrontal cortex (PFC). Red and green dots indicate detectors and sources, respectively, while the branches represent channels. The red channels indicate the regions of interest (ROI) in the medial prefrontal cortex (mPFC) and dorsolateral prefrontal cortex (DLPFC).
Data preprocessing was performed using the Homer2 toolbox (Huppert et al., 2009), which included light intensity detection, conversion to optical density, automatic removal, and correction of motion artifacts. A 0.01–0.5 Hz bandpass filter was applied to extract changes in oxygenated hemoglobin concentration (Keles et al., 2016). The time of interest was 1 to 5.5 s after stimulus onset, with a baseline of -2 to 0 s before stimulus onset. The activation value for each stimulus was calculated as the maximum activation value during the time of interest minus the baseline mean value.
Taking the dynamic changes of different brain regions into consideration, the trial level of HBO concentrations was added to the LMM models as the same as the behavioral data analysis. For example, in the acquisition stage, stimulus (i.e. CS+, CS-), trial, executive function as a continuous variable (z-score transformation for each subcomponent), and stimulus × trial × executive function interaction were included in the model with a random intercept for participants and the dependent variable was HBO concentrations of each channel (including mPFC, rDLPFC, and lDLPFC regions [23 ROI channels in total]). The models of the rest stages were the same as those in the acquisition stage. The functional connections between mPFC and DLPFC, and the relationships between principal component analysis (PCA) components and executive functions were attached in the supplementary materials.
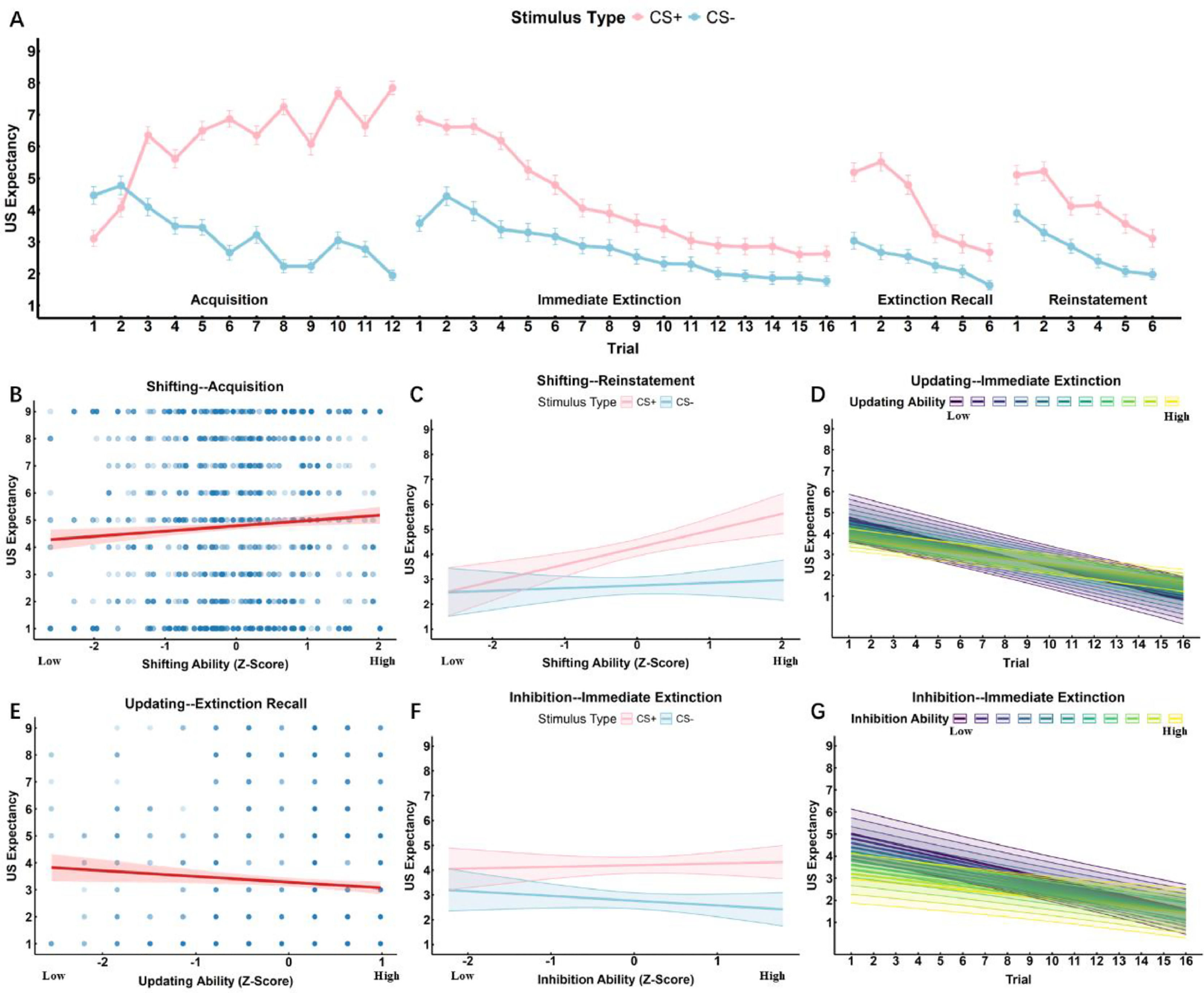
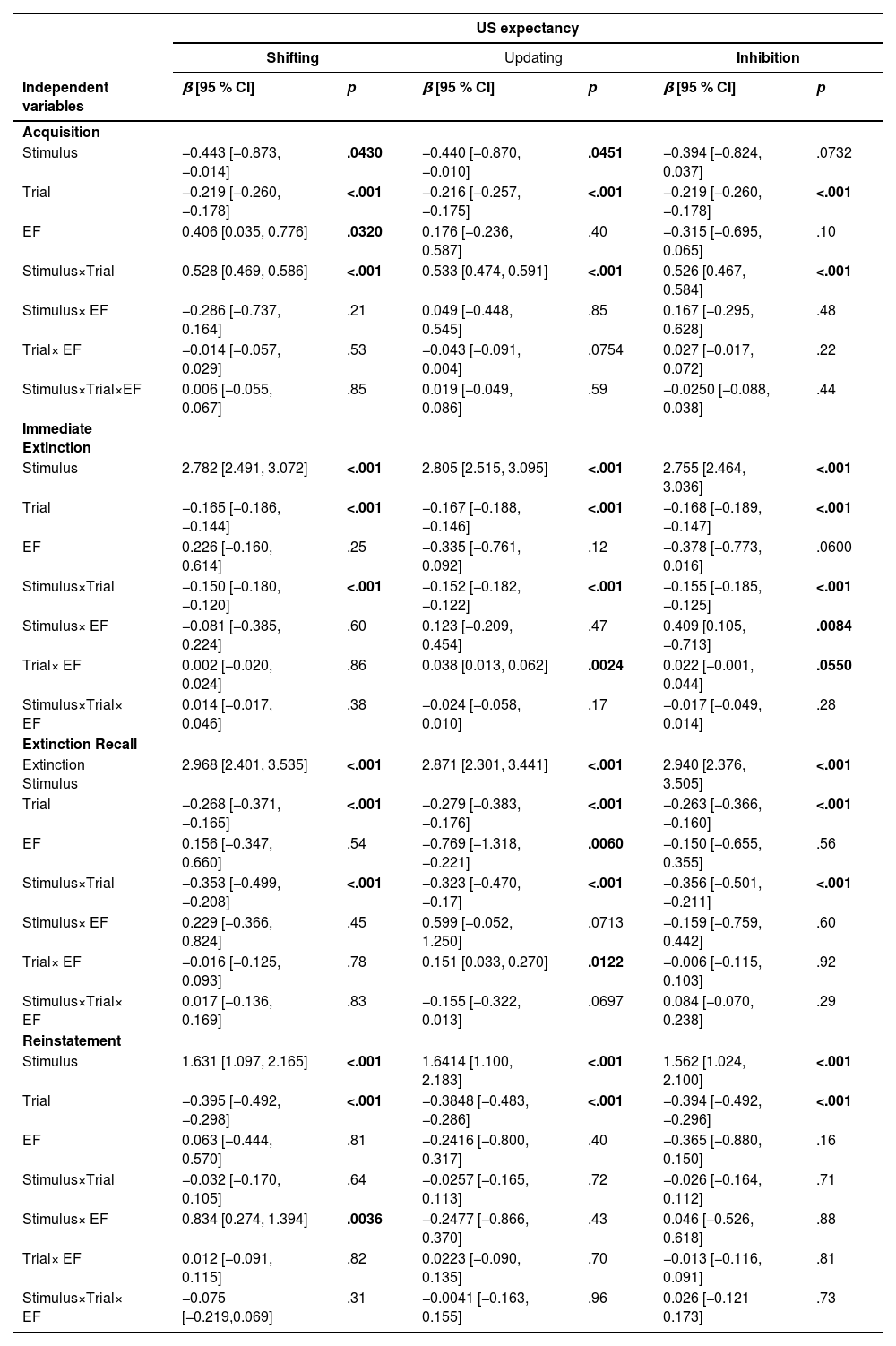
ResultsUS expectancy ratingsShifting functionAcquisition: Both Stimulus and Trial exhibited significant main effects on US expectancy ratings (β = -0.443, p = 0.043;β = -0.219, p < 0.001). Additionally, the main effect of shifting was significant (β = 0.406, p = 0.032), indicating that individuals with higher shifting ability had higher US expectancy ratings for electric shocks (see Fig. 5B). The interaction between stimulus type and trial was significant (β = 0.528, p < 0.001), showing that US expectancy ratings for CS+ gradually increased, whereas those for CS- gradually decreased (see Fig. 5A). However, the interactions of Stimulus × Shifting, Trial × Shifting, and Stimulus × Trial × Shifting did not reach statistical significance (see Table 3).
The effects of different trials and executive function subcomponents on US expectancy ratings. A Change in US expectancy ratings for CS+ and CS- across different phases: During the acquisition phase, US expectancy ratings for CS+ increased while those for CS- decreased. In the immediate extinction and extinction recall phases, US expectancy ratings for CS+ declined more rapidly. B Shifting function and US expectancy ratings: During the acquisition phase, individuals with lower shifting ability exhibited lower US expectancy ratings. C Shifting function in the reinstatement phase: Individuals with higher shifting ability had higher US expectancy ratings in the CS+ condition, indicating a greater degree of fear of reinstatement. D Updating function and the extinction: Individuals with higher updating ability showed a slower decline in US expectancy ratings, whereas those with lower updating ability exhibited faster extinction. E Updating function and extinction recall: Individuals with higher updating ability had lower overall US expectancy ratings, and their decline in ratings was slower. F Inhibition function and Stimulus: During the extinction phase, individuals with higher inhibition ability exhibited higher US expectancy ratings in the CS+ condition and lower ratings in the CS- condition. G Inhibition function and fear extinction: Individuals with lower inhibition ability showed a more rapid decline in US expectancy ratings.
Results of the linear mixed-effects model predicting US expectancy ratings across the acquisition, immediate extinction, extinction recall, and reinstatement phases.
Immediate Extinction: The main effects of Stimulus and Trial were significant (β = 2.784, p < 0.001; β = -0.165, p < 0.001). The interaction between Stimulus and Trial was significant (β = -0.150, p < 0.001), indicating that US expectancy ratings for both CS+ and CS- gradually decreased. However, with an increasing number of trials, the expectancy ratings for electric shocks in the CS+ condition declined more rapidly (see Fig. 5A). The main effect of Shifting, as well as the interactions of Stimulus × Shifting, Trial × Shifting, and Stimulus × Trial × Shifting, were not significant (see Table 3).
Extinction Recall: Both Stimulus and Trial exhibited significant main effects (β = 2.968, p < 0.001; β = -0.268, p < 0.001). Additionally, the interaction between Stimulus and Trial was significant (β = -0.353, p < 0.001), indicating that the expectancy ratings for electric shocks in both CS+ and CS- conditions showed an overall decreasing trend, but the decline was more rapid for CS+ (see Fig. 5A). However, the main effect of Shifting, as well as the interactions of Stimulus × Shifting, Trial × Shifting, and Stimulus × Trial × Shifting, did not reach statistical significance (see Table 3).
Reinstatement: Both Stimulus and Trial exhibited significant main effects on US expectancy ratings (β = 1.631, p < 0.001; β = -0.395, p < 0.001). Additionally, the interaction between Stimulus and Shifting was significant (β = 0.834, p = 0.004), indicating that individuals with higher shifting ability had higher US expectancy ratings in the CS+ condition, suggesting a greater degree of fear reinstatement related to CS+ (see Fig. 5C). However, the main effect of Shifting, as well as the interactions of Stimulus × Trial, Trial × Shifting, and Stimulus × Trial × Shifting, did not reach statistical significance (see Table 3).
Updating functionAcquisition: Both Stimulus and Trial exhibited significant main effects on US expectancy ratings (β = -0.440, p = 0.045; β = -0.216, p < 0.001). Additionally, the interaction between Stimulus and Trial was significant (β = 0.533, p < 0.001), showing that US expectancy ratings for CS+ gradually increased, while those for CS- gradually decreased (see Fig. 5A). The interaction between Trial and Updating approached significance (β = -0.043, p = 0.075). However, the main effect of Updating, as well as the interactions of Stimulus × Updating, Trial × Updating, and Stimulus × Trial × Updating, did not reach statistical significance (see Table 3).
Immediate Extinction: Both Stimulus and Trial exhibited significant main effects (β = 2.805, p < 0.001; β = -0.167, p < 0.001). Additionally, the interaction between Stimulus and Trial was significant (β = -0.152, p < 0.001), indicating that US expectancy ratings for both CS+ and CS- decreased with increasing trials, but the decline was more rapid for CS+ (see Fig. 5A). The interaction between Trial and Updating was significant (β = 0.038, p = 0.002), suggesting that individuals with higher updating ability showed a slower decline in US expectancy ratings, whereas those with lower updating ability exhibited faster fear extinction (see Fig. 5D). However, the main effect of Updating, as well as the interactions of Stimulus × Updating and Stimulus × Trial × Updating, were not significant (see Table 3).
Extinction Recall: Both Stimulus and Trial exhibited significant main effects (β = 2.871, p < 0.001; β = -0.279, p < 0.001). Additionally, the main effect of Updating was significant (β = -0.769, p = 0.006), indicating that higher updating ability was associated with lower overall US expectancy ratings (see Fig. 5E). The interaction between Stimulus and Trial was significant (β = -0.323, p < 0.001), showing that fear ratings for CS+ declined more rapidly with increasing trials (see Fig. 5A). The interaction between Trial and Updating was also significant (β = 0.151, p = 0.012), suggesting that individuals with higher updating ability exhibited a slower decline in US expectancy ratings across trials. Furthermore, the interaction between Stimulus and Updating approached significance (β = 0.599, p = 0.071), and the three-way interaction of Stimulus × Trial × Updating also demonstrated a marginally significant effect (β = -0.155, p = 0.070) (see Table 3).
Reinstatement: Both Stimulus and Trial exhibited significant main effects on US expectancy ratings (β = 1.641, p < 0.001; β = -0.385, p < 0.001). However, the main effects of Trial and Updating, as well as the interactions of Stimulus × Trial, Stimulus × Updating, Trial × Updating, and Stimulus × Trial × Updating, did not reach statistical significance (see Table 3).
Inhibitory functionAcquisition: The main effect of Stimulus on US expectancy ratings approached significance (β = -0.394, p = 0.073), while the main effect of Trial was significant (β = -0.219, p < 0.001). Additionally, the interaction between Stimulus and Trial was significant (β = 0.526, p < 0.001), indicating that US expectancy ratings for CS+ gradually increased, whereas those for CS- gradually decreased (see Fig. 5A). However, the main effect of Inhibition, as well as the interactions of Stimulus × Inhibition, Trial × Inhibition, and Stimulus × Trial × Inhibition, were not significant (see Table 3).
Immediate Extinction: Both Stimulus and Trial exhibited significant main effects (β = 2.750, p < 0.001; β = -0.168, p < 0.001). Additionally, the interaction between Stimulus and Trial was significant (β = -0.155, p < 0.001), indicating that US expectancy ratings for CS+ gradually decreased with increasing trials, whereas the decline in US expectancy ratings for CS- was more stable (see Fig. 5A). The interaction between Stimulus and Inhibition was significant (β = 0.409, p = 0.008), suggesting that individuals with higher inhibition function maintained higher US expectancy ratings in the CS+ condition but exhibited lower US expectancy ratings in the CS- condition (see Fig. 5F). Furthermore, the interaction between Trial and Inhibition approached significance (β = 0.022, p = 0.055), indicating that individuals with lower inhibition ability showed a faster decline in US expectancy ratings across trials (see Fig. 5G). The main effect of Inhibition also approached significance (β =-0.378, p = 0.060). However, the three-way interaction of Stimulus × Trial × Inhibition did not reach statistical significance (see Table 3).
Extinction Recall: Both Stimulus and Trial exhibited significant main effects (β = 2.940, p < 0.001; β = -0.263, p < 0.001). Additionally, the interaction between Stimulus and Trial was significant (β = -0.356, p < 0.001), indicating that with increasing trials, the fear expectancy ratings for CS+ declined faster than those for CS- (see Fig. 5A). However, the main effect of Inhibition, as well as the interactions of Stimulus × Inhibition, Trial × Inhibition, and Stimulus × Trial × Inhibition, did not reach statistical significance (see Table 3).
Reinstatement: Both Stimulus and Trial exhibited significant main effects (β = 1.562, p < 0.001; β = -0.394, p < 0.001). However, the main effect of Inhibition, as well as the interactions of Stimulus × Trial, Stimulus × Inhibition, Trial × Inhibition, and Stimulus × Trial × Inhibition, did not reach statistical significance (see Table 3).
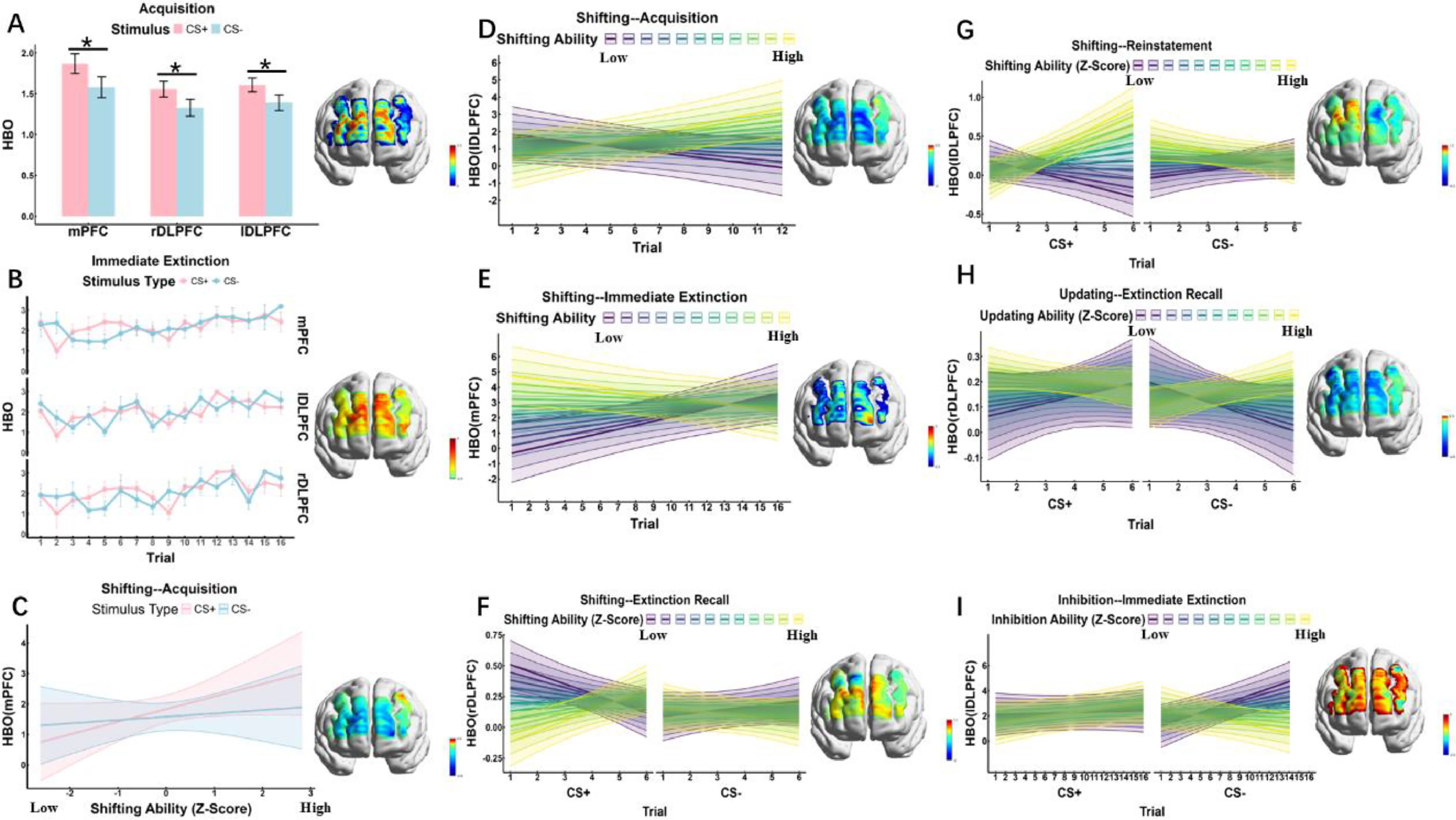
fNIRS resultsShifting functionAcquisition In the mPFC (channels 6, 7, 8, 9, 11, 25), rDLPFC (channels 21, 23, 24, 26, 39, 40), and lDLPFC (channels 27, 28, 30, 43, 45), the main effect of Stimulus was significant (ps < 0.0497), with CS+ eliciting significantly greater activity than CS- (see Fig. 6A). In the rDLPFC (channels 23, 39) and lDLPFC (channels 28, 30, 43, 45, 47), the main effect of Trial was significant (ps < 0.0407). Additionally, the interaction between Stimulus and Trial was significant (ps < 0.0312) in the mPFC (channels 6, 8, 9), rDLPFC (channels 21, 23, 24, 26, 39), and lDLPFC (channels 27, 28, 43, 45). In the mPFC (channels 8, 10), the interaction between Stimulus and Shifting was significant (ps < 0.0452), specifically showing that for CS+ stimuli, individuals with high shifting ability exhibited significantly greater activity in the mPFC and DLPFC compared to those with low shifting ability (see Fig. 6C). Furthermore, the interaction between Trial and Shifting was significant (ps < 0.0397) in the mPFC (channels 10, 11, 25), rDLPFC (channels 26, 39), and lDLPFC (channel 27), indicating that individuals with higher shifting ability showed greater activity intensity in the mPFC and DLPFC as trials progressed (see Fig. 6D). In the rDLPFC (channel 4), the three-way interaction of Stimulus × Trial × Shifting was significant (p = 0.022), though the main effect of Shifting was not significant.
fNIRS results across experimental phases. HbO activity in mPFC, rDLPFC, and lDLPFC during acquisition, with CS+ showing greater activity than CS-. B Time course of HbO changes during immediate extinction across brain regions. C Shifting ability modulates HbO activity in mPFC during acquisition. D Trial-based changes in rDLPFC HbO activity as a function of shifting ability during acquisition. E Effects of shifting ability on HbO activity during immediate extinction. F Influence of shifting ability on extinction recall, showing differential activity patterns in rDLPFC and lDLPFC.G Shifting ability modulates HbO activity during reinstatement, with high-shifting individuals exhibiting distinct activity patterns. H Updating ability modulates extinction recall, particularly in rDLPFC. Inhibitory control influenced HbO activity during the immediate extinction phase, with individuals exhibiting distinct neural activation patterns based on their levels of inhibitory ability.
Immediate Extinction In the mPFC (channels 8, 11, 25), rDLPFC (channel 26), and lDLPFC (channels 28, 29, 43, 45), the main effect of Trial was significant (ps < 0.0170), indicating that as trials progressed, both CS+ and CS-induced increased activity in the mPFC, rDLPFC, and lDLPFC (see Fig. 6B). In the mPFC (channels 6, 7, 9, 10, 11) and lDLPFC (channels 27, 45), the main effect of Shifting was significant (ps < 0.0396). Additionally, in the rDLPFC (channel 4), the interaction between Stimulus and Trial was significant (p = 0.0394). In the mPFC (channels 6, 9, 10, 11), rDLPFC (channel 26), and lDLPFC (channels 27, 28, 45), the interaction between Trial and Shifting was significant (ps < 0.0454), showing that in individuals with higher shifting ability, activity in the mPFC and DLPFC gradually decreased as trials progressed (see Fig. 6E). Furthermore, the main effect of Stimulus, the interaction between Stimulus and Shifting, and the three-way interaction of Stimulus × Trial × Shifting did not reach statistical significance.
Extinction Recall In the rDLPFC (channel 23), the main effect of Stimulus was significant (p = 0.0282). In the mPFC (channels 7, 10, 25), rDLPFC (channels 21, 23, 26, 37), and lDLPFC (channel 30), the interaction between Stimulus and Shifting was significant (ps < 0.0465). Furthermore, in the mPFC (channel 10), rDLPFC (channels 23, 36), and lDLPFC (channel 30), the three-way interaction of Stimulus × Trial × Shifting was significant (ps < 0.0345). Specifically, for CS+ stimuli, mPFC and DLPFC activation in individuals with low shifting ability gradually decreased as trials progressed. In contrast, for CS− stimuli, no significant difference in mPFC and DLPFC activation was observed between individuals with high and low shifting ability (see Fig. 6F). In addition, the main effects of Trial and Shifting, as well as the interactions of Stimulus × Trial and Trial × Shifting, did not reach statistical significance.
Reinstatement In the rDLPFC (channels 23, 24, 37, 40) and lDLPFC (channels 29, 30, 32, 43), the main effect of Stimulus was significant (ps < 0.0218). Additionally, in the mPFC (channel 10) and lDLPFC (channel 28), the main effect of the Trial was significant (ps < 0.0327). In the mPFC (channel 6) and lDLPFC (channels 29, 43, 45), the main effect of Shifting was significant (ps < 0.0357). In the rDLPFC (channels 23, 37) and lDLPFC (channels 27, 29, 30, 32, 43), the interaction between Stimulus and Trial was significant (ps < 0.0443). In the lDLPFC (channels 27, 43), the interaction between Stimulus and Shifting was significant (ps < 0.0032). In the mPFC (channel 6) and lDLPFC (channels 29, 43), the interaction between Trial and Shifting was significant (ps < 0.0407). In the mPFC (channel 11) and lDLPFC (channels 27, 30, 32, 43), the interaction between Trial and Shifting was significant (ps < 0.0308). Specifically, for CS+ stimuli, individuals with higher shifting ability exhibited stronger activation in the medial prefrontal cortex (mPFC) and the left dorsolateral prefrontal cortex (lDLPFC). In contrast, for CS− stimuli, individuals with higher shifting ability showed reduced activation in these regions (see Fig. 6G).
Updating functionAcquisition In the mPFC (channels 6, 7, 8, 9, 11, 25), rDLPFC (channels 21, 23, 24, 26, 39, 40), and lDLPFC (channels 27, 28, 30, 43, 45), the main effect of Stimulus was significant (ps < 0.0496), indicating that CS+ elicited significantly greater activity than CS- (see Fig. 6A). In the rDLPFC (channels 23, 39) and lDLPFC (channels 28, 30, 43, 45, 47), the main effect of Trial was significant (p = 0.0406). Additionally, in the mPFC (channels 6, 8, 9), rDLPFC (channels 21, 23, 24, 26, 39), and lDLPFC (channels 27, 28, 43, 45), the interaction between Stimulus and Trial was significant (ps < 0.0311). In the rDLPFC (channel 39), the interaction between Trial and Updating was significant (p = 0.0196). However, the main effect of Updating, the interaction between Stimulus and Updating, and the three-way interaction of Stimulus × Trial × Updating did not reach statistical significance.
Immediate Extinction In the mPFC (channels 8, 11, 25), rDLPFC (channel 26), and lDLPFC (channels 28, 29, 43, 45), the main effect of Trial was significant (ps < 0.0146), indicating that as trials progressed, both CS+ and CS-induced increased activity in the mPFC, rDLPFC, and lDLPFC (see Fig. 6B). Additionally, in the rDLPFC (channel 4), the interaction between Stimulus and Trial was significant (p = 0.0387). However, the main effects of Stimulus and Updating, as well as the interactions of Stimulus × Updating, Trial × Updating, and Updating × Trial × Shifting, did not reach statistical significance.
Extinction Recall In the rDLPFC (channel 23), the main effect of Stimulus was significant (p = 0.0284). In the rDLPFC (channel 37), the main effect of Updating was significant (p = 0.0119). Additionally, in the rDLPFC (channels 23, 37, 39) and lDLPFC (channel 45), the interaction between Stimulus and Updating was significant (ps < 0.0485). In the rDLPFC (channel 37), the interaction between Trial and Updating was significant (p = 0.0068). Furthermore, in the rDLPFC (channels 21, 23, 24, 37, 39) and lDLPFC (channel 28), the three-way interaction of Stimulus × Trial × Updating was significant (ps < 0.0458). Specifically, for the CS− stimulus, individuals with high updating ability exhibited significantly greater DLPFC activation compared to those with low updating ability; whereas for the CS+ stimulus, individuals with high updating ability showed lower DLPFC activation (see Fig. 6H). However, the main effect of the Trial and the interaction between Stimulus and Trial did not reach statistical significance.
Reinstatement In the rDLPFC (channels 23, 24, 37, 40) and lDLPFC (channels 29, 30, 32, 43), the main effect of Stimulus was significant (ps < 0.0215). Additionally, in the mPFC (channel 10) and lDLPFC (channel 28), the main effect of the Trial was significant (ps < 0.0332). In the rDLPFC (channels 23, 37) and lDLPFC (channels 27, 29, 30, 32, 43), the interaction between Stimulus and Trial was significant (ps < 0.0452). In the lDLPFC (channel 27), the three-way interaction of Updating × Trial × Shifting was significant (p = 0.0285). However, the main effect of the Trial, as well as the interactions of Stimulus × Updating and Trial × Updating, did not reach statistical significance.
Inhibitory functionAcquisition In the mPFC (channels 6, 7, 8, 9, 11, 25), rDLPFC (channels 21, 23, 24, 26, 39, 40), and lDLPFC (channels 27, 28, 30, 43, 45), the main effect of Stimulus was significant (ps < 0.0426), indicating that CS+ elicited significantly greater activity than CS- (see Fig. 6A). In the rDLPFC (channels 23, 39) and lDLPFC (channels 28, 30, 43, 45, 47), the main effect of Trial was significant (ps < 0.0405). Additionally, in the mPFC (channels 6, 8, 9), rDLPFC (channels 21, 23, 24, 26, 39), and lDLPFC (channels 27, 28, 43, 45), the interaction between Stimulus and Trial was significant (ps < 0.0312). In the mPFC (channel 7) and rDLPFC (channel 4), the interaction between Stimulus and Inhibitory was significant (ps < 0.0269). Furthermore, in the rDLPFC (channel 40) and lDLPFC (channel 30), the interaction between Trial and Inhibitory was significant (ps < 0.0389). In the mPFC (channel 7), the three-way interaction of Inhibitory × Trial × Shifting was significant (p = 0.0356). However, the main effect of Inhibitory did not reach statistical significance.
Immediate Extinction In the mPFC (channels 8, 11, 25), rDLPFC (channel 26), and lDLPFC (channels 28, 29, 43, 45), the main effect of Trial was significant (ps < 0.0132), indicating that as trials progressed, both CS+ and CS-induced increased activity in the mPFC, rDLPFC, and lDLPFC (see Fig. 6B). Additionally, in the rDLPFC (channel 4), the interaction between Stimulus and Trial was significant (p = 0.040). In the lDLPFC (channels 45, 47), the interaction between Trial and Inhibitory was significant (ps < 0.0068). In the mPFC (channel 6), rDLPFC (channel 24), and lDLPFC (channels 29, 45, 47), the three-way interaction of Stimulus × Trial × Inhibitory was significant (ps < 0.0421). Specifically, for CS-, individuals with high inhibitory ability exhibited reduced activity in them and DLPFC, whereas individuals with low inhibitory ability showed increased activity in these regions. However, for the CS+ stimulus, no significant differences in activation levels were observed between individuals with high and low inhibitory ability (see Fig. 6I). However, the main effects of Stimulus and Inhibitory, as well as the interaction between Stimulus and Updating, did not reach statistical significance.
Extinction Recall In the rDLPFC (channel 23), the main effect of Stimulus was significant (p = 0.0358). In the rDLPFC (channel 40), the interaction between Trial and Inhibitory was significant (p = 0.0090). However, the main effects of Trial and Inhibitory, as well as the interactions of Stimulus × Trial, Stimulus × Inhibitory, and the three-way interaction of Stimulus × Trial × Inhibitory, did not reach statistical significance.
Reinstatement In the rDLPFC (channels 23, 24, 37, 40) and lDLPFC (channels 29, 30, 32, 43), the main effect of Stimulus was significant (ps < 0.0164). Additionally, in the mPFC (channel 10) and lDLPFC (channel 28), the main effect of the Trial was significant (ps < 0.0326). In the rDLPFC (channels 23, 37) and lDLPFC (channels 27, 29, 30, 32, 43), the interaction between Stimulus and Trial was significant (ps < 0.0244). In the rDLPFC (channel 40), the main effect of inhibition was significant (p = 0.0172). However, the interactions of Stimulus × Inhibitory, Trial × Inhibitory, and the three-way interaction of Stimulus × Trial × Inhibitory did not reach statistical significance.
DiscussionWe investigated the impacts of executive function on fear extinction, reinstatement, and their neural correlates. The main findings indicate that different subcomponents of executive functions play a crucial role in modulating conditional fear extinction and reconsolidation. Our study further extends previous work, demonstrating that different subcomponents of executive functions regulate fear extinction and reconsolidation (Maren et al., 2013; Miyake et al., 2000; Raio et al., 2013; Schwabe & Wolf, 2013; Zarahn et al., 2007). Specifically:
Regarding shifting ability, our study found that individuals with higher shifting ability exhibited higher US expectancy ratings during the fear acquisition phase, indicating higher sensitivity to threatening stimuli. However, during the fear extinction and extinction recall phases, shifting ability did not significantly affect fear expectancy ratings, suggesting that individuals with higher shifting ability did not exhibit superior extinction performance in these phases. Notably, in the fear reinstatement phase, individuals with higher shifting ability exhibited stronger fear responses in the CS+ condition, suggesting heightened sensitivity to threatening stimuli in fear acquisition may lead to greater fear reinstatement. This finding aligns with previous research on the dual role of shifting ability: on one hand, individual differences in shifting ability are linked to adaptive regulation, with higher shifting ability facilitating quicker adjustments in emotional and cognitive strategies (Dajani & Uddin, 2015; Friedman & Miyake, 2017; Ionescu, 2012; Karbach & Kray, 2009). On the other hand, it may also heighten sensitivity to contextual changes in threatening environments, making individuals more prone to reinstating previous fear memories upon re-exposure to fear stimuli (Aldao & Nolen-Hoeksema, 2012; Kashdan & Rottenberg, 2010). The fNIRS results of this study further support this perspective. During the fear acquisition phase, individuals with higher shifting ability exhibited greater activity in the mPFC and DLPFC. The mPFC and DLPFC are related to fear discrimination function in the fear acquisition stage (Dou et al., 2020, Furlong et al., 2010; Kroes et al., 2019). Thus, the heightened mPFC and DLPFC for the individuals with higher shifting ability in our study might reflect the increased threatening discrimination to tCS. During the immediate extinction phase, individuals with higher shifting ability showed a gradual decrease in mPFC and DLPFC as trials progressed, which might indicate that extinction learning was reduced. This is because the trend of PFC activity was reversed compared with the trend of those we found in immediate extinction. More specifically, we found the mPFC and DLPFC activity were increased with trial increases, which is consistent with previous findings that the mPFC and DLPFC are involved in extinction learning (Delgado et al., 2006; Holmes et al., 2012; Milad et al., 2004). However, during the extinction recall phase, individuals with higher shifting ability exhibited a gradual increase in mPFC and DLPFC activity as trials progressed. This suggests that the old fear memory was reactivated during extinction recall, prompting these individuals to recruit more prefrontal cognitive resources for emotion regulation and inhibition of fear responses, thereby preventing fear relapse (Buhle et al., 2014; Schiller et al., 2008). During the fear reinstatement phase, individuals with higher shifting ability exhibited significantly greater activity in the mPFC and lDLPFC under CS+ conditions, consistent with the behavioral findings, suggesting that these individuals are more prone to fear recovery. This phenomenon may stem from heightened threat sensitivity to environmental changes, making individuals more likely to activate fear memories when facing potential threats (Schiller et al., 2010).
Regarding updating ability, we found that individuals with higher updating ability showed a slower decline in US expectancy during immediate extinction but exhibited overall lower ratings during the extinction recall phase. This suggests that individuals with high updating ability had poorer extinction learning but better extinction memory retention. Our findings are partially consistent with those of Stout et al. (2018), as both studies observed a positive effect of high updating ability on the fear extinction process. Stout et al. reported that individuals with higher working memory capacity exhibited stronger fear response inhibition during extinction learning, particularly in the later stages, reflecting better extinction learning outcomes. Similarly, our study found that individuals with higher updating ability demonstrated better extinction memory, as indicated by lower US expectancy ratings. However, it is worth noting that the two studies yielded different results regarding the extinction learning phase, possibly due to differences in task paradigms and measurement approaches. Stout et al. employed the fear-potentiated startle (FPS) paradigm, using physiological responses (startle reflex) as the primary measure, focusing on the gradual reduction of fear responses during extinction. In contrast, our study used US expectancy ratings as the behavioral measure, focusing on changes in individuals' subjective expectations of aversive outcomes. Additionally, the two studies differed in how they measured "extinction learning." Stout et al. used the magnitude of response reduction across extinction blocks as the core indicator, whereas we primarily focused on the rate of US expectancy decline. These methodological differences may account for the inconsistent findings regarding the effect of updating ability on fear extinction learning. At the neural level, individuals with high and low updating abilities exhibited distinct patterns, showing opposite activity characteristics in brain regions. Specifically, individuals with low updating ability showed higher activity in the DLPFC under the CS+ condition but lower activity under the CS− condition. This neural pattern may indicate that individuals with low updating ability struggle to effectively inhibit old fear memories during extinction recall, maintaining heightened attention toward threat cues. In contrast, individuals with high updating ability exhibited the opposite pattern, showing more effective activity of brain regions associated with safety information processing, thereby facilitating the encoding and retrieval of safety cues (Schweizer et al., 2013). Specifically, stronger activity in the DLPFC, particularly in the rDLPFC and lDLPFC, may be associated with enhanced cognitive control capacity, enabling individuals to flexibly regulate emotional responses. This ability allows them to rapidly suppress unnecessary fear responses under the CS- condition and reduce erroneous threat appraisals under the CS+ condition (Buhle et al., 2014; Etkin et al., 2015; Giustino & Maren, 2015; Kohn et al., 2014). Additionally, this result aligns with Hartley and Phelps (2010), who suggested that updating ability may play an important role in regulating emotional responses. The current study extends this perspective, suggesting that individuals with higher updating ability may exhibit stronger inhibitory learning and extinction memory retention abilities.
Regarding inhibitory ability, our study found that individuals with higher inhibition ability exhibited a greater difference in US expectancy ratings between CS+ and CS− during the immediate extinction phase, along with a slower decline in US expectancy ratings. This result suggests that individuals with high inhibition ability may face certain difficulties during fear extinction learning. However, during the immediate extinction phase, individuals with high inhibitory ability showed significantly lower US expectancy ratings under the CS− condition compared to those with low inhibitory ability. Additionally, the main effect of inhibitory ability approached significance (p = 0.055), with high inhibitory individuals exhibiting overall lower US expectancy ratings than low inhibitory individuals. These findings suggest that individuals with higher inhibitory ability may be better able to regulate emotional responses when faced with safety cues (CS−) (Geisler & Schröder-Abé, 2015), potentially more accurately differentiate between threat and non-threat information (Tang & Schmeichel, 2014), and possibly exhibit advantages in recognizing and maintaining safety signals. In contrast, individuals with lower inhibition ability showed a faster decline in US expectancy ratings during extinction, possibly reflecting greater sensitivity to environmental changes (e.g., CS+ no longer being paired with the US), allowing them to adjust fear responses more rapidly and thereby facilitating extinction learning. Neuroimaging results further supported these behavioral characteristics. Since this result is close to the significance level (p = 0.055), we believe it should be interpreted with caution and needs further validation in future research. During the extinction recall phase, individuals with high inhibitory ability exhibited significantly lower prefrontal cortex activation under the CS− condition compared to those with low inhibitory ability. This result may indicate that individuals with higher inhibition ability experienced more complete extinction of CS− during the immediate extinction phase, leading to reduced prefrontal activity for CS− during recall. In contrast, individuals with lower inhibition ability showed stronger prefrontal activity under the CS− condition, suggesting that the extinction of CS− was still ongoing, consistent with their faster behavioral adjustment and better extinction learning performance. Overall, the impact of inhibition ability on fear extinction appears to be complex and warrants further investigation in future studies.
Theoretically, the Integrated Model of Executive Function and Emotion Regulation may provide a comprehensive explanation for the findings of this study. This model posits that executive function and emotion regulation are interrelated cognitive processes, with their core neural foundation located in the prefrontal cortex (Hofmann et al., 2012; Ochsner & Gross, 2005; Zelazo & Cunningham, 2007). Specifically, inhibitory control may help individuals regulate automatic fear responses and reduce excessive reactions to unnecessary threat signals. Shifting ability facilitates flexible adjustment between threat and safety cues, enhancing adaptability. Updating ability is involved in integrating and updating safety memory representations to promote long-term fear suppression. The results of this study support the validity of this model, suggesting that different components of executive function may play crucial roles at various stages of fear extinction learning (Schweizer et al., 2013). Individuals with stronger inhibitory control exhibited greater differentiation between CS+ and CS- during extinction, indicating more precise emotion regulation and reduced erroneous threat assessment (Tang & Schmeichel, 2014). Individuals with stronger updating ability showed a slower decline in US expectancy ratings during the initial phase of extinction but had lower fear expectancy ratings during extinction recall, reflecting more effective storage and utilization of safety information (Schweizer et al., 2013). Individuals with stronger shifting ability exhibited a greater fear reinstatement effect, possibly due to heightened sensitivity to environmental changes, making fear memories more prone to reactivity (Aldao & Nolen-Hoeksema, 2012; Kashdan & Rottenberg, 2010). This theoretical framework not only helps explain the relationship between executive function and fear-related emotions but also provides a theoretical basis for interventions targeting emotion disorders through executive function enhancement (Joormann & Gotlib, 2008).
The results of this study support the influence of executive function subcomponents on fear extinction, but there are also some limitations. First, the sample size was relatively small (approximately 80 participants), and the control over potential influencing variables (such as gender and depression) was not stringent enough. Notably, although the STAI trait anxiety score in this study (M ± SD = 42.81 ± 8.48) is close to the normative data for Chinese college students (Li, 1995), the moderately high score may reflect anxiety effects that were not fully controlled. Individuals with high trait anxiety may show impaired extinction learning (Sehlmeyer et al., 2011),which could partially confound the relationship between executive function and extinction ability. Future studies should increase the sample size and rigorously control these factors when selecting participants (Graham & Milad, 2011; Milad et al., 2010). Secondly, the measurement of executive function in this study was relatively limited, as only one paradigm was used to assess each subcomponent. Different paradigms may involve different cognitive processes (Diamond, 2013). Future research should select more appropriate and comprehensive paradigms to assess executive function based on specific objectives. Furthermore, this study used fNIRS technology to measure brain region activity, but this technique is limited to detecting surface brain regions and cannot observe the activity of deeper regions such as the amygdala and hippocampus (Cui et al., 2011). Future research could consider using techniques such as functional magnetic resonance imaging (fMRI) to obtain more comprehensive brain activity data. Finally, some results reached only marginal significance. Although these results provide preliminary insights, their clinical relevance remains debated, and thus their interpretation should be cautious. Future research could further validate the reliability of these marginally significant results through larger sample sizes and stricter controls, providing a more solid evidence base for these preliminary findings.
ConclusionOur study explored the impact of executive function on conditioned fear extinction and reinstatement, as well as its neural correlates. The results indicate that different executive function subcomponents play distinct roles in fear extinction and reinstatement. Specifically, individuals with stronger shifting abilities were more likely to reinstate fear responses during the fear reinstatement phase. Those with stronger updating ability exhibited better retention of extinction memory during the extinction recall phase, while Individuals with stronger inhibitory ability demonstrated better discrimination between threat and safety cues during the immediate extinction phase and showed more effective emotional regulation in response to safety cues. Furthermore, fNIRS results revealed differences in activity patterns in the mPFC and DLPFC across different executive function subcomponents, further supporting the neural basis of executive function in fear extinction and reinstatement. Future research could further investigate how enhancing the specific executive function may improve an individual's ability to regulate fear extinction, with potential applications in treating pathological fear conditions such as anxiety disorders.
Data and code availability statementAll data, models, or codes used during the study are available from the corresponding author by request.
Artificial intelligenceNo artificial intelligence assisted technologies were used in this research or the creation of this article.
EthicsThis research received approval from a local ethics board (NO. SICNU-220118).
Statement of human rightsParticipants provided informed written consent and were given monetary compensation.
CRediT authorship contribution statementWenzhao Zhang: Data curation, Software, Writing – original draft, Writing – review & editing. Yixia Huang: Conceptualization, Data curation, Methodology, Software. Ying Mei: Writing – review & editing. Jinxia Wang: Writing – review & editing. Haoran Dou: Conceptualization, Visualization, Writing – review & editing, Supervision. Yi Lei: Conceptualization, Funding acquisition, Visualization, Writing – review & editing, Supervision.
All authors declare no conflicts of interest.
This work was supported by the STI 2030—Major Projects 2022ZD0210900; The Ministry of Education of Humanities and Social Science project [23YJC190003]; National Natural Science Foundation of China [NSFC 32271142, 32300928]; Natural Science Foundation of Sichuan Province [2025ZNSFSC1023]; Guangdong Key Project in "Development of new tools for diagnosis and treatment of Autism" [2018B030335001]; Ministry of Education Key Projects of Philosophy and Social Sciences Research [grant number 21JZD063]; Shenzhen Science and Technology Research Funding Program [JCYJ20200109144801736].