Edited by: Em. Professor Gualberto Buela-Casal
(University of Granada, Granada, Spain)
Dr. Katie Almondes
(Federal University of Rio Grande do Norte, NATAL, Brazil)
Dr. Alejandro Guillén Riquelme
(Valencian International University, Valencia, Spain)
Last update: December 2025
More infoLow sleep quality induces inflammation. Because anti-thyroid peroxidase antibody (TPO-Ab) is an autoantibody that induces inflammation in the thyroid, insufficient sleep may stimulate the production of TPO-Ab. However, the thyroid function is also associated with sleep. Therefore, to evaluate the association between TPO-Ab positivity and insufficient sleep, the target population should be limited to euthyroid individuals whose free triiodothyronine (T3), free thyroxine (T4), and thyroid-stimulating hormone (TSH) are within the normal ranges.
MethodThis cross-sectional study recruited 1324 euthyroid individuals who participated in annual health checkups. Insufficient sleep was assessed by using a questionnaire. Individuals with free T3, free T4, and TSH levels within the normal ranges were defined as euthyroid.
ResultsAmong the study population, 406 had insufficient sleep, and 242 were TPO-Ab-positive. Insufficient sleep was associated with a higher likelihood of TPO-Ab positivity. Sex and age adjusted odd ratios (95 % confidence intervals, p) of TPO-Ab positive for insufficient sleep was 1.47 (1.08, 2.01, p = 0.014). These associations remained unchanged even after further adjustment for free T4 and TSH, status of body mass index, smoking status, drinking status, mental distress, and physical activity; 1.53 (1.11, 2.10, p = 0.009).
ConclusionEuthyroid individuals with insufficient sleep may be at risk of autoimmune thyroiditis. Although further investigations are necessary, sleep disorder therapy might reduce the risk of the incidence of autoimmune thyroiditis.
Anti-thyroid peroxidase antibodies (TPO-Abs) are associated with Hashimoto's thyroiditis, a phenotype of autoimmune thyroiditis (Vargas-Uricoechea. 2023). However, life-related factors that induce TPO-Ab positivity have not yet been identified.
Previous studies revealed a close relationship between sleep quality and inflammation (Engert et al., 2023) (Zheng et al., 2023) (Besedovsky et al., 2019). Chronic sleep restriction has been shown to induce oxidative stress and inflammation (Zheng et al., 2023). Experimental sleep disturbances have been found to influence the ability to resolve inflammation (Engert et al., 2023). Individuals with insufficient sleep possess inflammatory disadvantage which induce chronic inflammation.
Additionally, poor sleep quality and short sleep duration are reportedly associated with rheumatoid arthritis (Wu et al., 2020). Rheumatoid arthritis is an autoimmune disease. Therefore, insufficient sleep may be a risk factor for autoimmune thyroiditis associated with TPO-Ab positivity.
Insufficient sleep, associated with insomnia, is a common social problem. A previous Japanese study of the general adult population reported that 21 % of patients had symptoms of insomnia (Liu et al., 2000). Fatigue is the most common concern among individuals with autoimmune disease (Zielinski et al., 2019). Because insufficient sleep might also be associated with fatigue, it could also be associated with TPO-Ab positivity.
However, the thyroid function is also associated with sleep (Nazem et al., 2021) (Yan et al., 2022) (Wu et al., 2021). Even subclinical hypothyroidism, with normal thyroid hormone levels, is associated with insufficient sleep (Yan et al., 2022) (Wu et al., 2021). Therefore, to evaluate the association between TPO-Ab positivity and insufficient sleep, the target population should be limited to euthyroid individuals whose free triiodothyronine (T3), free thyroxine (T4), and thyroid-stimulating hormone (TSH) are within the normal ranges.
To test our hypothesis, we conducted a cross-sectional study on a euthyroid population.
MethodsMaterials and methodsStudy populationMethods related to the present risk survey, including thyroid function and TPO-Ab, have been described previously (Shimizu et al., 2023).
The study population comprised 1883 Japanese individuals aged between 40 and 74 years from Saza town in western Japan who underwent an annual medical checkup in 2014.
Individuals without thyroid function data, such as free T3, free T4, or TSH (n = 20), were excluded. Individuals with an abnormal free T3 (normal range: 2.1–4.1 pg/mL), free T4 (normal range: 1.0–1.7 ng/dL) (both n = 78), and TSH (normal range: 0.39–4.01 μIU/mL) (n = 140) were also excluded. Individuals with a history of thyroid disease (n = 43) were excluded to avoid the influence of thyroid disease. Individuals without TPO-Ab data (n = 269) were excluded. Individuals without sleep status data (n = 3), smoking status data (n = 1), drinking status data (n = 1), body mass index (BMI) data (n = 1), and mental distress status (n = 3) were also excluded.
No significant differences in clinical characteristics were observed between the excluded and included individuals.
A total of 1324 individuals with a mean age of 60.8 years (standard deviation (SD): 9.0; range 40–74) were enrolled in the study.
Written consent forms were used to ensure that participants understood the objectives of the study when obtaining informed consent. Informed consent was obtained from all participants. This study was approved by the Ethics Committee of the Nagasaki University Graduate School of Biomedical Sciences (project registration number: 14,051,404). All procedures involving human participants were performed in accordance with the ethical standards of the Institutional Research Committee and the 1964 Declaration of Helsinki and its later amendments for comparable ethical standards. No personally identifiable information was available to any author.
Data collection and laboratory measurementsTrained interviewers obtained information on the clinical characteristics. Sleep status was assessed as follows: “Are you getting enough rest by sleep?”. If the participants replied “no” to this question, they were deemed to have insufficient sleep. Exercise status was also assessed as follows: “Do you engage in any physical activity to the extent that induced light perspiration at least twice a week for 30 min and have maintained this regime for over a year?”. If the participants replied “yes” to this question, they were considered physically active.
Both questions were promoted by the Ministry of Health, Labor, and Welfare. A report from a national lifestyle-related disease prevention program referenced the utility of the aforementioned sleep-related questions (Chin, 2016). The effectiveness of physical activity-related questions has been reported in a previous study (Fujihara 2020).
Fasting blood samples were collected from all patients. TSH, free T3, and free T4 levels were measured using a Chemiluminescent Immunoassay (CLIA) at the LSI Medience Corporation (Tokyo, Japan). Normal ranges of free T3 (2.1–4.1 pg/mL), free T4 (1.0–1.7 ng/dL), and TSH (0.39–4.01 μIU/mL) using this method have been reported previously (LSI Medience Corporation Information, website). TPO-Ab levels were also measured using standard procedures at the LSI Medience Corporation (Tokyo, Japan), and the normal (negative) range was <16 IU/mL (LSI Medience Corporation Information, 2025). Therefore, TPO-Ab positive was defined as levels ≥16 IU/mL.
Serum triglyceride, high-density lipoprotein cholesterol (HDLc), and hemoglobin A1c (HbA1c) levels were measured at SRL, Inc. using standard laboratory procedures.
Body weight and height were measured using an automatic body composition analyzer. The body mass index (BMI) was calculated. As a previous systematic-review and meta-analysis revealed that BMI<18.5 kg/m2 and BMI≥25.0 kg/m2 are associated with increased risk of mortality (Ogawa et al., 2024), these values were used as cut-off for categorizing BMI status. Therefore, a high BMI was defined as ≥25.0 kg/m2 and low BMI was defined as that <18.5 kg/m2.
The K6 was developed to identify mental distress (Kessler et al., 2002), and mental health treatment needs are recognized (K6 scale score: ≥5) (Prochaska et al., 2012). Therefore, in the present study we defined mental distress as K6 scale score: ≥5, similar to that in our previous study (Shimizu et al., 2015).
Statistical analysisCharacteristics of contentious values of the study population were expressed as the mean ± standard deviation (SD) except for TSH and triglycerides. Because TSH and triglycerides showed a skewed distribution, the characteristics of this study population were expressed as medians (first and third quartiles). Sex (male), high BMI, low BMI, current smoking status, current drinking status, physical activity, and mental distress were expressed as percentage values.
Differences among potential variables regarding sleep quality status (sufficient and insufficient sleep) were calculated. Significant differences were evaluated using Student's t-test for continuous variables and the χ2 test for proportional data.
Logistic regression models were used to calculate odds ratios (ORs) and 95 % confidence intervals (CIs) to determine the association between TPO-Ab positivity and insufficient sleep.
In addition to thyroid function, factors that influence sleep quality and thyroid function may confound the association between TPO-Ab levels and insufficient sleep. BMI status (Gonnissen et al., 2013) (Walczak et al., 2021), smoking status (Purani et al., 2019) (Wiersinga et al., 2013), drinking status (Thakkar et al., 2015) (Valeix et al., 2008), mental distress (Yasugaki et al., 2023) (Zach et al., 1988), and physical activity (Yang et al., 2012) (Klasson et al., 2022) are associated with quality of sleep and thyroid function.
Therefore, adjustment models were used in this study. Model 1 was adjusted for sex and age (sex- and age-adjusted model). In Model 2 (multivariate model), in addition to sex and age, thyroid function parameters such as free T4 and the logarithmic value of TSH were included for adjustment. Model 3 was adjusted for sex, age, high BMI (yes or no), low BMI (yes or no), current smoking status (yes or no), current drinking status (yes or no), mental distress (yes or no), and physical activity (yes or no). Furthermore, the normal range of TPO-Ab is positively associated with atherosclerosis, as evaluated using carotid intima-media thickness (CIMT) (Shimizu, Kawashiri et al., 2020a). As atherosclerosis is an established cardiovascular risk factor, it may have influenced the present study. Normal thyroid function can also influence cardiovascular risk factors (Shimizu et al., 2021). Therefore, adding known cardiovascular risk factors to adjusted model 3 might induce the problem of multi-covariance. Subsequently, we created another adjustment model (Model 4). A previous study that reported an association between the normal range of TPO-Ab and atherosclerosis used sex, age, BMI, systolic blood pressure, smoking status, drinking status, and TG, HDLc, and HbA1c levels as confounders (Shimizu, Kawashiri et al., 2020a). In Model 4, we adjusted for sex, age, BMI, systolic blood pressure, smoking status, drinking status, TG level, HDLc, and HbA1c level, as in the aforementioned study.
The goodness of fit for these models was validated using the Homer–Lemeshow test. The Variance Inflation Factor (VIF) was used to evaluate multicollinearity. All variance inflation factor values in the present analysis were < 1.40.
For the sensitivity analysis, sex- and age-adjusted ORs (95 %CIs) of insufficient sleep for free T3, free T4, and TSH levels were determined.
For additional analysis, continuous values of TPO-Ab titer and insufficient sleep were also evaluated.
All statistical analyses were performed using SAS software for Windows, version 9.4 (SAS Inc., Cary, NC, USA). Statistical significance was set at p < 0.05.
ResultsAmong the study population, 331 individuals had insufficient sleep, and 242 individuals were TPO-Ab-positive.
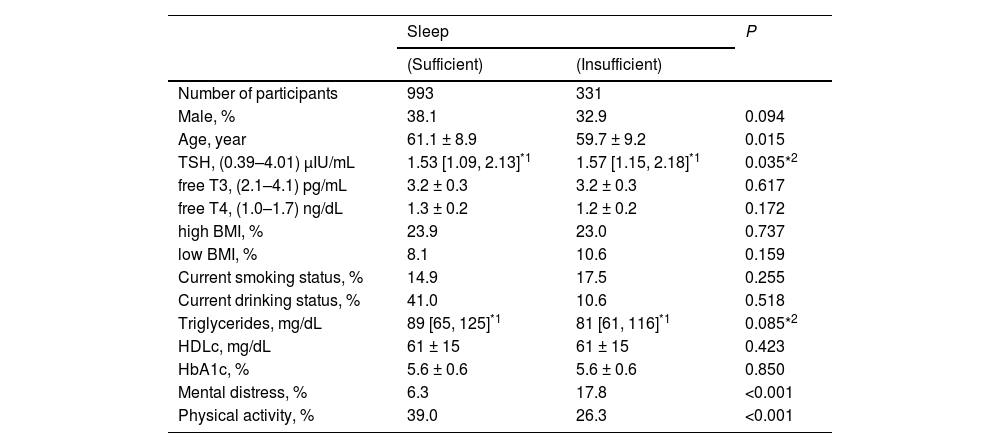
Characteristics of study populationThe characteristics of the study population with respect to insufficient sleep are shown in Table 1. Compared with individuals with sufficient sleep, those with insufficient sleep were significantly younger, had lower levels of physical activity, and had a higher prevalence of mental distress.
Characteristics of study population by status of sleep.
TSH; thyroid stimulating hormone, free T3; free triiodothyronine, free T4; free thyroxine. BMI; body mass index, HDLc; high density lipoprotein cholesterol, HbA1c; hemoglobin A1c. Values are presented as the mean ± standard deviation. *1: Values are median [the first quartile, third quartile]. *2: Logarithmic transformation was used. Normal range of measurements are presented in parenthesis.
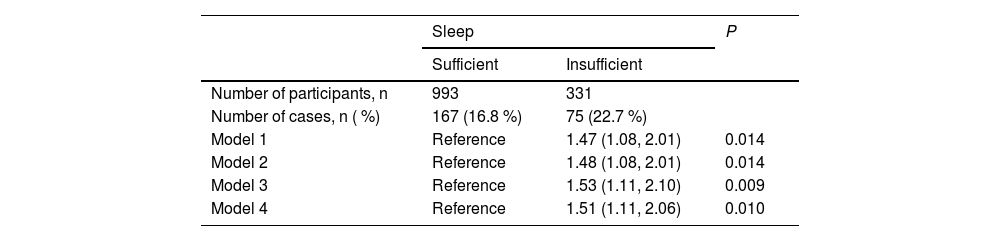
The association between TPO-Ab positivity and sleep insufficiency is shown in Table 2. Compared with individuals without insufficient sleep, those with insufficient sleep showed significantly higher ORs for TPO-Ab positivity. The adjusted ORs (95 %CIs) of TPO-Ab were 1.47 (1.08, 2.01, p = 0.014) for model 1, 1.48 (1.08, 2.01, p = 0.014) for model 2, and 1.53 (1.11, 2.10, p = 0.009) for model 3.
Association between TPO-Ab positivity and sleep insufficiency.
TPO-Ab: Antithyroid peroxidase antibodies. Model 1: adjusted only for sex and age. Model 2: adjusted for thyroid stimulating hormone (TSH) and free triiodothyronine (T3) in addition to sex and age. Model 3: adjusted for sex, age, high body mass index (BMI), low BMI, current smoking status, current drinking status, mental distress, and physical activity. Model 4: adjusted for sex, age, high BMI, low BMI, systolic blood pressure, current smoking status, current drinking status, triglycerides, high-density lipoprotein cholesterol, and hemoglobin A1c.
We also evaluated the influence of mental status on the association between insufficient sleep and TPO-Ab positivity by adjusting for the model variables. Even after further adjusting for mental disease defined as K6 ≥ 13 (Prochaska at al. 2012), the same association was observed [fully adjusted OR (95 %CI): 1.53 (1.11, 2.10), p = 0.009]. Furthermore, even when adjusting the continuous values of the K6 scale (logarithmic transformation values) instead of adjusting for mental distress (k 6 scale≥ 5) and mental disease (k6 scale ≥ 13), the association remained essentially the same [1.75 (1.05, 2.90), p = 0.031]. Therefore, mental status may not determine the association between insufficient sleep and TPO-Ab positivity.
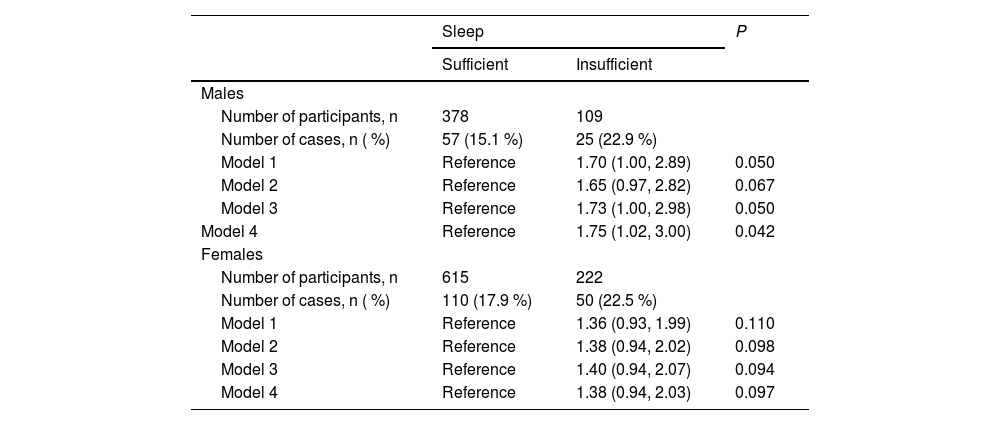
Sex specific association between TPO-Ab positivity and insufficient sleepThe sex-specific associations between TPO-Ab levels and insufficient sleep are shown in Table 3. For both males (n = 487) and females (n = 837), although the statistical power did not reach a significant value, a positive relationship between TPO-Ab positivity and insufficient sleep was observed. Among males, the adjusted ORs (95 % CIs, p) of TPO-positive were 1.70 (1.00, 2.89, p = 0.050) for model 1, 1.65 (0.97, 2.82, p = 0.067) for model 2, and 1.73 (1.00, 2.98, p = 0.050) for model 3. The corresponding values for females were 1.36 (0.93, 1.99, p = 0.110), 1.38 (0.94, 2.02, p = 0.098), and 1.40 (0.94, 2.07, p = 0.094).
Sex specific association between TPO-Ab positivity and sleep insufficiency.
TPO-Ab: Antithyroid peroxidase antibodies. Model 1: adjusted only for sex and age. Model 2: adjusted for thyroid stimulating hormone (TSH) and free triiodothyronine (T3) in addition to sex and age. Model 3: adjusted for sex, age, high body mass index (BMI), low BMI, current smoking status, current drinking status, mental distress, and physical activity. Model 4: adjusted for age, high BMI, low BMI, systolic blood pressure, current smoking status, current drinking status, triglycerides, high-density lipoprotein cholesterol, and hemoglobin A1c.
The OR values (95 %CI, p) of insufficient sleep in the sex- and age-adjusted model were 0.99 (0.87, 1.12, p = 0.875) for 1 SD increment of free T3 (0.3 pg/mL for both males and females), 0.93 (0.82,1.05, p = 0.249) for 1 SD increment of free T4 (0.2 ng/dL for males and 0.1ng/dL for females), and 1.15 (0.88, 1.52, p = 0.307) for logarithmic value of TSH.
Association between continuous values of TPO-Ab titer and insufficient sleepWe also evaluated the ORs (95 % CI) and p-values of insufficient sleep for contentious values of TPO-Ab (logarithmic transformation values) and explored nonlinear associations. The corresponding values were 1.12 (0.98, 1.28), p = 0.103 for model 1, 1.13 (0.98, 1.30), p = 0.082 for model 2, 1.13 (0.98, 1.30) for model 3, and 1.17 (0.94, 1.46), p = 0.160 for model 4.
DiscussionThe major finding of the present study is that independent of known sleep quality-related factors, insufficient sleep is positively associated with TPO-Ab positivity among euthyroid individuals.
However, the mechanisms underlying the association between TPO-ab positivity and insufficient sleep remain unclear.
In present study, the contentious value of TPO-Ab (logarithmic transformation values) did not increase the OR for insufficient sleep (dichotomized value), whereas insufficient sleep increased the OR for TPO-Ab positivity (dichotomized value). These associations partly indicate that elevated TPO-Ab levels might not increase the risk of insufficient sleep, but rather that insufficient sleep might increase the risk of TPO-Ab positivity.
Since essentially same associations were observed in both males and females, sex differences might not have strongly influenced the association between TPO-Ab positivity and insufficient sleep.
However, the prevalence of autoimmune thyroiditis is significantly higher in women than in men (Ortona et al., 2016). Therefore, women are at a higher risk of stimulating the production of TPO-Ab independent of insufficient sleep. This risk factor reduces the magnitude of the association between insufficient sleep and TPO-Ab levels.
Direct crosstalk between androgen and thyroid hormone axes has been reported (Flood et al. 2013). Androgen deprivation therapy also reduces sleep quality (Koskderelioglu et al. 2017), and poor sleep quality contributes to reduced testosterone concentration (Lateef et al. 2020). Since oral testosterone undecanoate reduces the titer of thyroid peroxidase among men with Hashimoto's thyroiditis and low levels of testosterone (Krysiak et al. 2019), androgens might positively influence the association between insufficient sleep and TPO-Ab. Therefore, in this study, the association between insufficient sleep and TPO-Ab levels was stronger in men than in women.
A previous cross-sectional study of 16,648 individuals reported that poor sleep quality and short sleep duration were associated with rheumatoid arthritis (Wu et al., 2020). Rheumatoid arthritis is a systemic autoimmune disease characterized by the presence of autoantibodies such as rheumatoid factor and antibodies against post-translationally modified proteins, such as citrullination (ACPA) and carbamylation (anti-CarP antibodies) (van Delft et al., 2020). Therefore, sleep disorders may trigger immune system abnormalities partly by inducing auto-antibody production (Wu et al., 2020). As TPO-Ab is an autoantibody, insufficient sleep could be positively associated with TPO-Ab positivity. Previous study with euthyroid individuals without obesity reported an independent positive association between TPO-Ab titer and inflammatory activity evaluated by high-sensitivity C-reactive protein (CRP) (Liu et al., 2018), which also supports the above-mentioned mechanism: insufficient sleep induces chronic inflammation, which also stimulates the production of autoantibodies.
T-helper 1 (Th1) cytokines contribute to cell-mediated immunity, whereas T-helper 2 (Th2) cytokines contribute to humoral immunity. Hyperactivity of cell-mediated immunity, which is similar to humoral immunity, damages tissues and organs. Th1 and Th2 cells suppress this activity each other. Therefore, maintaining a well-balanced Th1/Th2 ratio is crucial (Berger, 2000). A previous experimental study evaluating the association between the balance of Th1/Th2 and TPO-Ab synthesis reported that a shift towards Th1 is associated with reduced autoantibody synthesis (Guo et al., 1999). Another study reported that insomnia shifts the Th1/Th2 balance towards Th2 dominance (Sakami et al., 2003). Consequently, insufficient sleep might shift the Th1/Th2 balance towards Th2 dominance, which stimulates TPO-Ab synthesis, and vice versa.
In addition, interleukin-17 (IL-17), which activates the nuclear factor-kappa B (NF-κB) signaling pathway that stimulates the production of tumor necrosis factor-alfa (TNF-α), IL-6, and interferon-gamma (IFN-γ), was elevated in patients with autoimmune thyroiditis (Lu et al., 2022). As experimental sleep restriction activates the production of IL-17 (van Leeuwen et al., 2009), insufficient sleep can activate IL-17 and stimulate TPO-Ab production.
Atherosclerosis (Sasaguri et al., 2004), mental distress (Myint 2005)(Zhang 2022), and exercise (Santos et al., 2019)(Xiang et al., 2014), which are reported to be associated with the Th1/Th2 balance, are also associated with IL-17 (Ryu et al., 2015) (Lu et al., 2023)(Machado et al., 2021). Therefore, future longitudinal studies with information on Th1/Th2 cells and IL-17 levels may be an effective way to elucidate the mechanisms underlying the association between TPO-Ab positivity and sleep insufficiency. Furthermore, as moderate exercise decreases IL-17 (Heidarianpour et al., 2016), whereas strenuous exercise stimulates IL-17 production (Duzova et al., 2009), further studies focusing on the amount of exercise load with information on IL-17 could provide a scientific basis for designing interventions.
A significant association between thyroid function and sleep has been previously reported (Nazem et al., 2021)(Yan et al., 2022)(Wu et al., 2021). Even among patients with subclinical hypothyroidism, the proportion of patients with insufficient sleep is higher than that among those with normal thyroid function (Yan et al., 2022)(Wu et al., 2021). However, the thyroid function may not determine the association between TPO-Ab positivity and sleep insufficiency. In the present study, insufficient sleep was found to be significantly positively associated with TPO-Ab positivity even among individuals with normal thyroid hormone (free T3 and free T4) and TSH levels. In addition, in the present study, thyroid hormones (free T3 and free T4) and TSH levels were not significantly associated with insufficient sleep. Even after further adjustment for thyroid function, the positive association between TPO-Ab positivity and insufficient sleep remained significant. Therefore, the thyroid function may not determine the association between TPO-Ab positivity and sleep insufficiency.
However, the thyroid gland may also precisely regulate latent injury. In an additional analysis, no significant linear association was observed between continuous TPO-Ab levels and insufficient sleep. However, the current definition of insufficient sleep does not apply to a single situation (Chin, 2016). Even within the normal range, the TPO-Ab titer is reported to be associated with atherosclerosis (Shimizu, Kawashiri et al., 2020a) and active arterial wall thickening, as evaluated by the yearly progression of CIMT (Shimizu et al., 2022). Chronic inflammation has been reported to be associated with both the progression of CIMT (Li et al., 2024) and quality of sleep (Engert et al., 2023) (Zheng et al., 2023) (Besedovsky et al., 2019). Even in euthyroid populations, TPO-Ab stimulates the production of TSH (Shimizu et al., 2023). TPO-Ab is also inversely associated with thyroid cysts in euthyroid populations (Shimizu, Nabeshima-Kimura et al., 2020b), indicating that the absence of thyroid cysts is a marker of latent thyroid injury. Even in euthyroid populations, thyroid cysts have been shown to influence thyroid function (Shimizu et al., 2021). Thyroid function is also associated with sleep (Nazem et al., 2021) (Yan et al., 2022) (Wu et al., 2021). Therefore, numerous factors influence the thyroid gland and lead to a positive association between TPO-Ab positive and insufficient sleep.
Due to the regular consumption of seaweed, iodine intake is considered high among Japanese people, and excess iodine intake has been associated with thyroid function (Farebrother et al. 2019). However, in this study, iodine intake did not correlate with such results, perhaps because the study population was comprised of euthyroid patients. In the present study, the TSH level of insufficient sleepers was slightly but significantly higher than that of sufficient sleepers. Therefore, the latent thyroid hormone-producing capacity should be higher for participants with sufficient sleep than for those with insufficient sleep. This finding indicates that insufficient sleep may increase the risk of excess iodine intake.
Marine polyphenols, which are rich in seaweeds, have been reported as candidates for the treatment of insomnia (Kim et al. 2022). Furthermore, more than adequate iodine intake is reported to inversely associate with TPO-Ab positively (Teng et al. 2020). Therefore, without adjusting for the iodine intake status, the association between insufficient sleep and TPO-Ab positivity should weaken; however, a significant association was observed. Further analysis with non-Japanese individuals who do not regularly take iodine is recommended to improve our understanding of the mechanism underlying these associations and determine the generalizability of our findings to the global population.
The clinical implication of the present study is that euthyroid individuals with insufficient sleep should be evaluated for TPO-Ab levels. A bidirectional relationship between sleep and inflammation has been reported; sleep influences inflammatory activity, and vice versa (Veler. 2023). Therefore, improvements in sleep quality may reduce the inflammatory activity. Although further investigation is necessary, sleep disorder therapy might reduce the risk of the incidence of autoimmune thyroiditis. Further, the measurement of TPO-Ab might not only be an efficient tool for risk estimation for autoimmune thyroiditis but also an efficient tool to evaluate the effect of sleep disorder therapy.
For public health implications, although further investigations are necessary, a simple, easily understandable questionnaire could be an efficient tool to risk estimation of autoimmune thyroiditis that is also efficient tool for conducting a strategy for preventing autoimmune thyroiditis.
The limitations of this study warrant further investigation. Although chronic inflammation may play a vital role in the association between TPO-Ab positivity and insufficient sleep, no data evaluating the activity of inflammation were available in this study. Further investigation using inflammatory data, such as high-sensitivity C-reactive protein levels, is necessary. Owing to the small sample size, a meaningful sex-specific study could not be established. However, the same association was observed in both males and females. The current definition of insufficient sleep is based on a single questionnaire that cannot evaluate the multidimensional aspects of sleep quality, such as sleep duration, patterns (e.g., daytime napping), and objective measurements (e.g., actigraphy). However, the National Lifestyle-Related Disease Prevention Program reported the utility of this single questionnaire (Chin 2016). Further investigations using data from validated scales, such as the Pittsburgh Sleep Quality Index (PSQI), are necessary. As this was a cross-sectional study, causal relationships could not be established, and well-managed longitudinal studies are required to establish causal relationships.
ConclusionIndependent of established sleep quality-related factors, insufficient sleep was associated with an increased probability of TPO-Ab positivity in euthyroid individuals. Although further investigation is necessary, this finding indicates that measuring TPO-Ab might not only be an efficient tool to estimate the risk of autoimmune thyroiditis but also to evaluate the effect of sleep disorder therapy.
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Yuji Shimizu reports financial support was provided by Japan Society for the Promotion of Science. Takahiro Maeda reports financial support was provided by Japan Society for the Promotion of Science. Yuji Shimizu reports financial support was provided by Japan Agency for Medical Research and Development. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.