The aim of this work was to investigate the FUT 2 gene, the secretor status and the expression of CD44 protein in epithelial cells obtain from saliva samples from patients with oral lesions (benign, pre-cancerous and cancerous lesions, n=94). We analyzed polymorphisms of the FUT2 gene by allele specific oligonucleotide–polymerase chain reaction. The FUT2 gene encodes the α(1,2) fucosyltransferase (Se enzyme) that regulates the expression of ABH antigens in secretions. Generally speaking, being a non-secretor has several disadvantages with regard to metabolism and immune function. In this study, we found that oral pre-cancerous and cancerous lesions were increased among individuals with non-secretor status and nonsense mutation 428G→A. Fifty-one percent of the patients with oral pre-malignant and malignant lesions were non-secretors, in contrast with the healthy population (OR=3.44). We observed a marginal association between secretor status and these lesions. Our study suggests that the lack of wild type FUT2 gene and a non-secretor status appear to be an associated risk marker for the development of oral cancer in patients with oral lesions.
One of the genes involved in tumour processes is CD44, which appears to be one of the most promising candidates as a cancer diagnosis marker. We investigated, using confocal microscopy, the expression of CD44 protein in epithelial cells obtained from saliva samples from patients with oral lesions. The results obtained showed fluorescence corresponding to the presence of CD44 protein in samples from patients diagnosed with cancer and pre-cancer. These findings indicate that overexpression of CD44 molecule analyzed could be considered as a marker of risk in individuals with oral lesions.
El objetivo del presente trabajo fue investigar la expresión del gen FUT 2 y del marcador CD44 en células epiteliales obtenidas a partir de muestras de saliva de pacientes con lesiones orales (benignas, pre-cáncer y cáncer). Analizamos el polimorfismo del gen FUT 2 mediante la reacción de cadena de la polimera alelo específica. Este gen codifica para la α(1,2) ucosyltransferasa (enzima secretora), que regula la e3xpresión de los antígenos ABH en las secreciones. Hemos hallado una mayor intensidad de reacción en el grupo no secretor (OR=3.44). El 51% de los pacientes con pre-cáncer y cáncer oral fueron no secretores, en contraste de la población control. Además hemos observado un asociación marginal entre el estado secretor y estas lesiones. Nuestro estudio sugiere que la pérdida del tipo alelo salvaje del gen FUT 2 y el carácter no secretor parecerían estar asociado a marcadores de riesgo en el desarrollo del cáncer oral en pacientes con estas lesiones.
Uno de los genes involucrados en el proceso tumoral es el que codifica para la proteína CD44, la cual parece ser un candidato importante como marcador de diagnóstico de cáncer. Hemos investigado por microscopía confocal la expresión de esta proteína en células epiteliales obtenidas de muestras de saliva de estos pacientes con lesiones orales. Los resultados obtenidos muestran la fluorescencia correspondiente a la presencia del marcador CD44 en los pacientes con diagnóstico de cáncer y pre-cáncer. Estos hallazgos indicarían que la sobreexpresión del la molécular CD44 podría ser considerada como una herramienta complementaria para el pronóstico de cáncer oral.
Cancer incidence in humans has gradually increased over the last century. Surgical, radio, chemotherapeutic and biological treatments have experienced important advances, with concomitant reduction in the morbidity associated with the radical surgical practices of the past. The term “oral cancer” includes a diverse group of tumors arising from the oral cavity.1 Although globally oral cancer represents an incidence of 3% (males) and 2% (females) of all malignant neoplasm, it has one of the lowest survival rates—50%, within a five-year period.2
It is important to diagnose oral cancer in its early stages, since the management of small and localized tumors involves less morbidity and mortality than more advanced-stage disease, where treatment must be more aggressive. Indeed, the stage in which the disease is diagnosed is directly correlated to long-term survival.3 It is generally accepted that when diagnosed in its early stages, a favourable prognosis is expected.
Historical studies associating the Lewis system antigens and/or ABH system secretory antigens with disease are varied and generally inconclusive. The Lewis histo-blood group antigens Lewis a (Lea) and Lewis b (Leb) are carbohydrate structures that form epitopes on glycolipids and glycoproteins.4 Two independent genes determine the Lewis phenotype: the Lewis gene (Le and le), and the secretor gene (Se and se). Conventional Lewis blood grouping is difficult (e.g., in cancer patients and pregnant women) because of the presence of nongenuine Lewis-negative individuals.4,5 Secretor status in Lewis-negative individuals is determined with a labor-intensive hemagglutination inhibition technique that uses heat-inactivated saliva. In Lewis-positive individuals, secretor status is deduced from the Lewis phenotype: Le(a−b+) individuals are secretors, and Le(a+b-) individuals are nonsecretors.5
The H antigen, which is a precursor of A and B antigens, is synthesized by α(1,2) fucosyltransferase. It has been demonstrated that two distinct α(1,2) fucosyltransferases are present in human tissues.6 One is the H gene (FUT1)-encoded α(1,2) fucosyltransferase (H enzyme) that regulates expression of ABH antigens in erythrocytes, and the other is the Secretor gene (FUT2)-encoded α(1,2) fucosyltransferase (Se enzyme) that regulates expression of ABH antigens in the gastrointestinal tract and secretions.7 Secretors, who have ABH antigens in saliva, have at least one functional Se allele, and nonsecretors, who fail to express ABH antigens in their saliva, are homozygous for the nonfunctional se allele.8,9
The FUT2 gene has a significant polymorphism with typical ethnic specificity. The nonsense mutation 428G→A (Trp143→stop) is characteristic for the dominating nonsecretor allele (se428) in Europeans and appears in about 20% of the Caucasian population.10
The functional significance for ABO antigen expression on erythrocytes has not been defined, but ABO-related structures may play a role in other systems. Alterations in the expression of fucosylated oligosaccharides have also been observed in several pathological processes, including cancer and atherosclerosis.9–12
In carcinomas, altered expression of the various carbohydrate epitopes of this family occur, and are often strongly associated with either a good or bad prognosis. In human tumors, blood group antigens change in the same general direction as other glycosphingolipids do in tumors.12,13 The mechanisms of aberrant expression of blood-group antigens are not clear in all cases.12,14–16 It has been demonstrated in a number of earlier studies on the etiology and pathogenesis of certain diseases that the patients’ secretor status (ABO (H) blood group antigens) may probably be a factor influencing the development of systemic oral diseases.12,17,18Since FUT2 regulates the expression of a carbohydrate on the several epithelium, we decided to investigate the possible association between secretor status and disease progression specially in oral pre-cancer.
Altered blood group antigens in malignant oral tissues may indicate increased cell migration. This hypothesis is supported by studies showing that normal migrating oral epithelial cells like malignant cells show lack of expression of A/B antigens, and by studies that target ABH antigens to key receptors controlling adhesion and motility, such as integrins, cadherins, and CD-44.15
It is well established that the large array of functions that a tumour cell has to fulfill to settle as a metastasis in a distant organ requires cooperative activities between the tumour and the surrounding tissue and that several classes of molecules are involved, such as cell–cell and cell–matrix adhesion molecules and matrix degrading enzymes, to name only a few. Cell adhesion molecules are found on the surfaces of all cells, where they bind to extracellular matrix molecules or to receptors on other cells. Cell adhesion is critical in the dynamic processes necessary for tissue morphogenesis in development and the maintenance of complex differentiated tissues in adult organisms. Adhesion molecules have originally been thought to be essential for the formation of multicellular organisms and to tether cells to the extracellular matrix or to neighbouring cells.19 CDD44 is the major human cell surface receptor for hyaluronate and functions in a diverse range of physiological processes. CD44 may play a role in stimulating in vivo aggressiveness of tumors through hyaluronate-rich stroma.20 Expression of CD44 has been described to correlate with metastasis formation in various tumors, although evidence in oral cavity cancers is inconclusive.
Several studies have provided evidence that the expression of CD44 is specifically altered in many types of tumours. They show aberrant expression and processing of CD44 transcripts and cell surface expression of CD44 appears to change profoundly during tumour metastasis, particularly during the progression of various carcinomas.21 Numerous studies based on immunohistochemical analyses of paraffin-embedded or frozen tissue sections using different monoclonal antibodies to CD44 isoforms and molecular biological techniques have provided evidence that in many types of tumours there is overexpression of CD44 isoforms.
In the present study we investigate the FUT 2 gene, the secretor status and the expression of CD44 protein in epithelial cells obtain from saliva samples from patients with oral lesions (benign, pre-cancerous and cancerous lesions).
Materials and methodsPatients with benign oral lesions showed hyperplasia caused by diverse agents such as infectious, inflammatory, traumatic, hormonal, and drugs. The premalignant lesions included leukoplakia and lichen planus. The malignant lesions studied were squamous cell carcinoma.
Appropriate informed consent was obtained from all subjects and all procedures were performed according to the ethical standards established by the University of Rosario.
In total 188 subjects were examined, half of whom suffered from oral lesions (benign, pre-cancerous and cancerous ones), while the other half were the healthy control group. All were subjected to clinical oral examinations and standard evaluation tests in order to establish the secretor status of their saliva (agglutination inhibition technique).22 In the group of patients with oral benign, pre-cancerous and cancerous lesions (experimental group), a pathohistological examination of the oral mucosa was performed.
Serological studiesSaline erythrocyte suspensions were used for serological studies.
The Lewis phenotypes of fresh blood samples were determined by a hemagglutination method,22 using anti-Lea and anti-Leb monoclonal antibodies.
In order to establish the secretor status we analyzed their saliva by the agglutination inhibition technique.22
Inhibition test for secretor status2 or 3mL of saliva were collected into wide mouthed tubes. In order to eliminate the mucine protein they were treated with thermal shocks. They were centrifuged and the supernatant were transferred to a clean test tube and placed in boiling water bath for 10min to inactivate salivary enzymes.
To 1 drop of appropriately diluted blood grouping reagent (anti-A, anti-B or ulex europeaus) we added 1 drop of patient's saliva. We incubated 10minutes at room temperature and then we added 2 drops of 2–5% saline suspension of washed indicator red cells. Then, the tube was incubated 30min and centrifuged in order to inspect cell button macroscopically for agglutination.
Agglutination of indicator cells by antibody in tubes containing saliva indicates that the saliva does not contain the corresponding antigen (non secretor status). Failure of known antibody to agglutinate indicator cells after incubation with saliva indicates that the saliva contains the corresponding antigen (secretor status).
Molecular studiesDNA isolationGenomic DNA was isolated from saliva samples with a modified salting-out procedure. The DNA concentration was measured spectrophotometrically at 260nm and diluted in sterile water to a concentration of 100ng per μL.23
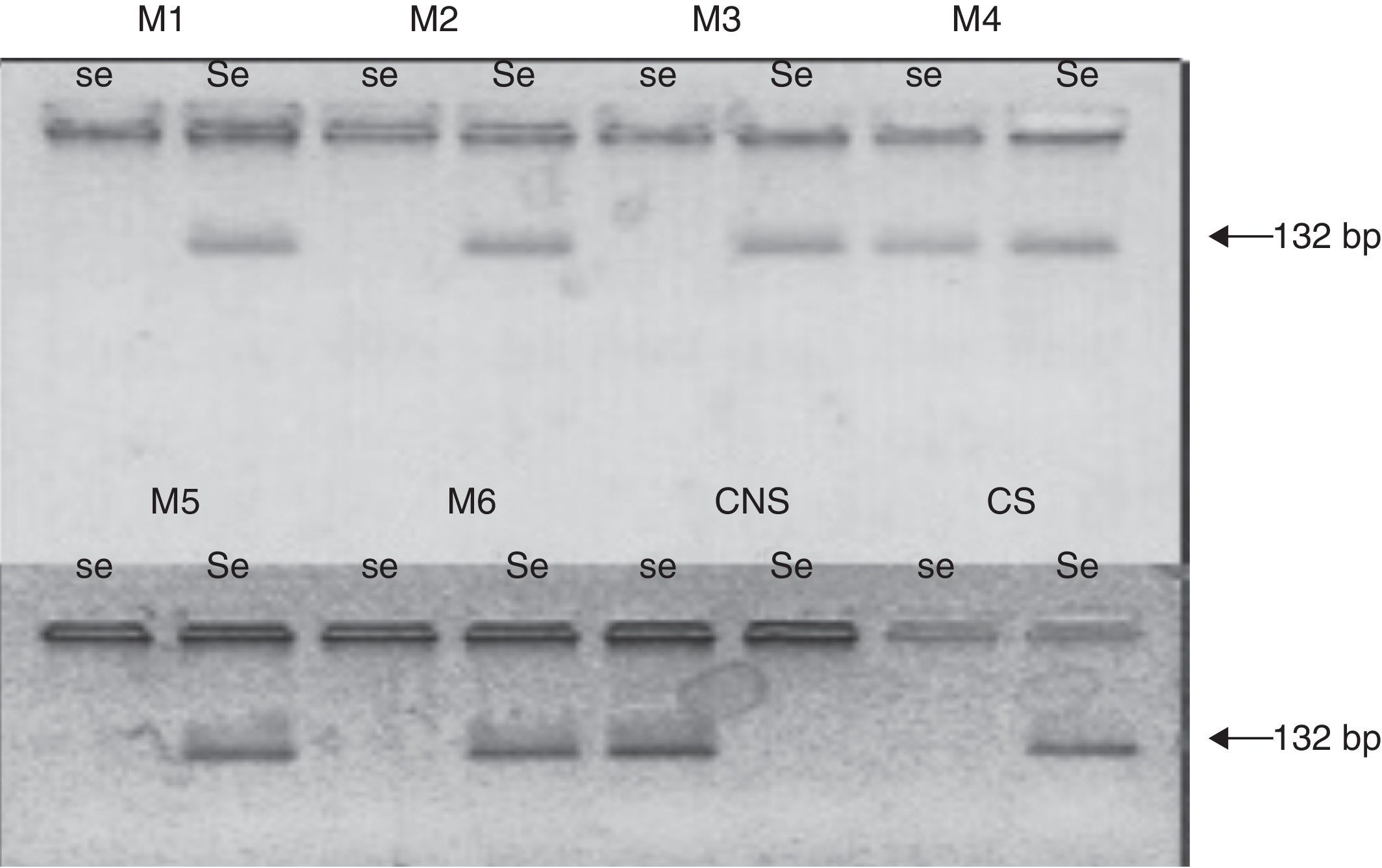
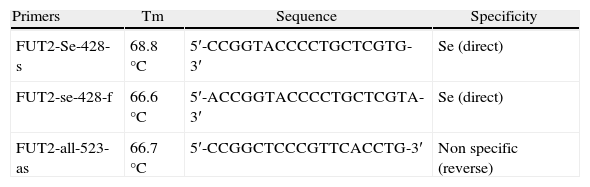
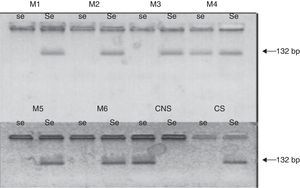
G428A polymorphysmThe DNA samples were analyzed by ASO-PCR (allele specific oligonucleotid–polymerase chain reaction) with specific primers (Operon Lab) for G428A allele and the wild type allele of FUT2 gene (Table 1). A fragment of 132bp was amplified as described by Henry et al.,23 except for modifications of the annealing temperature according to the Tm of the primers.
Statistical analysisThe categorical data were examined with a λ2 test, and the ORs were estimated using an unconditional logistic model.
CD44
We investigated by confocal microscopy, the expression of CD44 protein in epithelial cells obtained from saliva samples from patients with oral lesions. We studied 48 patients with various oral lesions (benign, pre-cancerous and cancerous), and a control group (n=32) who had no alterations. We worked with saliva samples subjected to thermal shock and washed with phosphate buffered saline. They were concentrated by centrifugation. Then 106 cells were incubated with anti-CD44 antibody suitable dilution for 30min at room temperature. After washing with phosphate buffered saline, it was incubated with secondary antibody labeled with allophycocyanin (APC). Parallel internal controls were processed for each sample. The different cell suspensions were washed with phosphate buffered saline and observed by confocal microscopy (Nikon C1) using 639nm red laser.
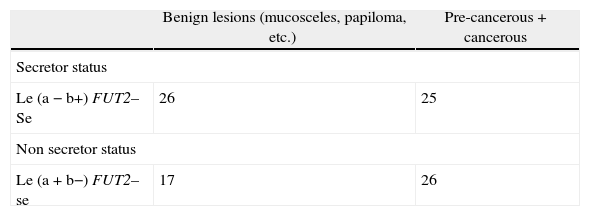
ResultsThe 79.5% of the healthy individuals studied × the Se gene (FUT 2) that governs the secretion of water-soluble ABH antigens into saliva. These secreted antigens can be demonstrated in saliva by agglutination inhibition tests with ABH antisera and molecular biology through analysis of the FUT 2 gene.The 51% (n=26) of the patients with oral pre-cancerous and cancerous lesions were non-secretors, OR=2.44; II 95% (0.7836; 7.5534) (p=0.1196) in contrast with the healthy population (Table 2), We observed a marginal association between secretor status and these lesions.
The molecular analysis showed that 28.38% of patients were homozygous for the G428A mutation (the mutation present in the 2 alleles), and the other patients were homozygous for the secretor status (none of them presented the allele G428A), or heterozygous secretor (1 allele presented with the mutation G428A).
We found that oral pre-cancerous and cancerous lesions were increased among individuals with non-secretor status and nonsense mutation 428G→A, OR=3.44; CI 95% (1.0682; 11.0729) (p=0.0346). Fifty-one percent of the patients with oral pre-malignant and malignant lesions were non-secretors, in contrast with the healthy population. In our population the nonsense mutation (428 G–A) in the FUT2 gene is the most frequent polymorphism. We studied the possible association between the 428 G-A in the FUT2 gene and oral disease progression. The genotyping revealed that 20 (21.3%) of the 94 blood donors were found to be non-secretors (se_/_) and 78.7% of the healthy individuals studied presented the Se gene (FUT 2) that governs the secretion of water-soluble ABH antigens into saliva (control group). These secreted antigens can be demonstrated in saliva by agglutination inhibition tests with ABH antisera and molecular biology through analysis of the FUT 2 gene. In contrast, thirty-three patients (57%) with oral pre-cancerous and cancerous lesions were non-secretors, OR=2.43; CI 95% (1.03; 5.71) (p=0.0407) (Table 2). We found a higher intensity of oral disease in the non-secretor group, OR=3.44; CI 95% (1.0682; 11.0729) (p=0.0346), and epithelial dysplasia was found exclusively in this group.
The molecular analysis showed that 48.62% of patients (n=53), were homozygous for the G428A mutation (the mutation present in the 2 alleles), while the other patients were homozygous for the secretor status (none of them presented the allele G428A), or heterozygous secretor (1 allele presented with the mutation G428A) (Fig. 1).
CD44
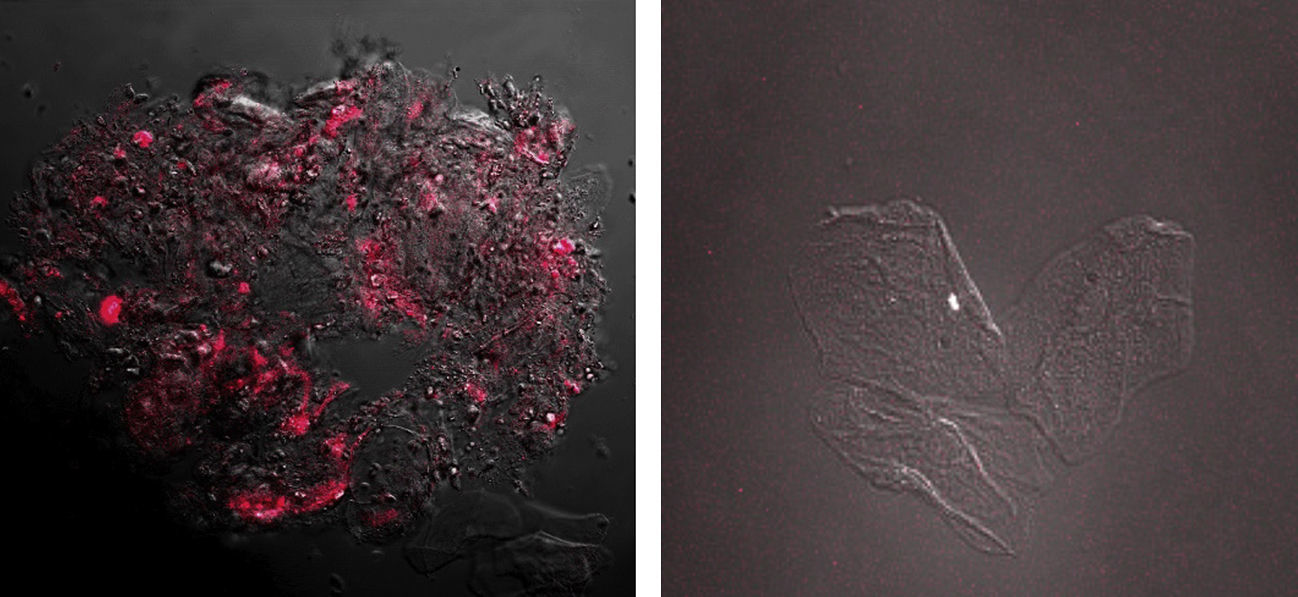
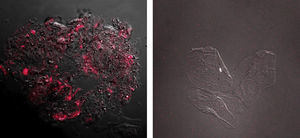
The results obtained showed fluorescence corresponding to the presence of CD44 protein in samples from patients diagnosed with cancer and precancer (n=28) OR=2.51; CI 95% (1.01; 6.52) (p=0.0397). A higher intensity was observed in individuals with a pathological diagnosis of squamous cell carcinoma (Fig. 2). In contrast, samples from patients with benign lesions showed no fluorescence images as samples of the control group (Fig. 2).
Image of squamous carcinoma cells obtained by confocal microscopy. The over expression of CD44 protein is noted by the red fluorescence observed on cell membranes and in cytoplasms. Image of benign lesions cells obtained by confocal microscopy. No red fluorescence is observed indicating the absence of CD44 protein expression.
All the biological properties of CD44 are essential to the physiological activities of normal cells, but they are also associated with the pathologic activities of cancer cells.
CD44s is expressed ubiquitously, but the expression of CD44v is far more restricted in normal tissues. In tumor tissue and cells, there is an increased level of CD44s and expression of CD44v.
DiscussionMany studies have demonstrated that during malignant development there are changes in cell surface carbohydrates associated with blood group antigens.
The secretor enzyme (FUT2), an α-1,2-fucosyltransferase, is responsible for the fucose transfer in an α-1,2 linkage to form the terminal H type 1 structure.5,6 The cell-surface fucosylated oligosacharides participate in several biological processes, such as embryogenesis, tissue differentiation, tumour metastasis, inflammation and bacterial adhesion.15,16 About 20% of the Caucasian population is non-secretor.10 Several disease correlations have been linked to non-secretor status. In general, being non-secretor results in several disadvantages regarding metabolism and immune function
Our results have demonstrated that the large majority of the individuals examined in the healthy group were secretors (have the FUT2 gene) (78.7%) and there were significant difference between secretors and non-secretors in the experimental group. We have also found a higher intensity of oral disease in the non-secretor group, and the occurrence of epithelial dysplasia was mostly found in the non-secretor group. This study evaluated the association between oral lesions and polymorphisms of the Se genes. We found that oral pre-cancerous and cancerous lesions were increased among individuals with non-secretor status and nonsense mutation 428G→A (Trp143→stop) (58.33%). We found 25 patients diagnosed anatomopathologically as malignant lesions despite the secretory status.
The studies of patients with premalignant and malignant oral lesions, in which non-secretor status predominates, appear to be an associated risk marker for the development of oral cancer. Leukoplakia and erythroplakia are clinical changes in the oral mucosa regarded as potentially malignant lesions.1,13 Certain histopathological changes may indicate a malignant potential in a lesion. However, the presence of such changes is not a reliable predictor of malignant transformation, and their absence does not mean that the patient is out of risk of developing a tumour.1,14
Although the relationship between epithelial dysplasia in a leukoplakia and malignant transformation of the lesion is debatable, many workers consider that the finding of epithelial dysplasia indicates a higher likelihood to develop malignancy. It is, however, more probable that the antigen changes found in the dysplastic lesions are associated with other factors, such as cell movement and growth rate, rather than malignancy per se.25
Our study evaluated the association between oral lesions and polymorphisms of the Se genes and secretor status. We found that oral pre-cancerous and cancerous lesions were increased among individuals with non-secretor status and nonsense mutation 428G→A (Trp143→stop). We also demonstrated that the Le (a+b−) antigen expression was present in the population showing greater risk. The studies of patients with premalignant and malignant oral lesions, in which non-secretor status predominates, show that this status appears to be an associated risk marker for the development for oral cancer.
CD44 is a multistructural and multifunctional cell surface molecule involved in cell proliferation, cell differentiation, cell migration, angiogenesis, presentation of cytokines, chemokines, and growth factors to the corresponding receptors, and docking of proteases at the cell membrane, as well as in signalling for cell survival. All these biological properties are essential to the physiological activities of normal cells, but they are also associated with the pathologic activities of cancer cells
They show aberrant expression and processing of CD44 transcripts and cell surface expression of CD44 appears to change profoundly during tumour metastasis, particularly during the progression of various carcinomas.21,24 Numerous studies based on immunohistochemical analyses of paraffin-embedded or frozen tissue sections using different monoclonal antibodies to CD44 isoforms and molecular biological techniques have provided evidence that in many types of tumours there is overexpression of CD44 isoforms.
The results obtained showed fluorescence corresponding to the presence of CD44 protein in samples from patients diagnosed with cancer and precancer. These findings indicate that overexpression of CD44 molecule analyzed could be considered as a marker of risk in individuals with oral lesions.
In summary, our results indicate that at the same time as the morphological changes that occur during the process of oral carcinogenesis, another series of events occurs. Further follow-up studies are required to clarify the role of predictive markers of risk in precursor lesions of oral cancer.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of DataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and /or subjects mentioned in the article. The author for correspondence is in possession of this document.
Conflict of interestsThe authors declare no conflict of interest.